Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 9 » Issue 1
Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2–4 COPD: results from two replicate 48-week studies
Authors Ferguson G , Feldman G, Hofbauer P, Hamilton A, Allen L, Korducki L, Sachs P
Received 7 February 2014
Accepted for publication 2 May 2014
Published 16 June 2014 Volume 2014:9(1) Pages 629—645
DOI https://doi.org/10.2147/COPD.S61717
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Gary T Ferguson,1 Gregory J Feldman,2 Peter Hofbauer,3 Alan Hamilton,4 Lisa Allen,5 Lawrence Korducki,5 Paul Sachs6
1Pulmonary Research Institute of Southeast Michigan, Livonia, MI, 2S Carolina Pharmaceutical Research, Spartanburg, SC, USA; 3Pneumologie, Weinheim, Germany; 4Boehringer Ingelheim, Burlington, ON, Canada; 5Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, CT, 6Pulmonary Associates of Stamford, Stamford, CT, USA
Background: Olodaterol is a long-acting β2-agonist with a 24-hour bronchodilator profile. Two replicate, randomized, double-blind, placebo-controlled, parallel-group, Phase III trials were performed as part of a comprehensive clinical program to investigate the long-term safety and efficacy of olodaterol in patients with moderate to very severe chronic obstructive pulmonary disease (COPD) receiving usual-care background therapy.
Methods: Patients received olodaterol 5 µg or 10 µg or placebo once daily for 48 weeks. Coprimary end points were forced expiratory volume in 1 second (FEV1) area under the curve from 0 to 3 hours (AUC0–3) response (change from baseline), and trough FEV1 response at 12 weeks. Secondary end points included additional lung function assessments, use of rescue medications, FEV1 AUC response from 0 to 12 hours, and Patient Global Rating over 48 weeks.
Results: Overall, 624 and 642 patients were evaluated in studies 1222.11 and 1222.12, respectively. In both studies, olodaterol 5 µg and 10 µg significantly improved the FEV1 AUC0–3 response (P<0.0001) and trough FEV1 (study 1222.11, P<0.0001; study 1222.12, P<0.05, post hoc) at week 12, with an incidence of adverse events comparable with that of placebo. Secondary end points supported the efficacy of olodaterol.
Conclusion: These studies demonstrate the long-term efficacy and safety of once-daily olodaterol 5 µg and 10 µg in patients with moderate to very severe COPD continuing with usual-care maintenance therapy.
Keywords: chronic obstructive pulmonary disease, bronchodilator, olodaterol
Introduction
Long-acting bronchodilators, such as long-acting β2-agonists (LABAs) and long-acting muscarinic antagonists (LAMAs), are considered the cornerstone of maintenance therapy for patients with chronic obstructive pulmonary disease (COPD),1,2 because they improve lung function and reduce dyspnea, hyperinflation, and frequency of exacerbations.3–5 The first available inhaled long-acting bronchodilators, such as salmeterol and formoterol, were administered twice daily. Tiotropium, the first 24-hour inhaled long-acting bronchodilator, and a LAMA, was introduced a decade ago. LABAs with 24-hour dosing profiles, such as indacaterol, have recently become available, allowing for once-daily dosing.6 Once-daily dosing may be associated with improved adherence and clinical outcomes.6,7 Olodaterol is a LABA with a high affinity for, and almost full intrinsic activity at, β2 receptors and low-affinity partial agonist activity at β1 receptors.8,9 Preclinical and early clinical studies in COPD and asthma indicated that olodaterol has a 24-hour bronchodilatory effect10–13 and is well tolerated at single doses up to 20 μg,11,13 and over 3 and 4 weeks at doses up to 20 μg per day.12,14,15 Based on these Phase II clinical trials, olodaterol 5 μg and 10 μg were selected as the most appropriate doses to investigate further in the Phase III COPD program.12,15
Studies 1222.11 and 1222.12 form part of a comprehensive Phase III clinical development program for olodaterol in COPD, comprising five pairs of replicate studies designed to assess lung function efficacy and safety over 48 weeks, symptomatic benefit, 24-hour bronchodilator profile versus formoterol and tiotropium, and exercise capacity. Replicate studies were conducted to provide independent substantiation of results.
