Back to Journals » Journal of Pain Research » Volume 14
Efficacy and Safety of Pregabalin for Fibromyalgia in a Population of Chinese Subjects
Authors Zhang X, Xu H , Zhang Z, Li Y, Pauer L, Liao S, Zhang F
Received 11 September 2020
Accepted for publication 26 January 2021
Published 25 February 2021 Volume 2021:14 Pages 537—548
DOI https://doi.org/10.2147/JPR.S281483
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael Schatman
Xiao Zhang,1 Huji Xu,2 Zhiyi Zhang,3 Yang Li,4 Lynne Pauer,5 Shanmei Liao,6 Fengchun Zhang7
1Department of Rheumatology, Guangdong General Hospital, Guangdong, People’s Republic of China; 2Department of Rheumatology and Immunology, Shanghai Changzheng Hospital, Affiliated to Second Military Medical University, Shanghai, People’s Republic of China; 3School of Clinical Medicine, The First Affiliated Hospital of Harbin Medical University, Harbin, People’s Republic of China; 4Department of Rheumatology and Immunology, The Second Affiliated Hospital of Harbin Medical University, Harbin, People’s Republic of China; 5Global Research and Development, Pfizer, Groton, CT, USA; 6Pfizer China Statistics Department, Global Innovative Pharma Business, Shanghai, People’s Republic of China; 7Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Beijing, People’s Republic of China
Correspondence: Fengchun Zhang
Peking Union Medical College Hospital, No. 9, Dongdan Santiao, Dongcheng District, Beijing, People’s Republic of China
Tel/Fax +8610-69155416
Email [email protected]
Purpose: Fibromyalgia (FM) may go underdiagnosed and untreated in China in part due to a lack of awareness and understanding of the condition, and limited available treatments.
Patients and Methods: This randomized, double-blind, Phase III local registration trial compared the efficacy and safety of pregabalin (flexibly dosed 300– 450 mg/day) versus placebo for the management of pain in Chinese adults diagnosed with FM according to American College of Rheumatology 1990 criteria, across 22 centers within China. Patients reported pain score of ≥ 40 mm on 100-mm scale (from 0 “no pain” to 100 “worst possible pain”). The primary efficacy endpoint was change from baseline to Week 14 in mean pain score (MPS). Secondary endpoints included measures of sleep and sleep interference. Safety and tolerability were monitored throughout.
Results: Median pregabalin dose was 335 mg/day. A significant reduction from baseline to Week 14 in weekly MPS was seen for patients treated with pregabalin (n=170) versus placebo (n=164) (least-squares mean difference [95% confidence interval]: – 0.73 [– 1.10 to – 0.36]; P=0.0001). Significantly greater proportions of patients experienced ≥ 30% and ≥ 50% reductions in MPS at Week 14 with pregabalin versus placebo. Pregabalin-treated subjects demonstrated improvements in measures of sleep and sleep interference. Pregabalin was generally well tolerated. The most common adverse events were dizziness and somnolence; no serious adverse events (SAEs) occurred in pregabalin-treated subjects. Nine placebo-treated subjects experienced SAEs.
Conclusion: Pregabalin (300– 450 mg/day) is a safe and effective treatment for reducing pain and improving sleep in native Chinese subjects with FM.
ClinicalTrials.gov Identifier: NCT01387607.
Keywords: China, chronic pain, FM, Lyrica, pain management, sleep
Introduction
Fibromyalgia (FM) is a leading cause of chronic pain globally1–5 and imposes personal, social, and economic burdens on individual patients, their families, and society as a whole.6,7 The key characteristic of FM is chronic, widespread musculoskeletal pain with multiple tender points, which can be associated with unrefreshing sleep, fatigue, somatic symptoms, and, in some patients, cognitive problems.8,9 However, patients also demonstrate a higher prevalence of anxiety and depression than the general population,9,10 adding further to the burden of disease in patients with FM.
Diagnosis of FM has always been complicated by symptoms overlapping with those of other conditions, such as myofascial pain syndrome, chronic fatigue syndrome, thyroid disorders, or autoimmune disease.11,12 As such, FM is often diagnosed and treated as a rheumatic condition when other causes of widespread pain have been systematically ruled out.11–13 Due in part to these complexities around diagnosis, “fibromyalgia” as a condition is not widely accepted in certain countries, and not accepted in some provinces of China.14 Patients are consequently seen in a variety of clinical settings depending on the patient’s symptoms or preference. As a result, patients are seen by a diverse group of Chinese healthcare providers who may not be experienced in FM recognition or treatment.14–16 There is, therefore, a real need to increase awareness of FM across healthcare professionals in China in order to improve diagnosis and management, regardless of the place of initial presentation.
