Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Efficacy and Safety of Paliperidone Palmitate 6-Month versus Paliperidone Palmitate 3-Month Long-Acting Injectable in European Patients with Schizophrenia: A Post Hoc Analysis of a Global Phase-3 Double-Blind Randomized Non-Inferiority Study
Authors Giron‐Hernandez C, Han JH, Alberio R, Singh A , García-Portilla MP, Pompili M , Knight RK, Richarz U, Gopal S , Antunes J
Received 20 December 2022
Accepted for publication 22 March 2023
Published 13 April 2023 Volume 2023:19 Pages 895—906
DOI https://doi.org/10.2147/NDT.S400342
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Cesar Giron‐Hernandez,1 Joong Hee Han,2 Roberta Alberio,3 Arun Singh,2 Maria Paz García-Portilla,4 Maurizio Pompili,5 R Karl Knight,2 Ute Richarz,6 Srihari Gopal,2,7 José Antunes8
1EMEA Medical Affairs, Janssen-Cilag, Issy-les-Moulineaux, France; 2Janssen Research & Development, LLC, Titusville, NJ, USA; 3Medical Affairs, Janssen-Cilag, Milan, Italy; 4Department of Psychiatry, Universidad de Oviedo, Instituto Sanitario Del Principado de Asturias (ISPA) and CIBERSAM, Oviedo, Spain; 5Department of Neurosciences, Mental Health, and Sensory Organs, Sant’Andrea Hospital, Sapienza University of Rome, Rome, Italy; 6Janssen Global Services LLC, Cilag Int., Zug, Switzerland; 7Regeneron Pharmaceuticals, Tarrytown, NY, USA; 8EMEA Medical Affairs, Janssen-Cilag, Porto Salvo, Portugal
Correspondence: José Antunes, EMEA Medical Affairs, Janssen-Cilag, Porto Salvo, Portugal, Email [email protected]
Purpose: To examine efficacy and safety of paliperidone palmitate (PP) 6-month (PP6M) vs PP3-month (PP3M) long acting injectable (LAI) in patients with schizophrenia from European sites previously stabilized on PP3M or PP1-month (PP1M).
Methods: This post-hoc subgroup analysis used data from a global phase-3 double-blind (DB) randomized non-inferiority study (NCT03345342). Patients were randomized (2:1, respectively) to receive dorsogluteal injections of PP6M (700 mg eq. or 1000 mg eq.) or PP3M (350 mg eq. or 525 mg eq.) in the 12-month DB phase. Primary endpoint was time-to-relapse during the DB phase, using a Kaplan–Meier cumulative survival estimate (non-inferiority margin 95% CI lower bound larger than prespecified as − 10%). Treatment emergent adverse events (TEAEs), physical examinations, and laboratory tests were also evaluated.
Results: A total of 384 patients who entered the DB phase were included in European sites (PP6M, n = 260; PP3M, n = 124) with a mean age similar in both groups (mean age [SD] years: PP6M, 40.0 [11.39]; PP3M, 38.8 [10.41]). Baseline characteristics were similar across both groups. The number of patients who experienced a relapse during DB phase were PP6M: 18 (6.9%) vs PP3M: 3 (2.4%) with percentage relapse-free difference of − 4.9% (95% CI: − 9.2%, − 0.5%), thus achieving non-inferiority criteria. Secondary efficacy endpoints indicated comparable improvements. Incidence of TEAEs was similar between PP6M (58.8%) and PP3M (54.8%) groups. Nasopharyngitis, headache, increased weight, and injection-site pain were the most common TEAEs.
Conclusion: The efficacy of PP6M was non-inferior to that of PP3M in preventing relapse in the European subgroup previously treated with PP1M or PP3M, which was consistent with the global study. No new safety signals were identified.