The lung function benefits of olodaterol 5 μg and 10 μg were examined in two pairs of replicate pivotal studies in patients with moderate to very severe COPD. One pair of studies assessed the lung function efficacy and symptomatic benefits of olodaterol in comparison with placebo and formoterol after 24 weeks. The pair of studies presented here evaluated the lung function efficacy of olodaterol 5 μg and 10 μg once daily after 12 weeks in comparison with placebo. The patient population included in these studies was chosen to closely represent the range of patients seen in clinical practice with regard to disease severity, background therapies, and comorbidities.
Materials and methods
Study design
Studies 1222.11 (ClinicalTrials.gov identifier NCT 00782210) and 1222.12 (ClinicalTrials.gov identifier NCT 00782509) were replicate, multicenter, multinational, randomized, double-blind, placebo-controlled, parallel-group Phase III trials (Figure 1). Following an initial screening visit and a 2-week baseline period, patients were randomized to receive olodaterol 5 μg or 10 μg once daily or placebo; randomization was stratified based on concomitant tiotropium use to ensure balanced assignment across treatment arms. Olodaterol was administered as two actuations of the Respimat® inhaler (Boehringer Ingelheim, Ingelheim, Germany). The treatment period was 48 weeks, with a final follow-up 2 weeks later. Primary efficacy evaluations were carried out at 12 weeks, in line with US Food and Drug Administration requirements,16 and longer-term safety and efficacy end points were studied over the 48-week period.
  | Figure 1 Trial design for studies 1222.11 and 1222.12. |
The studies were carried out in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Harmonised Tripartite Guideline for Good Clinical Practice, and written informed consent was obtained from each patient.
Patients
Patients with a diagnosis of moderate to very severe COPD (Global initiative for chronic Obstructive Lung Disease [GOLD] stage 2–4) were recruited if they met the following inclusion criteria: post-bronchodilator forced expiratory volume in 1 second (FEV1) less than 80% predicted; FEV1/forced vital capacity (FVC) less than 70% predicted; age at least 40 years with a smoking history of more than 10 pack-years. There was no lower limit for FEV1. Key exclusion criteria were: history of asthma; significant disease other than COPD (a disease that may put the patient at risk because of participation, may cause concern about the patient’s ability to take part, or may influence the results); history of myocardial infarction or hospitalization due to heart failure in the previous year; unstable or life-threatening cardiac arrhythmia; regular use of daytime oxygen therapy (if patients were considered unable to abstain from use during clinic visits).
Patients continued with usual background maintenance therapy other than LABAs for the study duration, including LAMAs and short-acting muscarinic antagonists, inhaled corticosteroids, and methylxanthines. A 48-hour washout period was required for LABAs and patients receiving such medications were allowed to switch to short-acting muscarinic antagonists. All patients were provided with salbutamol for use as rescue medication, as needed, during the baseline, treatment, and follow-up periods.
Study outcomes
The coprimary efficacy end points were FEV1 area under the curve from 0–3 hours (AUC0–3) response (change from baseline) and trough FEV1 response (measured as predose FEV1) at 12 weeks. Secondary end points included FEV1 AUC from 0–12 hours (AUC0–12) response at 12 weeks (subset of patients only), and rescue medication use, Patient Global Rating (PGR),17 and additional lung function assessments over 48 weeks (FEV1 AUC0–3 response, trough FEV1 response, FVC AUC0–3 response, and peak expiratory flow rate). Safety end points included adverse events, vital signs, clinical laboratory testing, and electrocardiogram.