With possible barriers to diagnosis and acceptance of FM in China, estimates of the prevalence vary,17–19 and data remain limited. For example, a nationwide survey of rheumatologists in China in 2010 demonstrated that even among specialists, awareness and understanding of FM were low.19 An epidemiological survey estimated the overall prevalence of FM among Chinese residents of Hong Kong at 0.82%,18 which is at the lower end of the worldwide prevalence estimates of between 1% and 10%.1–5 Conversely, a provincial survey in China estimated a much lower prevalence of 0.07% among residents of multistory buildings in China.17 The real burden of FM in China is therefore unclear and inconsistent, but these studies provided “unequivocal support” for the presence of FM in China.17
Pregabalin is approved for the treatment of FM-associated pain in at least 39 countries, including the United States,20 Japan,21 and South Korea,22 and in 2018 was approved for use in China. Studies conducted in the United States,23–25 as well as other regions including Japan,26,27 have demonstrated the efficacy and safety of pregabalin for the management of pain and improving sleep in patients with FM. Although these studies demonstrate the efficacy and safety of pregabalin for patients with FM from different ethnic backgrounds, at the time the present study was planned, no medical treatments were approved in China for FM-associated pain. Given the historically low acceptance of FM in China,14 it is imperative that data specifically from Chinese patients living within China can be presented to physicians to aid their awareness and acceptance of FM and, moreover, to allow them to treat these patients effectively. Accordingly, this local registration study sought to be the first to determine the efficacy and safety of pregabalin for treatment of FM in Chinese patients living within China.
Patients and Methods
Study Design
A multicenter, randomized, double-blind, flexibly dosed, parallel-group, placebo-controlled trial (ClinicalTrials.gov identifier: NCT01387607) was conducted at 22 centers in China between February 2012 and October 2016. The study included a 1-week screening/single-blind placebo run-in phase, a 14-week double-blind treatment phase (2-week titration phase and 12-week fixed-dose phase), and a 1-week taper phase (Supplementary Figure S1). Patients meeting inclusion/exclusion criteria during screening/baseline entered the 1-week run-in phase. The study was conducted in accordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, the International Conference on Harmonisation Good Clinical Practice Guidelines, and the Declaration of Helsinki. Subjects provided written informed consent to participate in the trial. The study protocols were approved by the internal review boards and ethics committees of all 22 study sites (primary site: Peking Union Medical College Hospital; approval number 2010L03879-2010L03885).
Inclusion Criteria
Men or women (nonpregnant, nonlactating), ≥18 years of age, who met American College of Rheumatology (ACR) 1990 criteria for FM (widespread pain present for ≥3 months, pain in ≥11 of 18 specific tender points28) and had a score of ≥40 mm at screening and randomization on a 100-mm visual analog scale (VAS; where 0 indicates “no pain” and 100 indicates “being in the worst possible pain”), were eligible for inclusion. Subjects also completed ≥4 pain diaries within 7 days prior to randomization, recording a mean daily numeric rating scale (NRS) pain score ≥4 (0 = “no pain” to 10 = “worst possible pain”). The VAS and NRS assessments were not part of the diagnosis criteria for FM.28
Exclusion Criteria
Subjects were excluded if they demonstrated a high placebo response (≥30% decrease on 100-mm VAS at randomization relative to screening). Other exclusion criteria included: pain due to other conditions that might confound assessment; prior participation in a pregabalin clinical trial; history of failed pregabalin treatment; current pregabalin use; diagnosis of severe depression; active malignancy; or an immunocompromised status. Subjects with creatinine clearance ≤60 mL/min were also excluded.
Study Medication
Subjects were randomized (1:1) to pregabalin (300–450 mg/day, flexible dose) or placebo. Randomization occurred using a computer-generated pseudorandom code with random permuted blocks. Study medication was administered orally BID, starting at 150 mg/day (75 mg BID) in Week 1 and increasing to 300 mg/day for Week 2. At the end of Week 2, the dose was either maintained at 300 mg/day (150 mg BID) or increased to 450 mg/day (225 mg BID) at entry into the 12-week fixed-dose treatment period, depending on tolerability and response. Subjects who could not tolerate dose escalation above 150 mg/day were discontinued from the study. Subjects were permitted to take aspirin (≤325 mg/day) for cardiovascular prophylaxis, or acetaminophen (≤4 g/day) for additional pain relief.
Efficacy Measures
The primary efficacy endpoint was change from baseline to Week 14 in mean pain score (MPS), calculated from the past 7 days of pain diary entries (using the 11-point NRS from 0 “no pain” to 10 “worst possible pain”). Weekly MPS from daily pain diaries was also assessed in the primary efficacy analysis. The original protocol was updated from including co-primary endpoints (pain score, Patient Global Impression of Change [PGIC], and Fibromyalgia Impact Questionnaire [FIQ]) to the single primary endpoint (weekly MPS) in accordance with US Food and Drug Administration guidance provided to the sponsor.
The efficacy of pregabalin versus placebo treatment on sleep was assessed using: the Medical Outcomes Study Sleep Scale (MOS-SS), a subject-rated questionnaire consisting of 12 items that assess key constructs of sleep; the sleep interference score, recorded using a daily sleep diary on an 11-point NRS (scored from 0 “does not interfere with sleep” to 10 “completely interferes [cannot sleep due to pain]”), over a 24-hour recall period; and the Subjective Sleep Questionnaire (SSQ; not a validated measure), administered to each subject approximately 30–60 minutes after arising in the morning, each day. The SSQ consists of patient-reported estimates of subjective waking after sleep onset (sWASO), subjective latency to sleep onset (sLSO), subjective number of awakenings after sleep onset (sNAASO), subjective total sleep time (sTST), and rated quality of sleep.