Keywords: Europe, long-acting injectable, paliperidone palmitate 6-month, relapse-free, schizophrenia
Introduction
In patients with schizophrenia, nonadherence to antipsychotic (AP) medications is a major concern leading to relapse and rehospitalization, with risk elevations shown to occur as soon as 10 days after medication discontinuation. Studies have shown that patients with a treatment gap of 30 days or more are at nearly 5 times higher risk of hospitalization than those with a treatment gap of 0–10 days.1 Greater medication adherence is reported in patients with schizophrenia using long acting injectable (LAI) APs vs oral APs (OAP), resulting in fewer hospitalizations and lower overall health-care costs in real-world studies.2–5 Recent meta-analyses suggest that earlier and wider use of LAIs can reduce the risk of relapse and stabilize patients following the diagnosis of schizophrenia.4–6
Paliperidone palmitate (PP) LAIs as once-month (PP1M) and 3-month (PP3M) formulations have been reported to significantly control symptoms, prevent relapses, and reduce hospitalizations in patients with schizophrenia.7,8 Recently, PP 6-month (PP6M) was approved by the US Food and Drug Administration (August 31st, 2021), and European Medicines Agency (November 23rd, 2021). PP6M is the only 6-month LAI AP currently available; once-every-6-months makes it a unique treatment option, both in the early and the advanced phases of illness. Prior to transitioning to PP6M, patients must be adequately treated with PP1M for at least four months, or PP3M for at least one 3-month injection cycle.9
The European population represents an important geographic subgroup and displays differences in genetic factors.10,11 Ethnicity and genetic variations are among the major determinants of treatment response and tolerability. Patients with schizophrenia display variations in the manifestation of symptoms based on differences in sociocultural and contextual factors.12 A patient’s adherence to a specific drug may be affected by cultural or psychosocial factors, as well as limited or inadequate access to medical care.13 Thus, it is crucial to determine any potential differences in the efficacy and safety of PP6M across geographical regions. A previous global study, where efficacy and safety of PP6M (700 mg eq. or 1000 mg eq.) was compared with PP3M (350 mg eq. or 525 mg eq.) in a double-blind (DB), randomized, parallel-group, multi-center global study, demonstrated that PP6M was non-inferior to PP3M in preventing relapse in patients with schizophrenia adequately treated with corresponding doses of PP1M or PP3M. On transitioning from PP1M or PP3M to PP6M, there were no significant differences in efficacy or safety.9 This current post hoc subgroup analysis aims to assess the efficacy and safety of PP6M vs PP3M in European patients from that global study with schizophrenia previously stabilized on PP3M or PP1M as it is important to provide to clinicians, the evidence demonstrating the treatment efficacy and safety in European patients.
Materials and Methods
Study Population
This post hoc subgroup analysis used data from a global phase-3 double-blind (DB) randomized active-controlled non-inferiority study (NCT03345342) conducted at 121 sites across 20 countries (including 10 European countries). Data from the primary study are previously reported elsewhere.9 In the current analysis, we focus on the subgroup of patients recruited in investigational sites from European countries, including Bulgaria, France, Czech Republic, Hungary, Poland, Italy, Russian Federation, Turkey, Spain, and Ukraine. In this study, adult patients (18–70 years) of either gender with schizophrenia who were stabilized on PP1M for at least four months, or PP3M for at least one 3-month injection cycle, were screened based on eligibility criteria and enrolled into the study as described in detail in the primary study9 and elaborated in Supplementary Material 1.
As this study was a post hoc analysis of a subset of previously published data, no additional ethics committee or institutional review board approvals were required. The study protocol, informed consent forms, and applicable study documents were approved by an independent ethics committee or the institutional review board at each study site. The study was conducted in compliance with the principles of the Declaration of Helsinki, Good Clinical Practices, and applicable regulatory requirements. All patients provided written informed consent prior to study participation (Supplementary Material 2).