Assessments
Qualifying pulmonary function tests (PFTs) were conducted at screening. Predose PFTs (FEV1 and FVC) were performed at 10 minutes prior to study drug inhalation (−0:10) on day 1 and after 2, 6, 12, 24, and 48 weeks of treatment. An additional 1 hour predose (−1:00) PFT was performed on day 1 and at weeks 6 and 12. Thus, trough FEV1 was the −0:10 measurement or the mean of the −0:10 and −1:00 PFTs, depending on the visit. Post-dose PFTs were performed at 0:05, 0:15, 0:30, 1:00, 2:00, and 3:00 on day 1, and after 2, 6, 12, 24, and 48 weeks. For the subset of patients included in the FEV1 AUC0–12 assessment at week 12, additional post-dose PFTs were performed at 4:00, 5:00, 6:00, 8:00, 10:00, and 12:00. Spirometric tests were performed in accordance with American Thoracic Society and European Respiratory Society criteria, and the highest FEV1 and FVC were recorded.18 Patients recorded their peak expiratory flow rate twice daily using an electronic peak flow meter for the duration of the study. The electronic device was also used as a diary to record rescue salbutamol and study drug use. Patients were asked to rate their respiratory health using a seven-point scale at weeks 6, 12, 24, and 48 to provide the PGR score.
All adverse events and serious adverse events were recorded. Pulse rate and blood pressure were recorded at every clinic visit, and blood chemistry, hematology, and urinalysis were assessed at weeks 6 and 12. A 12-lead electrocardiogram was performed at screening, baseline, and at week 6, 12, 24, and 48 study visits predose and 40 minutes post-dose; 24-hour Holter monitoring was carried out in a subset of patients prior to randomization and then after clinic visits at 12, 24, 40, and 48 weeks (total of 393 patients across both studies).
Statistical analysis
A sample size of 200 patients per group for each study was planned to provide 90% power to detect a difference in means of 75 mL in trough FEV1 response, with an expected standard deviation of 0.226 L between olodaterol groups and placebo at the one-sided alpha of 0.025 (corresponding to a two-sided alpha of 0.05).
The full analysis set was defined as all randomized patients who received at least one dose of treatment and had a baseline and at least one post-randomization measurement at or before 12 weeks for either coprimary efficacy variable. The full analysis set was used for all analyses presented here.
The mean change from baseline in the two coprimary end points was analyzed using a mixed model for repeated measurements with fixed, categorical effects of treatment, tiotropium use stratum, test day, treatment by test-day interaction, treatment by tiotropium use stratum interaction, tiotropium use stratum by test-day interaction, and treatment by tiotropium use stratum by test-day interaction, as well as the continuous, fixed covariates of baseline and baseline by test-day interaction. These were tested in hierarchical order as detailed in Table S1. Tiotropium use stratum was originally included as a covariate in the statistical model to meet International Conference on Harmonisation E9 guidelines.19 However, inclusion of the tiotropium stratum interaction terms within the original statistical model weighted the strata equally rather than proportional to stratum size when in fact the stratum sizes were very different. In addition to inappropriately weighting the strata, this also resulted in increased variability. As this issue was not recognized until after unblinding, the planned analysis was amended to remove the treatment-by-tiotropium stratum interaction from the analysis. We demonstrated that removal of treatment-by-tiotropium stratum interaction had little effect on the overall analysis (Table S2). The same model was used for spirometry and the same model without the covariates was used for PGR. An analysis of covariance model was used for each week to analyze peak expiratory flow rate and rescue medication use with the fixed categorical effects of treatment, tiotropium stratum, and baseline. For FEV1 AUC0–12, as only one measurement was taken, an analysis of covariance model was used.
Prespecified analyses based on the combined dataset from studies 1222.11 and 1222.12 were conducted to assess the FEV1 AUC0–12 and Holter electrocardiogram monitoring outcomes, as these assessments were performed in only a subset of patients in each study. Combined analysis of other end points, including lung function results, PGR, and rescue medication use, was performed in addition to analyses for each individual study; selected combined data are presented here for simplicity.