Other secondary assessments included the difference between treatment with pregabalin versus placebo at end of study/early termination (before the 1-week taper period, unless otherwise stated), on the PGIC 7-point scale (from 1 “very much improved” to 7 “very much worse”), with responses defined as either: (i) much/very much improved (PGIC ≤2); or (ii) any improvement (PGIC ≤3); the FIQ self-administered questionnaire, which included questions on FM symptoms and function, with scores ranging from 0 (“no impact”) to 100 (“maximum impact”); and the 100-mm pain VAS, ranging from 0 (“no pain”) to 100 (“worst possible pain”). Health, functioning, fatigue, and mood were assessed using the 36-Item Short Form Health Survey (SF-36), the Multidimensional Assessment of Fatigue (MAF) scale, and the Hospital Anxiety and Depression Scale (HADS).
Safety and Tolerability
Adverse events (AEs) were monitored and evaluated from the time of first dose of study treatment until the last subject visit, and were classified in terms of severity (mild/moderate/severe), whether they were treatment emergent (TEAEs), and/or whether they were serious (SAEs; life-threatening, resulted in hospitalization/incapacity or death). Laboratory values (hematology, chemistry, urinalysis); vital signs; physical, ophthalmological, and neurological examinations; and 12-lead electrocardiogram (ECG) were assessed either during or at the end of study, as appropriate. All subjects who were randomized and took at least one dose of study medication were included in the safety analysis.
Sample Size
A sample size of 160 subjects per group was determined to have 90% power to detect a treatment difference of 0.8 in endpoint MPS at a significance level of 0.05 (for a two-sided test). The study used a placebo run-in phase and it was assumed that ~50% of screened subjects would not be randomized after the placebo run-in phase; it was anticipated that ~648 subjects would be screened. It was also assumed that ~1% of randomized subjects would not take study medication, and therefore ~162 subjects per group would be randomized in a 1:1 ratio (~324 subjects in total). Based on previous evidence from studies of patients with FM treated with pregabalin,23,24,27 the expected standard deviation for endpoint MPS was ~2.2 points, and treatment difference in endpoint MPS between pregabalin versus placebo ~0.8 points. While the study was in progress, a completed pregabalin study in Japanese patients with FM showed a smaller treatment difference in MPS with pregabalin versus placebo treatment.26 Following a protocol amendment, an unblinded interim analysis was performed to re-estimate the sample size, but no adjustment in sample size was required.
Statistical Analysis
Unless otherwise stated, all efficacy and safety analyses were based on the full analysis set (FAS), defined as all randomized subjects who received ≥1 dose of study medication. Endpoint refers to Week 14 (before the 1-week taper period) for all efficacy measures. For assessments based on daily diary entries (MPS, sleep interference, and SSQ scores), endpoint mean value was calculated from the last 7 days of diary entries before the taper.
Change from baseline in weekly MPS was the primary endpoint, analyzed using a mixed model repeated measures (MMRM) approach, with model terms for treatment, center, week, treatment-by-week interaction, and baseline MPS as the covariates. The MMRM used “week” as the repeated factor and utilized a compound symmetry covariance matrix. Two sensitivity analyses were performed. First, final weekly MPS during the double-blind phase was derived from MPS from the last seven pain diary entries using the last observation carried forward (LOCF) imputation method. The LOCF-imputed final weekly MPS was then compared between treatment groups using an analysis of covariance (ANCOVA), with treatment and center as factors and baseline MPS as a covariate. Second, weekly MPS was analyzed using the same MMRM approach as the primary analysis, based on the per-protocol analysis set (PPAS), a subset of the FAS containing only subjects without any major protocol deviations that would affect efficacy assessments. Definitions of major protocol deviations used to define the PPAS are given in Supplementary Table S1. Pain responder rates were calculated for proportions of subjects with ≥30% or ≥50% improvement in MPS from baseline to endpoint.
For secondary efficacy analyses: the two predefined recategorizations of PGIC-observed data were each analyzed using the Cochran–Mantel–Haenszel (CMH) test stratified by center. Responder status was imputed by the LOCF approach for missing data and analyzed with CMH test stratified by center. The following scales were imputed with the LOCF approach and analyzed by ANCOVA, with treatment and center as factors and baseline score as covariate: FIQ total score, pain VAS, MAF, SF-36, HADS, six of the MOS-SS subscales (sleep disturbance, snoring, short of breath/headache at awakening, quantity of sleep, sleep adequacy, somnolence), and MOS-SS overall sleep problems index. The MOS-SS optimal sleep subscale was imputed with the LOCF approach and analyzed using a logistic regression model with model terms of treatment and center, and the baseline score as a covariate. The weekly mean sleep interference and weekly mean SSQ for each domain were analyzed using MMRM in the same way as the weekly MPS.