Study Design
The study was divided into 3 phases: (a) screening phase (up to 28 days); (b) open-label (OL) transition (conditional) and maintenance phases (1–3 months depending on either PP1M or PP3M received); and (c) DB phase (12 months). Eligible patients who completed the screening phase entered the OL phase. During the conditional OL-transition phase (1–4 months) patients who were receiving an OAP, injectable risperidone, or previously initiated on PP1M but were not yet stabilized, received additional doses of deltoid or gluteal PP1M. Patients previously stabilized for ≥3 months on PP1M or for at least one 3-month injection cycle PP3M entered an OL-maintenance phase and received 1 injection cycle of PP1M (100 mg eq. or 150 mg eq.) or PP3M (350 mg eq. or 525 mg eq.). During the DB phase, patients were randomized (2:1) to receive PP6M (700 mg eq. or 1000 mg eq.) or PP3M (350 mg eq. or 525 mg eq.). The doses of PP6M for the patients in the PP6M group were chosen equivalent to the most prescribed doses of PP1M and PP3M. Patients in the PP3M group who received OL PP1M doses (100 mg eq. or 150 mg eq.) were given DB PP3M doses (350 mg eq. or 525 mg eq., respectively), whereas those on OL PP3M doses (350 mg eq. or 525 mg eq.) continued the same treatment. Patients on OL PP1M (100 mg eq.) or PP3M (350 mg eq.) treatment received PP6M (700 mg eq.), whereas those undergoing OL PP1M (150 mg eq.) or PP3M (525 mg eq.) received PP6M (1000 mg eq.). To maintain blinding, patients in the PP6M group received 3-month matched 20% Intralipid® placebo injections as a substitute for active treatment. All injections in the DB phase were administered dorsogluteally by an unblinded drug administrator (with no other responsibilities in the study), due to differences in syringe sizes between PP6M and PP3M.
Assessments
The primary non-inferiority efficacy endpoint for this study was the percentage of European patients who remained relapse-free at 12 months between PP6M vs PP3M based on the differences in the 12-month Kaplan–Meier estimate of survival. Relapse was defined as in the primary study9 (Supplementary Material 1).
Secondary endpoints included changes from baseline during the 12 month DB phase in the Positive and Negative Syndrome Scale (PANSS) total and subscale scores, Clinical Global Impression-Severity (CGI-S), Personal and Social Performance (PSP) scale, the proportion of patients who met criteria for symptomatic remission (defined as having a score of ≤3 on all of the following eight PANSS items: P1, P2, P3, N1, N4, N6, G5, and G9 for the last 6 months of DB treatment, with one excursion allowed), and the proportion of patients who met both symptomatic and functional remission (PSP >70).
Safety assessments comprised evaluation of rates of treatment-emergent adverse events (TEAEs), including serious TEAEs and TEAEs leading to drug withdrawal, physical examinations, clinical laboratory tests, vital signs, assessments of body weight, electrocardiograms, and injection-site assessment. Extrapyramidal symptoms (EPS) were assessed using the Barnes Akathisia Rating Scale, the Simpson-Angus Scale, and the Abnormal Involuntary Movement Scale. Suicidal ideation and behavior was assessed using the Columbia Suicide Severity Rating Scale.
Statistical Analysis
The primary and the secondary efficacy analyses in the European subgroup were conducted on the DB intent-to-treat (ITT) population; safety (DB safety) analyses included patients who received at least one dose of treatment (PP3M or PP6M) during the DB phase (ITT-DB analyses set). Non-inferiority was concluded if the lower limit of the 2-sided 95% confidence interval (CI) of the difference in the relapse-free rates between PP6M and PP3M exceeded the pre-specified margin of −10%. Safety results were summarized descriptively.