Results
Patient disposition and baseline characteristics
In study 1222.11, 859 patients were enrolled from the USA, Germany, Australia, New Zealand, China, and Taiwan; of these, 625 were randomized and 624 were treated (Figure 2A). In study 1222.12, 892 patients were enrolled from the USA, Germany, China, and Taiwan; 644 of these were randomized and 642 were treated (Figure 2B). There was an overall discontinuation rate of 19% in study 1222.11, with a higher rate in the placebo group (24%) compared with the active treatment groups (17% at both doses). In study 1222.12, the overall discontinuation rate was 16%, again with a higher rate in the placebo group (19%) than in the active treatment groups (11% and 17% with olodaterol 5 μg and 10 μg, respectively) (Figure 2; reasons for discontinuations provided in Figure S1). Patient baseline characteristics were comparable between treatment groups in both studies (Table 1). More than 70% of patients in both studies were taking pulmonary medication at baseline; 21%–25% of patients across groups in study 1222.11 and 15%–22% of patients in study 1222.12 were receiving tiotropium (Table 1). A total of 12.0% of patients in study 1222.11 and 13.1% in study 1222.12 were categorized as having GOLD stage 4 disease.
Efficacy
Lung function
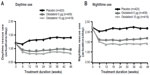
After 12 weeks, statistically significant improvements compared with placebo were demonstrated in the coprimary end point of FEV1 AUC0–3 response for both olodaterol 5 μg and 10 μg once daily in studies 1222.11 and 1222.12 (P<0.0001, Table 2 and Table S3). Statistically significant improvements versus placebo in the coprimary end point of trough FEV1 response were also observed at week 12 with both doses of olodaterol in study 1222.11 (P<0.0001) and in study 1222.12 (5 μg, P<0.05; 10 μg, P<0.01; Table 2 and Table S3). These improvements were also evident at weeks 24 and 48 (Table 2 and Table S3). There were no statistically significant differences between olodaterol 5 μg and 10 μg in either coprimary end point. Comparison of lung function analysis by original and revised statistical models demonstrated similar results (Table S2). Analysis of the combined data set found no statistically significant treatment-by-tiotropium stratum interaction (results of the coprimary end points in the tiotropium and non-tiotropium strata are provided in Table S4).
Over the 48-week treatment period, the FEV1 AUC0–3 response with olodaterol 5 μg and 10 μg once daily was significantly improved compared with placebo at all time points in studies 1222.11 and 1222.12 (P<0.0001, Figure 3). Trough FEV1 response was significantly improved compared with placebo with both doses of olodaterol at all time points measured over the 48-week period (P<0.0001) in study 1222.11 and at all but one time point in study 1222.12 (P<0.0001–P=0.0186 except day 225, olodaterol 10 μg, P=0.1614).
  | Figure 3 FEV1 AUC0–3 (A) and trough FEV1 (B) over 48 weeks in study 1222.11 and study 1222.12. |
FVC AUC0–3 response mirrored the FEV1 results, and was significantly improved compared with placebo with both olodaterol doses at all time points over 48 weeks for both studies (P<0.0001, Table 3). In study 1222.11 (but not study 1222.12), the trough FVC response was statistically significantly improved at all time points over 48 weeks with both doses of olodaterol once daily versus placebo (P<0.0001–P<0.05, Table 3).
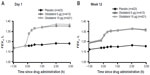
Improvements in FEV1 with olodaterol were evident from 5 minutes after the first dose. In the combined data set, mean differences from placebo on day 1 at 5, 15, and 30 minutes were 0.118, 0.152, and 0.160 L (P<0.0001 versus placebo), respectively, with olodaterol 5 μg, and 0.117, 0.149, and 0.160 L with 10 μg (P<0.0001 versus placebo). FEV1 results from –1:00 predose to 3:00 post-dose are shown for the individual studies in Figure 4 and the combined data are shown in Figure S2.
  | Figure 4 FEV1 over time from 1 hour pre-dose to 3 hours post-dose in study 1222.11 and study 1222.12 at day 1 (A) and week 12 (B). |
A prespecified analysis of combined data from studies 1222.11 and 1222.12 for the subset of patients who underwent PFTs up to 12 hours post-dose was performed, with FEV1 AUC0–12 response significantly improved with olodaterol 5 μg (0.137 L; P<0.0001) and olodaterol 10 μg (0.124 L; P<0.0001) at week 12 compared with placebo (n=562).