Results
Patient Population
Of the 431 subjects screened for eligibility, 343 subjects were randomized (n=170 pregabalin, n=164 placebo) (Figure 1). Of the 334 patients assigned to treatment (FAS), 48 patients allocated to pregabalin (28.2%) and 39 to placebo (23.8%) discontinued treatment. Reasons for discontinuation are given in Figure 1. Of the pregabalin-treated subjects, 33 (19.4%) received a fixed dose of 300 mg/day and 119 (70.0%) received a fixed dose of 450 mg/day; the remaining subjects did not reach the fixed-dose phase. For both groups, median treatment duration was 106 days. The mean pregabalin dose was 335 mg/day (titration phase included). The majority of subjects were female (n=286; 86.0%) and the average age was 44 years. Pregabalin and placebo groups were comparable with respect to demographic and baseline characteristics (Table 1). Mean duration since onset of FM was 6.0 years (pregabalin) and 5.6 years (placebo).
 |
Figure 1 Patient dispositiona. Note: aThe per-protocol analysis set (PPAS) was a subset of the full analysis set (FAS) containing only subjects without any major protocol deviations that would affect efficacy assessments (see Table S1). |
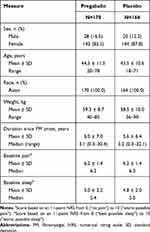 |
Table 1 Patient Demographics and Baseline Characteristics |
Efficacy Outcomes
The primary efficacy endpoint (MMRM FAS) showed a statistically significant reduction from baseline to endpoint in MPS for pregabalin compared with placebo (least-squares [LS] mean difference: –0.73; P=0.0001) (Table 2). Improvement in weekly MPS was significantly greater with pregabalin than with placebo at every time point from Week 1 through Week 14 (Figure 2). The two sensitivity analyses conducted in the FAS (ANCOVA, LOCF) and in the PPAS (MMRM) populations also showed significant improvement in endpoint MPS with pregabalin versus placebo treatment (Table 2).
 |
Table 2 Primary Efficacy Outcome (FAS MMRM) of Change from Baseline in Mean Pain Score at Endpoint Between Patients Treated with Pregabalin vs Placebo, and Associated Sensitivity Analyses |
Pregabalin subjects demonstrated improvements in many secondary efficacy endpoints, although not all reached statistical significance compared with placebo (Table 3). Significantly greater proportions of patients experienced ≥30% (47.5% vs 32.7%; P=0.0044) and ≥50% reductions (27.2% vs 17.0%; P=0.0189) in MPS at endpoint with pregabalin compared with placebo, respectively (Table 3).
 |
Table 3 Summary of Secondary Efficacy Outcome Measures, FAS |
Significantly greater improvements from baseline to endpoint were evident for pregabalin versus placebo in mean sleep interference score (LS mean difference = –0.88; P<0.0001); MOS-SS subscales of sleep adequacy (LS mean difference = 9.03; P=0.0003), quantity of sleep (LS mean difference = 0.286; P=0.0106), and somnolence (LS mean difference = 4.194; P=0.0112); and SSQ subscales of sWASO (LS mean difference = –17.17; P=0.0273), sNAASO (LS mean difference = –0.66; P<0.0001), and sleep quality (LS mean difference = 0.77; P=0.0003) (Table 3). There were no statistically significant differences between the pregabalin and placebo groups in the MOS-SS subscales of optimal sleep, sleep disturbance, snoring, short of breath/headache at awakening, and overall sleep problems index, nor SSQ subscales of sLSO and sTST (Table 3).
In terms of mood, HADS depression total score was significantly improved with pregabalin versus placebo (LS mean difference = –0.83; P=0.0226), whereas HADS anxiety total score did not differ with pregabalin versus placebo (Table 3). For the two predefined recategorizations of PGIC, greater proportions of pregabalin subjects gave responses of “much or very much improved” and “any improvement” in comparison with placebo subjects, but the differences in responses did not reach statistical significance (Table 3). Improvements from baseline to endpoint in FIQ total score with pregabalin were numerically but not significantly greater than with placebo (Table 3). Likewise, change at endpoint with pregabalin versus placebo did not reach statistical significance for pain VAS, SF-36 mental and physical components (MPC), and MAF index scores (Table 3).
Safety and Tolerability Evaluations
Pregabalin was generally well tolerated. All-causality TEAEs were reported by 70.0% (119/170) of subjects in the pregabalin group and 62.8% (103/164) of subjects in the placebo group (Table 4). The majority of these TEAEs were rated by investigators as mild (pregabalin, 67.3%; placebo, 74.7%). Among pregabalin-treated subjects, the most common all-causality TEAEs were dizziness (41.8%) and somnolence (17.6%). In the placebo group, dizziness and somnolence occurred in 18.3% and 7.9% of subjects, respectively. The majority of reports of dizziness in pregabalin-treated subjects (72% [51/71]) were of mild severity. Fifteen (8.8%) pregabalin-treated subjects discontinued due to dizziness, seven of whom reported mild and eight moderate dizziness. Incidences of severe AEs were similar with pregabalin (9.4% [16/170]) and placebo (9.1% [15/164]). Early discontinuations due to AEs occurred in 12.9% of the pregabalin group and 6.7% of the placebo group. No SAEs occurred in pregabalin-treated subjects. Nine SAEs occurred in placebo-treated subjects, of which two were considered to be treatment-related (atrial tachycardia in a 71-year-old female that was not resolved by end of study; cerebral hemorrhage in a 55-year-old female that resolved without sequelae). No notable findings were observed for any other safety parameters (physical examination, neurological examination, vital signs, laboratory test results, and ECG).
 |
Table 4 Overview of Common Treatment-Emergent AEs (All-Causality Safety Analysis) |
Discussion
This is the first Phase III, placebo-controlled, double-blind study to demonstrate the clinical efficacy and safety of pregabalin (300–450 mg/day) for reducing pain and improving sleep in Chinese subjects diagnosed with FM, using data from within China. Moreover, in addition to reducing pain and improving measures of sleep and sleep interference, pregabalin treatment improved other symptoms of FM, including mood, when compared with placebo treatment. Pregabalin was also generally well tolerated and there were no unexpected safety findings.20 These multidimensional observations demonstrate the potential for pregabalin treatment to improve a range of FM-related symptoms in native Chinese subjects.