Results
Patient Demographics and Disposition
Overall, 838 eligible patients were enrolled in the OL phase. Of these, 702 were randomized in the DB phase to the PP6M (n = 478) and PP3M (n = 224) groups; global primary results are published elsewhere.9 In the screening phase (up to 28 days), 496 patients were screened in European sites, of which n = 439 were enrolled or dosed in the OL phase (over 1 or 3 months depending on either PP1M or PP3M received), and 384 patients of which entered the DB phase (PP6M, n = 260; PP3M, n = 124) with a mean age similar in both groups (mean age [SD]: PP6M, 40.0 [11.39] years; PP3M, 38.8 [10.41] years). The majority of patients in both groups were men (71%) and white (PP6M, 96.5%; PP3M, 98.4%). Baseline characteristics were similar across both groups (Table 1). In total, 384 European patients continued and completed the DB phase. The DB ITT analysis set findings were similar for both the treatment groups for completion. For the total European study population, 321/384 (83.6%) patients in DB phase completed the study (PP6M, 82.3% [214/260]; PP3M, 86.3% [107/124]). The most common reason for patient discontinuation in the DB phase was the withdrawal of consent (Figure 1).
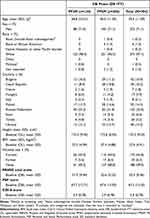 |
Table 1 Demographic and Baseline (OL) Characteristics (DB ITT Sets) |
Prior Therapies
Prior to the OL phase, the percentage of patients on psychotropic medication was similar between the PP3M and PP6M groups. At DB phase entry, 383/384 (99.7%) patients received psychotropic medications (Supplementary Material 3). Prior to study entry, 383/384 (99.7%) patients received psychotropic medications; 205/384 (53.4%) patients received oral atypical APs most commonly: risperidone (81, 21.1%), PP (51, 13.3%), olanzapine (47, 12.2%), quetiapine (36, 9.4%), aripiprazole (14, 3.6%). Depot APs were received by 188/384 (49.0%) patients; PP1M and PP3M (153, 39.8%) and risperidone (32, 8.3%). Anti-EPS medications were taken by 44 (11.5%) patients, of which, 42 (10.9%) received antidepressants, 36 (9.4%) benzodiazepines, 17 (4.4%) beta blockers, 10 (2.6%) mood stabilizers and antiepileptics, 7 (1.8%) non-benzodiazepines hypnotics and anxiolytics and 4 (1.0%) antihistamines.
Extent of Drug Exposure
A similar trend in drug exposure was observed in the DB ITT analysis set between patients from both treatment groups. Patients received similar mean (SD) doses of PP6M and PP3M. In the DB phase, the mean (SD) duration of exposure was 334.6 (85.71) days in the PP6M group and 339.3 (79.48) days in the PP3M group. The mean (SD) dose was 847.7 (150.27) mg eq. for PP6M and 440.3 (87.81) mg eq. for PP3M. In total, 227 (87.3%) patients received two active injections in the PP6M group, and 105 (84.7%) patients received four active injections in the PP3M group.
Primary Efficacy Endpoint
A total of 18 (6.9%) patients in the PP6M group and three (2.4%) patients in the PP3M group experienced a relapse event during the DB phase (DB ITT). The percentages of patients who remained relapse-free at the end of the 12-month DB phase as seen in the difference (95% CI) in the Kaplan–Meier estimate between the treatment groups (PP6M−PP3M) was −4.9% (95% CI: −[−9.2, −0.5]). The percentage of relapse-free patients at the end of the DB phase was 92.6% in the PP6M group and 97.5% in the PP3M group. Thus, the efficacy of PP6M was non-inferior to PP3M based on the lower limit bound of the 2-sided 95% CI being larger than the prespecified non-inferiority margin of −10% (Figure 2 and Table 2).