The weekly mean morning and evening peak expiratory flow rate were supportive of the FEV1 results and were statistically significantly improved with both olodaterol doses compared with placebo in study 1222.11 (P<0.0001–P=0.0063) and study 1222.12 (P<0.0001–P=0.0325) over 48 weeks (Figure S3).
Rescue medication use
Weekly mean daytime and nighttime rescue medication use with olodaterol was significantly reduced versus placebo over the 48 weeks of treatment in the combined data set; at week 48, daytime rescue medication use was reduced for olodaterol 5 μg by 0.46 actuations/day (P<0.0001) and for 10 μg by 0.57 actuations/day (P<0.0001); night-time rescue medication use was reduced for olodaterol 5 μg by 0.50 actuations/day (P<0.0001) and for 10 μg by 0.78 actuations/day (P<0.0001; data from combined analysis and individual trials presented in Figure S4 and Table S5, respectively).
Patient Global Rating
PGR scores,17 based on the combined data set (post hoc analysis), were statistically significantly improved versus placebo with both olodaterol doses (P<0.0001 at weeks 6, 12, and 24; P=0.0041, 5 μg and P<0.0001, 10 μg at week 48). At week 12, mean PGR scores were 3.0 with both olodaterol doses and 3.3 with placebo. Results are presented for the individual studies in Table S6.
Safety
In both studies, the incidences of adverse events, serious adverse events, deaths, and adverse events leading to discontinuations with olodaterol 5 μg and 10 μg once daily were similar to those for placebo (Table 4). The most common adverse event was exacerbation of COPD, which occurred in 34.0%, 24.0%, and 32.4% of patients receiving placebo, olodaterol 5 μg, and olodaterol 10 μg, respectively, in study 1222.11, and in 25.5%, 22.0%, and 27.2% of patients, respectively, in study 1222.12. Serious adverse events were reported by 16.3%, 18.8%, and 20.8% of patients with placebo, olodaterol 5 μg, and olodaterol 10 μg, respectively, in study 1222.11, and by 14.8%, 15.3%, and 17.1% of patients, respectively, in study 1222.12.
  | Table 4 Summary of AEs in Studies 1222.11 and 1222.12 |
Analysis of laboratory parameters indicated an upward shift in creatine phosphokinase in the olodaterol groups compared with placebo in studies 1222.11 and 1222.12 using maximum on-treatment values (change from baseline in study 1222.11: placebo –24 U/L, olodaterol 5 μg +8 U/L, olodaterol 10 μg +59 U/L, and in study 1222.12: placebo −14 U/L, olodaterol 5 and 10 μg +7 U/L). One patient receiving olodaterol 10 μg in study 1222.11 and no patients in study 1222.12 discontinued early due to increased blood creatine phosphokinase. No other notable changes in mean values for other clinical laboratory results, vital signs, electrocardiogram results, Holter monitoring, or physical examinations were found.
In study 1222.11, there were five deaths during the treatment period (three in the olodaterol 5 μg group [all due to COPD exacerbation], one in the 10 μg group [lung cancer], and one in the placebo group [aortic aneurysm]). There were four post-study deaths reported in the vital status follow-up during the planned observation period (48 weeks plus 2 weeks); all had discontinued the study (two in the placebo group, one in the olodaterol 5 μg group, and one in the olodaterol 10 μg group), and one death outside of the planned observation period in a patient who had completed the study. In study 1222.12, there were five deaths during the treatment period (one in the placebo group [COPD exacerbation] and four in the olodaterol 10 μg group [pneumonia, lung cancer, COPD exacerbation, and unknown cause]). Four post-study deaths were reported during the planned observation period; all patients involved had discontinued the study (two in the placebo group, one in the olodaterol 5 μg group, and one in the olodaterol 10 μg group). None of these deaths in either study were considered related to the study drug. Further safety data from these trials have already been presented.20
Discussion
In this replicate pair of Phase III studies, analyses indicated that the coprimary end points were met, with olodaterol 5 μg and 10 μg once daily demonstrating significant improvements in FEV1 AUC0–3 response and trough FEV1 response compared with placebo at 12 weeks in patients with GOLD 2–4 COPD receiving usual-care maintenance therapy. We detected no difference in efficacy between olodaterol 5 μg and 10 μg. These coprimary end points demonstrate the full effect of olodaterol over the 24-hour dosing interval considering both peak and trough lung function.