Historically, the term “fibromyalgia” has not been widely accepted in China,17 despite FM being an accepted condition in other countries for decades.28,29 Even though some Chinese subjects living in other countries have been included within the clinical evidence that supported approval of pregabalin for FM,20 there was a real need for high-quality, local clinical evidence to allow Chinese physicians to evaluate data in patients directly comparable to patients they would see in daily clinical practice and to support local approval. This was the first study to confirm an improvement in MPS in a population of native Chinese subjects with FM following pregabalin treatment, and observations are broadly consistent with data from other international cohorts of patients with FM, including those from Asian patients,26 and from the United States.23–25 For example, in a cohort of Japanese subjects with FM, a reduction in MPS was also seen within 1 week, and at each week, with pregabalin treatment, although the endpoint difference in MPS with pregabalin (vs placebo) was higher in the present cohort of Chinese subjects with FM compared with this earlier study in Japan (–0.73 vs –0.44 after 15-weeks’ treatment in Japanese FM patients26). Our study focused on pain as a key component of patients’ FM, and we demonstrate a significant reduction in pain using both the 11-point NRS, within patient-reported pain diaries, as well as on the physician-administered 100-mm VAS. The present study also demonstrated that more Chinese patients achieved ≥30% and/or ≥50% pain responder status following treatment with pregabalin, based on weekly pain diaries, in line with previous studies from Japan and the United States.24,26 As a pain reduction of ≥30% on the 11-point NRS is considered clinically meaningful,30 our observations support the positive clinical implications of pregabalin treatment for the management of pain in Chinese patients with FM.
Since the design of the present study, the acceptance and understanding of FM has increased substantially worldwide, including within China, with more treatments available for FM in certain countries,31–33 although data beyond the use of traditional Chinese medicines are still limited in native Chinese patients with FM.14,34,35 With greater understanding, ACR diagnosis criteria have been updated and now focus on widespread pain (the widespread pain index [WPI]) alongside other symptoms (using measures such as the symptom severity scale),8,9 rather than focusing on tender point analysis, which the present study used as part of the screening criteria for eligibility.28 Updated 2010/2016 ACR guidelines have been validated in many countries,36 although the importance of tender points in the diagnosis of FM is still debated by some.37,38 The increasing acceptance of FM within China can be demonstrated by an ongoing study aiming to translate, adapt, and validate a Chinese-language version of the 2010 ACR preliminary diagnosis criteria and the 2016 revisions.39 Translation and validation of the updated ACR diagnostic criteria for FM will be invaluable to future studies in native Chinese subjects with FM.
Sleep disturbance is a key clinical domain of FM, particularly as there is a reciprocal relationship between increased pain and poor sleep, with poor sleep also associated with reduced physical function and risk of depression.40 In the current study, certain measures of sleep interference, sleep quality, and sleep adequacy were improved following treatment with pregabalin, in line with observations from other international studies,24,25 including patients from Japan.26 However, some assessments of sleep, including subscales on the MOS-SS, did not reach significance with pregabalin treatment. Interestingly, the MOS-SS somnolence assessment was improved with pregabalin treatment in the present study, despite somnolence being a commonly reported AE with pregabalin both in the present study and in the prescribing information.20 Although not conducted to compare safety, the safety profile of pregabalin seen in this native Chinese population with FM was generally consistent with the Phase II and Phase III clinical studies reported in other patient populations,24,25,27 and with the prescribing information.20 While Chinese subjects in the present study also demonstrated numerical improvements in other measures of general health and functioning with pregabalin compared with placebo, not all outcomes reached significance. For example, there was no significant improvement in PGIC, FIQ total score, SF-36 scores, or MAF. These observations are somewhat in contrast to US studies of pregabalin in patients with FM, which have demonstrated certain improvements in overall global measures of function and fatigue.24,27 In addition, among Japanese subjects with FM, significant improvements in mean change from baseline FIQ score with pregabalin compared with placebo were noted.26 A study is also underway to translate and validate a Chinese-language version of the FIQ, alongside validating the 2010/2016 ACR diagnostic criteria in Chinese subjects.39 It will be of interest to see the outcomes of these validations and the application of the FIQ when translated.