 |
Table 2 Time to Relapse During DB Phase and Number of Patients Who Remained Relapse Free at End of DB Phase (DB ITT Analysis Set) |
Secondary Efficacy Endpoints
The baseline characteristic of illness assessed by age, PANSS, PSP, and CGI-S scores in the PP6M and PP3M treatment groups were similar at first diagnosis. Comparable improvements in PANSS total and PANSS subscale, PSP, and CGI-S scores were consistent with the primary efficacy analysis (Table 3; Supplementary Material 4). Patients maintained persistent clinical stability over the 12-month DB period as seen in improvements in CGI-S and PSP scores. The percentage of patients with improvement in PANSS total scores (≥20%, ≥30% and ≥40%) from DB baseline to DB endpoint, was numerically higher in the PP6M group; 103 (39.9), 69 (26.7), 46 (17.8) compared with PP3M group; 39 (32.2), 29 (24.0), 20 (16.5), respectively (Table 4). The proportion of patients with symptomatic 6-month remission during the DB phase was similar in both the groups (PP6M: 179 [68.8] vs PP3M: 88 [71.0]) (Table 4).
 |
Table 3 Results of Secondary Endpoints: PANSS, CGI-S and PSP Scores (Change from Baseline (DB) to End of 12 Month [DB]) (DB ITT Sets) |
 |
Table 4 Summary of Treatment-Emergent Adverse Events During DB Phase (ITT Safety Analysis Set) |
Safety
In the DB phase, 153 (58.8%) European patients in the PP6M group, and 68 (54.8%) in the PP3M group experienced ≥1 TEAEs (Table 4). The majority of the TEAEs were mild or moderate in severity, and the most common TEAEs (≥2% in either group) were increased weight, headache, nasopharyngitis, and injection-site pain. Serious TEAEs (≥1% in either group) were experienced by 16 (6.2%) patients in the PP6M group and 6 (4.8%) patients in the PP3M group. The most common serious TEAEs (>5 patients) were related to the worsening of schizophrenia (6 [1.0%], PP6M and none for PP3M). A total of 8 (3.1%) patients in the PP6M group and 2 (1.6%) patients in the PP3M group withdrew from the DB phase due to TEAEs. In European PP6M vs PP3M groups, the incidence of EPS-related TEAEs including parkinsonism, akathisia, dyskinesia, dystonia, and tremor was 4.6% vs 2.4%, 3.1% vs 2.4%, 1.9% vs 0, 0.8% (each) and 0 vs 0.4%, respectively; akathisia being the only one significant. There were no cases of neuroleptic malignant syndrome. Among European PP6M and PP3M groups injection-site-related TEAEs were observed in fewer than 6% of patients, were mostly mild in severity, and included injection-site induration (1.5%, 1.6%), swelling (1.5%, 0.8%), discomfort (0.4%, 0.8%), hemorrhage (0.4%, 0), and edema (0.4%, 0). No cases of injection-site reaction were reported as serious, and none led to discontinuation of treatment. The most common events were related to the worsening of schizophrenia (PP6M: 4 [1.5%]; PP3M: 0). One (0.8%) death due to pulmonary embolism was reported in the PP3M group and was considered unrelated to the study drug by the investigator.
Discussion
This post hoc subgroup analysis confirmed the non-inferiority of PP6M to PP3M in the European subpopulation and the findings were consistent with those in the global population. The efficacy or safety while transitioning from PP1M or PP3M to PP6M was similar and consistent. The majority (>90%) of patients in both the PP6M and PP3M treatment groups completed the 48-week DB phase without a relapse. The results from the secondary efficacy endpoints also suggested that PP6M displayed similar efficacy to PP3M in the treatment of European patients with schizophrenia. The low relapse rates and the efficacy of PP6M in the European subpopulation in delaying relapse and improving symptoms were consistent with those reported in the global study. Efficacy results in the European subpopulation were similar as most patients (53.4%) as compared to global (46.4%) had not received any LAIs prior to entering the study as they were receiving OAPs.