Reasons for the different profile of secondary efficacy measures between the international and Japanese study and this study of patients from China are unclear. The present study was not designed or powered to detect significant treatment differences on secondary endpoints, only on weekly MPS, and secondary observations are all considered exploratory. Subjects with a large placebo response during screening were excluded from the present study, but a high and enduring placebo response is known to occur in clinical trials of subjects with chronic pain.41 Therefore, we cannot exclude the possibility that a placebo response may have contributed to some of the evident differences in secondary outcomes between the present study and other international studies,24,26,27 nor exclude any impact of including a placebo control arm in observations from both the placebo and active treatment arms.42 Specifically designed studies would be needed to confirm any additional benefits on general health or wellbeing for Chinese patients with FM when treated with pregabalin, beyond the reductions in pain and sleep presented in this, the first placebo-controlled study of pregabalin conducted within China.
The study has several limitations in addition to those discussed. As noted, this study, with a relatively small sample size, was powered to detect a difference in endpoint weekly MPS with pregabalin versus placebo treatment, and any other observations should be considered descriptive. A fixed-dose titration schedule was used to ensure patients were receiving the recommended dose of pregabalin for FM as approved in other countries (ie, 300–450 mg/day).20 As a result, 18 patients were excluded due to inadequate titration to a higher dose. However, many patients treated with pregabalin may not be up-titrated to recommended doses,43 due in part to concerns of side effects or other challenges with prescribing and titrating pregabalin.44 In a real-world clinical setting outside of the structured requirements of a clinical trial, the prescribing physician may use a “low and slow” approach to titrate pregabalin,44 so patients individually adopt their own titration tempo to limit the negative impact of side effects and early discontinuation of therapy prior to a therapeutic benefit.44 Further study in the real-world clinical setting would be needed to confirm whether Chinese patients with FM respond to lower-than-recommended doses of pregabalin. Our study employed a placebo run-in period to ensure that all patients were still eligible for inclusion following receipt of placebo for 1 week. When this study was designed, a placebo run-in was seen to be particularly useful for studies with subjective endpoints to allow initial high responders to placebo to be excluded, as an unexpected favorable placebo response may result in a specifically designed trial becoming underpowered.42 In more recent years, since the present study was designed, the use of a placebo run-in period has been questioned,45 and observations presented should be considered in light of these conclusions. Other specifications of inclusion and exclusion criteria, while integral to clinical trial design, may mean that the results from a 14-week trial cannot be extrapolated to treatment of longer duration, or generalized to all patients in China with FM, such as, to patients with only mild pain (ie, VAS <40 mm), comorbidities, and/or taking concomitant medication. For example, patients with severe depression were excluded from the present analysis, although depression is a well-known comorbidity in FM.35 However, patients with severe depression may be taking psychiatric drugs with sedative effects, which could have affected the objective evaluation of the effect of pregabalin. Although no difference would be anticipated based on international analyses,46 further study would be needed to determine the influence of severe depression and other baseline comorbidities on the efficacy of pregabalin in Chinese patients with FM. Despite these limitations, the results of this study indicate that pregabalin may offer an important treatment option for patients with FM living and being treated within China.
Conclusions
Native Chinese subjects with a diagnosis of FM, and at least moderate pain, showed clinically meaningful reductions in pain and improvements in assessments of sleep and sleep interference following 14-weeks’ treatment with pregabalin (300–450 mg/day) versus placebo. Improvements in secondary measures of pain responder status and several other subjective measures of sleep were also observed with pregabalin treatment (vs placebo). These multidimensional observations suggest that pregabalin may be an effective treatment for a range of symptoms associated with FM in Chinese patients. Further study is needed to confirm the reproducibility of these observations in a real-world clinical setting in China.
Abbreviations
ACR, American College of Rheumatology; AE, adverse event; ANCOVA, analysis of covariance; BID, twice daily; CI, confidence interval; CMH, Cochran–Mantel–Haenszel; ECG, electrocardiogram; FAS, full analysis set; FIQ, Fibromyalgia Impact Questionnaire; FM, fibromyalgia; HADS, Hospital Anxiety and Depression Scale; LOCF, last observation carried forward; LS, least-squares; MAF, Multidimensional Assessment of Fatigue; MMRM, mixed model repeated measures; MOS-SS, Medical Outcomes Study Sleep Scale; MPC, mental and physical components; MPS, mean pain score; NRS, numeric rating scale; PGIC, Patient Global Impression of Change; PPAS, per-protocol analysis set; SAE, serious adverse event; SD, standard deviation; SE, standard error; SF-36, 36-Item Short Form Health Survey; sLSO, subjective latency to sleep onset; sNAASO, subjective number of awakenings after sleep onset; SSQ, Subjective Sleep Questionnaire; sTST, subjective total sleep time; sWASO, subjective waking after sleep onset; TEAE, treatment-emergent adverse event; VAS, visual analog scale.
Data Sharing Statement
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices: (1) for indications that have been approved in the US and/or EU; or (2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Ethics Approval and Informed Consent
The study was conducted in accordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, the International Conference on Harmonisation Good Clinical Practice Guidelines, and the Declaration of Helsinki. Subjects provided written informed consent to participate in the trial. The study protocols were approved by the internal review boards and ethics committees of all 22 study sites (primary site: Peking Union Medical College Hospital; approval number 2010L03879-2010L03885).
Acknowledgments
Medical writing support was provided by Karen Burrows, MPhil, CMPP, of Engage Scientific Solutions (Horsham, UK), and was funded by Pfizer. Parts of these data were presented as a poster at the 2017 Annual Scientific Meeting of the American College of Rheumatology and Association of Rheumatology Health Professionals (ACR/ARHP), San Diego, CA, USA (November 3–8, 2017), and as a poster at the 37th Annual Scientific Meeting of the American Pain Society (APS) (March 4–6, 2018).
Author Contributions
All authors made significant contribution to the work reported, including contributing to data analysis, and drafting and/or revising the manuscript. All authors agreed on the journal to which the article was submitted, gave final approval of the version to be published, and take responsibility for all aspects of the work.
Funding
This study was sponsored by Pfizer. The sponsor was involved in aspects of study design, data collection, and analysis.
Disclosure
LP is an employee of Pfizer and has stock options in Pfizer. SL was employed by Pfizer during the study and initial development of the manuscript. All other authors were investigators of the study and have no conflicts of interest to declare regarding the content of this article.
References
1. Branco JC, Bannwarth B, Failde I, et al. Prevalence of fibromyalgia: a survey in five European countries. Semin Arthritis Rheum. 2010;39(6):448–453. doi:10.1016/j.semarthrit.2008.12.003
2. Carmona L, Ballina J, Gabriel R, Laffon A. The burden of musculoskeletal diseases in the general population of Spain: results from a national survey. Ann Rheum Dis. 2001;60(11):1040–1045. doi:10.1136/ard.60.11.1040
3. Walitt B, Nahin RL, Katz RS, Bergman MJ, Wolfe F. The prevalence and characteristics of fibromyalgia in the 2012 National Health Interview Survey. PLoS One. 2015;10(9):e0138024. doi:10.1371/journal.pone.0138024
4. Wolfe F, Brahler E, Hinz A, Hauser W. Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: results from a survey of the general population. Arthritis Care Res. 2013;65(5):777–785. doi:10.1002/acr.21931
5. McNally JD, Matheson DA, Bakowsky VS. The epidemiology of self-reported fibromyalgia in Canada. Chronic Dis Can. 2006;27(1):9–16.
6. Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013;17(8):356. doi:10.1007/s11916-013-0356-5
7. Schaefer C, Mann R, Masters ET, et al. The comparative burden of chronic widespread pain and fibromyalgia in the United States. Pain Pract. 2016;16(5):565–579. doi:10.1111/papr.12302
8. Wolfe F, Clauw DJ, Fitzcharles MA, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46(3):319–329. doi:10.1016/j.semarthrit.2016.08.012
9. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Care Res. 2010;62(5):600–610. doi:10.1002/acr.20140
10. Wolfe F, Walitt B. Culture, science and the changing nature of fibromyalgia. Nat Rev Rheumatol. 2013;9(12):751–755. doi:10.1038/nrrheum.2013.96
11. Arnold LM, Clauw DJ, Dunegan LJ, Turk DC. A framework for fibromyalgia management for primary care providers. Mayo Clin Proc. 2012;87(5):488–496. doi:10.1016/j.mayocp.2012.02.010
12. Arnold LM, Clauw DJ, McCarberg BH. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin Proc. 2011;86(5):457–464. doi:10.4065/mcp.2010.0738
13. Choy E, Perrot S, Leon T, et al. A patient survey of the impact of fibromyalgia and the journey to diagnosis. BMC Health Serv Res. 2010;10:102. doi:10.1186/1472-6963-10-102
14. Mist SD, Jones KD. Randomized controlled trial of acupuncture for women with fibromyalgia: group acupuncture with traditional Chinese medicine diagnosis-based point selection. Pain Med. 2018;19(9):1862–1871. doi:10.1093/pm/pnx322
15. Mist S, Wright C, Jones KD, Carson JW, Shih J. Traditional Chinese medicine for fibromyalgia. Pract Pain Manag. 2010;10(7):1–7.
16. Mist SD, Wright CL, Jones KD, Carson JW. Traditional Chinese medicine diagnoses in a sample of women with fibromyalgia. Acupunct Med. 2011;29(4):266–269. doi:10.1136/acupmed-2011-010052
17. Russell IJ. The prevalence of the fibromyalgia syndrome in China. J Musculoskelet Pain. 2006;14(2):1–2.
18. Scudds RA, Li EKM, Scudds RJ. The prevalence of fibromyalgia syndrome in Chinese people in Hong Kong. J Musculoskelet Pain. 2006;14(2):3–11. doi:10.1300/J094v14n02_02
19. Mu R, Li C, Zhu JX, et al. National survey of knowledge, attitude and practice of fibromyalgia among rheumatologists in China. Int J Rheum Dis. 2013;16(3):258–263. doi:10.1111/1756-185X.12055
20. Lyrica® (pregabalin), capsule and solution [prescribing information]. New York: Pfizer Inc; 2004.
21. Pfizer Japan Inc, Eisai Co, Ltd [Internet]. New indication approved in Japan for Lyrica® capsules; 2013. Available from: https://www.eisai.com/news/pdf/enews201311pdf.pdf.