Safety and tolerability profiles of PP6M and PP3M during the DB phase in the European subpopulation were comparable. The incidence of TEAEs was slightly lower in the European subpopulation as compared with that reported in the global study. Headache in the PP6M group and weight gain and nasopharyngitis in the PP3M group were the most common TEAEs observed. Serious TEAEs observed were similar in both treatment groups, mostly related to worsening psychiatric-related symptoms. No new safety findings were reported. It is previously noted that to optimize AP treatments for individual patients, it is useful to collect information on demographic and clinical characteristics of patients and it would be helpful to identify the most effective therapy appropriate to the needs of each subgroup.14 Though differences in health-care systems along with disparities in race and ethnicities can influence efficacy and safety responses in patients treated with APs, this study did not reveal any major differences in the European population compared to the global population. We note that European data comprised 54.7% of the global population data.
Among the existing LAIs, PP6M provides the longest dosing interval with twice-yearly dosing. Assured medication delivery displays sustained therapeutic effects; the reduced frequency of injections required with PP6M can help meet treatment needs in terms of improved patient adherence and a reduced risk of relapse compared with oral or other LAI APs that require more frequent administrations. Real-world evidence indicates an improvement in the long-term treatment continuation upon a decrease in the frequency of treatment injections.15 The convenience of LAIs with long dosing intervals is expected to have positive impact on social functioning and quality of life that contribute to overall clinical stability in patients with schizophrenia. Furthermore, by reducing hospitalizations and improving medication adherence, LAIs can improve long-term functionality and facilitate better social integration and patient empowerment. In a real-world observational study, early introduction of PP3M in recently diagnosed schizophrenia was associated with clinically meaningful improvements in social functioning and high level of treatment satisfaction, while maintaining symptomatic stability.16 Another real-world observational study from Hungary, with larger sample size reports greater 1-year and 1.5-year longer median time to discontinuation and treatment continuation rates for second-generation AP LAIs compared with OAPs. Additionally, the study also suggested that the time to treatment discontinuation of PP3M was observed to be lower than previous LAIs.17
Longer intervals of medication may also be desirable for patients who are socially isolated or less connected to the healthcare system.18–22 It also can be advantageous during a health emergency such as the COVID-19 pandemic.23,24 PP6M can reduce the need for patients with schizophrenia to attend the clinic every month or 3-months for injections, thus allowing more time for a physician to intervene and focus on other aspects of treatment to further improve quality of life.
As PP6M requires only twice-yearly injections, it may offer an advantage for patients with schizophrenia who are vulnerable to relapse by potentially allowing greater time for a clinician to intervene and resume treatment. This represents a significant advancement over existing treatments; thus, patients, particularly those who are difficult to reach, would have longer medication coverage. Additionally, switching patients to LAIs may be beneficial in the early course of schizophrenia. Furthermore, after patients are stabilized on once-month LAIs, advancement to progressively longer-acting LAIs may be facilitated. Longer follow-up studies are warranted to assess PP6M treatment safety and impact on patients’ adherence, functioning, caregiver burden, quality of life, and satisfaction in real-world patients switching from PP1M or PP3M to PP6M.
The post hoc nature of this analysis with a small number of patients is the major limitation of this study. Patients entering the DB phase were already responsive to or stable with PP1M or PP3M treatment, which may also be a reason for the high rates of completion and the low rates of relapse. Unlike real-life practice, patients treated with PP6M and PP3M had no opportunities for dose adjustments. To maintain blinding, all patients in the study received 3-month injections, including those in the PP6M cohort, so they did not experience the full convenience of a 6-month regimen. Some varied responses observed in the study may be attributed to the occurrence of different ethnicities, as well as differences in healthcare delivery within the European populations.
Conclusion
The results in the European subgroup are comparable to the global study population. Efficacy and safety results showed no significant differences when transitioning directly from PP1M or PP3M to PP6M. Efficacy of PP6M was non-inferior to that of PP3M in preventing relapse in patients with schizophrenia previously stabilized on doses of PP1M or PP3M. No new safety signals particularly for PP6M emerged specifically for the European subgroup. In European PP6M vs PP3M groups, the incidence of EPS-related TEAEs was similar except for akathisia. No cases of injection-site reaction were reported as serious, and none led to discontinuation of treatment. The most common events were related to the worsening of schizophrenia. Along with PP1M and PP3M, PP6M provides flexible dosing regimens for increased adherence, especially in cases where reducing the frequency of injections is desirable. These flexible dosing regimens can aid in optimizing therapy management for patients with schizophrenia from European regions.
Acknowledgments
Salgo Merin Ricki Elenjikamalil, PhD (SIRO Clinpharm Pvt. Ltd., India) provided writing assistance and Ellen Baum, PhD (Janssen Global Services, LLC) provided additional editorial support. The authors also thank the patients and investigators for their support.
Disclosure
Cesar Giron-Hernandez is an employee of Janssen-Cilag and hold stocks and stock options, Joong Hee Hanc is an employee of Janssen Research & Development, LLC, USA and hold stocks and stock options. Roberta Alberio is an employee of Janssen-Cilag and hold stocks and stock options. Arun Singh is an employee of Janssen Research & Development, LLC, USA and hold stocks and stock options. Maria Paz García-Portilla has been a consultant to and/or received honoraria/grants from Alter, Alianza, Cassen-Recordati, Otsuka-Lundbeck, Angelini, Idorsia, Janssen-Cilag, Lundbeck, Otsuka, and SAGE Therapeutics. Maurizio Pompili has received lecture or advisory board honoraria or engaged in clinical trial activities with Angelini, Janssen, Lundbeck, MSD, Pfizer, and Recordati. Karl Knight is an employee of Janssen Research & Development, LLC, USA and hold stocks and stock options. Ute Richarz is an employee of Janssen-Cilag and hold stocks and stock options. Srihari Gopal was employed by Janssen Research & Development, LLC, USA when the study was conducted and is now employed by Regeneron. José Antunes is an employee of Janssen-Cilag and hold stocks and stock options. The authors report no other conflicts of interest in this work.
References
1. Kozma CM, Weiden PJ. Partial compliance with antipsychotics increases mental health hospitalizations in schizophrenic patients: analysis of a national managed care database. Am Health Drug Benefits. 2009;2(1):31–38.
2. Taipale H, Mehtala J, Tanskanen A, Tiihonen J. Comparative effectiveness of antipsychotic drugs for rehospitalization in schizophrenia-a nationwide study with 20-year follow-up. Schizophr Bull. 2018;44(6):1381–1387. doi:10.1093/schbul/sbx176
3. Tani H, Suzuki T, Wolfgang Fleischhacker W, Tomita M, Mimura M, Uchida H. Clinical characteristics of patients with schizophrenia who successfully discontinued antipsychotics: a literature review. J Clin Psychopharmacol. 2018;38(6):582–589. doi:10.1097/JCP.0000000000000959
4. Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603–619. doi:10.1093/schbul/sbx090
5. Ostuzzi G, Bertolini F, Tedeschi F, et al. Oral and long-acting antipsychotics for relapse prevention in schizophrenia-spectrum disorders: a network meta-analysis of 92 randomized trials including 22,645 participants. World Psychiatry. 2022;21(2):295–307. doi:10.1002/wps.20972
6. Ostuzzi G, Bertolini F, Del Giovane C, et al. Maintenance treatment with long-acting injectable antipsychotics for people with nonaffective psychoses: a network meta-analysis. Am J Psychiatry. 2021;178(5):424–436. doi:10.1176/appi.ajp.2020.20071120