22. Häuser W, Walitt B, Fitzcharles M-A, Sommer C. Review of pharmacological therapies in fibromyalgia syndrome. Arthritis Res Ther. 2014;16(1):201. doi:10.1186/ar4441
23. Arnold LM, Russell IJ, Diri EW, et al. A 14-week, randomized, double-blinded, placebo-controlled monotherapy trial of pregabalin in patients with fibromyalgia. J Pain. 2008;9(9):792–805. doi:10.1016/j.jpain.2008.03.013
24. Crofford LJ, Rowbotham MC, Mease PJ, et al. Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2005;52(4):1264–1273. doi:10.1002/art.20983
25. Mease PJ, Russell IJ, Arnold LM, et al. A randomized, double-blind, placebo-controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgia. J Rheumatol. 2008;35(3):502–514.
26. Ohta H, Oka H, Usui C, Ohkura M, Suzuki M, Nishioka K. A randomized, double-blind, multicenter, placebo-controlled phase III trial to evaluate the efficacy and safety of pregabalin in Japanese patients with fibromyalgia. Arthritis Res Ther. 2012;14(5):R217. doi:10.1186/ar4056
27. Pauer L, Winkelmann A, Arsenault P, et al. An international, randomized, double-blind, placebo-controlled, phase III trial of pregabalin monotherapy in treatment of patients with fibromyalgia. J Rheumatol. 2011;38(12):2643–2652. doi:10.3899/jrheum.110569
28. Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–172. doi:10.1002/art.1780330203
29. Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38(1):19–28. doi:10.1002/art.1780380104
30. Farrar JT, Young JP
31. Arnold LM, Whitaker S, Hsu C, Jacobs D, Merante D. Efficacy and safety of mirogabalin for the treatment of fibromyalgia: results from three 13-week randomized, double-blind, placebo- and active-controlled, parallel-group studies and a 52-week open-label extension study. Curr Med Res Opin. 2019;35(10):1825–1835. doi:10.1080/03007995.2019.1629757
32. Clauw DJ, Mease P, Palmer RH, Gendreau RM, Wang Y. Milnacipran for the treatment of fibromyalgia in adults: a 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trial. Clin Ther. 2008;30(11):1988–2004. doi:10.1016/j.clinthera.2008.11.009
33. Lian YN, Wang Y, Zhang Y, Yang CX. Duloxetine for pain in fibromyalgia in adults: a systematic review and a meta-analysis. Int J Neurosci. 2020;130(1):71–82. doi:10.1080/00207454.2019.1664510
34. Han M, Cui J, Xiao Y, et al. Acupuncture for primary fibromyalgia: study protocol of a randomized controlled trial. Trials. 2020;21(1):538. doi:10.1186/s13063-020-04317-y
35. Cao H, Liu J, Lewith GT. Traditional Chinese medicine for treatment of fibromyalgia: a systematic review of randomized controlled trials. J Altern Complement Med. 2010;16(4):397–409. doi:10.1089/acm.2009.0599
36. Galvez-Sánchez CM, Reyes Del Paso GA. Diagnostic criteria for fibromyalgia: critical review and future perspectives. J Clin Med. 2020;9(4):1219. doi:10.3390/jcm9041219
37. Sarzi-Puttini P, Atzeni F, Masala IF, Salaffi F, Chapman J, Choy E. Are the ACR 2010 diagnostic criteria for fibromyalgia better than the 1990 criteria? Autoimmun Rev. 2018;17(1):33–35. doi:10.1016/j.autrev.2017.11.007
38. Vanderschueren S, Van Wambeke P, Morlion B. Fibromyalgia: do not give up the tender point count too easily: comment on the article by Wolfe et al. Arthritis Care Res (Hoboken). 2010;62(11):1675. doi:10.1002/acr.20293
39. Guang’anmen Hospital of China Academy of Chinese Medical Sciences [Internet]. Chinese version of fibromyalgia criteria and severity scales study. Available from: https://clinicaltrials.gov/ct2/show/NCT03381131.
40. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. doi:10.1016/j.jpain.2013.08.007
41. Klinger R, Stuhlreyer J, Schwartz M, Schmitz J, Colloca L. Clinical use of placebo effects in patients with pain disorders. Int Rev Neurobiol. 2018;139:107–128.
42. Enck P, Klosterhalfen S, Weimer K, Horing B, Zipfel S. The placebo response in clinical trials: more questions than answers. Philos Trans R Soc Lond B Biol Sci. 2011;366(1572):1889–1895. doi:10.1098/rstb.2010.0384
43. Serpell M, Latymer M, Almas M, Ortiz M, Parsons B, Prieto R. Neuropathic pain responds better to increased doses of pregabalin: an in-depth analysis of flexible-dose clinical trials. J Pain Res. 2017;10:1769–1776.
44. Freynhagen R, Baron R, Kawaguchi Y, et al. Pregabalin for neuropathic pain in primary care settings: recommendations for dosing and titration. Postgrad Med. 2021;133(1):1–9. doi:10.1080/00325481.2020.1857992
45. Laursen DRT, Paludan-Muller AS, Hrobjartsson A. Randomized clinical trials with run-in periods: frequency, characteristics and reporting. Clin Epidemiol. 2019;11:169–184. doi:10.2147/CLEP.S188752
46. Silverman SL, Backonja M, Pauer L, et al. Effect of baseline characteristics on the pain response to pregabalin in fibromyalgia patients with comorbid depression. Pain Med. 2018;19(3):419–428. doi:10.1093/pm/pnx091
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