7. Xeplion®. Summary of Product Characteristics. Beerse, Belgium: Janssen-Cilag international NV; 2021.
8. Trevicta®. Summary of Product Characteristics. Beerse, Belgium: Janssen-Cilag international NV; 2021.
9. Najarian D, Sanga P, Wang S, et al. A randomized, double-blind, multicenter, noninferiority study comparing paliperidone palmitate 6-month versus the 3-month long-acting injectable in patients with schizophrenia. Int J Neuropsychopharmacol. 2022;25(3):238–251. doi:10.1093/ijnp/pyab071
10. Li M, Luo XJ, Xiao X, et al. Allelic differences between Han Chinese and Europeans for functional variants in ZNF804A and their association with schizophrenia. Am J Psychiatry. 2011;168(12):1318–1325. doi:10.1176/appi.ajp.2011.11030381
11. Bauer SM, Schanda H, Karakula H, et al. Culture and the prevalence of hallucinations in schizophrenia. Compr Psychiatry. 2011;52(3):319–325. doi:10.1016/j.comppsych.2010.06.008
12. Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;383(9929):1677–1687. doi:10.1016/S0140-6736(13)62036-X
13. Mucci A, Kawohl W, Maria C, Wooller A. Treating schizophrenia: open conversations and stronger relationships through psychoeducation and shared decision-making. Front Psychiatry. 2020;11:761. doi:10.3389/fpsyt.2020.00761
14. Papageorgiou G, Canas F, Zink M, Rossi A. Country differences in patient characteristics and treatment in schizophrenia: data from a physician-based survey in Europe. Eur Psychiatry. 2011;26(1 Suppl 1):17–28. doi:10.1016/S0924-9338(11)71710-2
15. Lin D, Thompson-Leduc P, Ghelerter I, et al. Correction to: real-world evidence of the clinical and economic impact of long-acting injectable versus oral antipsychotics among patients with schizophrenia in the United States: a systematic review and meta-analysis. CNS Drugs. 2021;35(8):923. doi:10.1007/s40263-021-00850-9
16. Garcia-Portilla MP, Benito Ruiz A, Gomez Robina F, Garcia Dorado M, Lopez Rengel PM. Impact on functionality of the paliperidone palmitate three-month formulation in patients with a recent diagnosis of schizophrenia: a real-world observational prospective study. Expert Opin Pharmacother. 2022;23(5):629–638. doi:10.1080/14656566.2021.2023496
17. Takács P, Kunovszki P, Timtschenko V, et al. Comparative effectiveness of second generation long-acting injectable antipsychotics based on nationwide database research in Hungary: an update. Schizophr Bull Open. 2022;3:1. doi:10.1093/schizbullopen/sgac013
18. Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892–909. doi:10.4088/jcp.v63n1007
19. Lefebvre P, Muser E, Joshi K, et al. Impact of paliperidone palmitate versus oral atypical antipsychotics on health care resource use and costs in Veterans with schizophrenia and comorbid substance abuse. Clin Ther. 2017;39(7):1380–1395 e4. doi:10.1016/j.clinthera.2017.05.356
20. Moreno C, Wykes T, Galderisi S, et al. How mental health care should change as a consequence of the COVID-19 pandemic. Lancet Psychiatry. 2020;7(9):813–824. doi:10.1016/S2215-0366(20)30307-2
21. Sajatovic M, Ramirez LF, Fuentes-Casiano E, et al. A 6-month prospective trial of a personalized behavioral intervention + long-acting injectable antipsychotic in individuals with schizophrenia at risk of treatment nonadherence and homelessness. J Clin Psychopharmacol. 2017;37(6):702–707. doi:10.1097/JCP.0000000000000778
22. Yoshimatsu K, Elser A, Thomas M, et al. Recovery-oriented outcomes associated with long-acting injectable antipsychotics in an urban safety-net population. Community Ment Health J. 2019;55(6):979–982. doi:10.1007/s10597-019-00412-w
23. Gannon JM, Conlogue J, Sherwood R, et al. Long acting injectable antipsychotic medications: ensuring care continuity during the COVID-19 pandemic restrictions. Schizophr Res. 2020;222:532–533. doi:10.1016/j.schres.2020.05.001
24. Ifteni P, Dima L, Teodorescu A. Long-acting injectable antipsychotics treatment during COVID-19 pandemic - A new challenge. Schizophr Res. 2020;220:265–266. doi:10.1016/j.schres.2020.04.030
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