The results of the secondary lung function end points support these data and also show the longer-term efficacy of olodaterol over 48 weeks. Olodaterol showed a rapid onset of action, with improvements in FEV1 at 5 minutes post-dose. Over 48 weeks, use of rescue medication was also significantly reduced and PGR improvements were maintained.
The incidence of adverse events was similar to that of placebo and no safety concerns were highlighted. The only laboratory parameter showing a notable change with olodaterol treatment was blood creatine phosphokinase concentration, possibly as a result of the pharmacologic effect of olodaterol. This is generally associated with mild or no symptoms21 and there was no increase in adverse events among affected patients. The incidence of COPD exacerbation was similar with olodaterol and placebo. While previous studies of LABAs suggest a reduction of exacerbations, it should be noted that the inclusion of background medications in this study would make comparison of exacerbations difficult and, in fact, this study was not designed to investigate exacerbation rate.
The effect sizes observed were in line with expectations for populations that include patients with very severe COPD, who are often excluded from COPD studies,22 and patients continuing with all non-LABA background bronchodilatory medications, corticosteroid medications, or combination therapies taken at baseline. This is consistent with comparable responses between olodaterol and formoterol in complementary trials in the Phase III program that also recruited from this broader population base.23,24 The importance of considering patient characteristics when interpreting effect size has previously been identified.25 The rationale for employing these expanded inclusion criteria in the olodaterol Phase III program was to more closely represent the patients who are commonly encountered in clinical practice, such as those with more severe disease and patients being treated with usual-care medication,26 including short-acting muscarinic antagonists and LAMAs.
Although the study design permits conclusions about the benefits of olodaterol in COPD patients receiving usual-care background therapy, including simultaneous use of other respiratory treatments, it does not permit specific conclusions about the efficacy of olodaterol in combination with muscarinic antagonists. Randomization was stratified by tiotropium use to ensure a balance across treatment groups and, accordingly, the results for the individual tiotropium strata are presented here. Olodaterol demonstrated a benefit versus placebo regardless of whether patients did or did not receive tiotropium. Perhaps unsurprisingly, the effect sizes were smaller in patients receiving tiotropium. However, evaluation of the efficacy of olodaterol in combination with tiotropium requires a trial design where all patients are coadministered olodaterol and tiotropium either as a free combination (studies presently being conducted: NCT01694771, NCT01696058) or as a fixed combination (Phase II studies completed; Phase III studies completed: NCT01533935, NCT01533922, NCT01431287, NCT01431274, NCT01559116,27 NCT01536262, NCT01525615).
The results of these two trials, 1222.11 and 1222.12, demonstrating the efficacy and safety of olodaterol over 48 weeks, are consistent with the findings of the broader Phase III clinical development program for olodaterol. Other trials have demonstrated that olodaterol improves lung function in a manner comparable with that of other bronchodilators, provides symptomatic benefit over 48 weeks, has a 24-hour bronchodilator profile similar to that of formoterol and tiotropium, and improves exercise capacity.23,24,28–30 In line with Phase III trials that rigorously assessed the 24-hour bronchodilation profile,28,29 a bronchodilatory effect with olodaterol over 12 hours was observed. Together with improvements in trough FEV1 response, this confirms that olodaterol is an effective once-daily agent. This has clinical significance in terms of offering improved convenience for patients, which can, in turn, improve adherence and clinical outcomes.6,7 Unlike other licensed LABAs, which are administered in dry powder form, olodaterol was administered via the Respimat® inhaler. This provides an additional option for clinicians when prescribing bronchodilators, allowing treatments to be better tailored to individuals.
Conclusion
These studies demonstrate that olodaterol 5 μg and 10 μg once daily significantly improve lung function (FEV1 AUC0–3 and trough FEV1) in comparison with placebo, with no detected difference between doses, and are well tolerated in patients with moderate to very severe COPD who continue to receive usual-care maintenance therapy.
Disclosure
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. They take full responsibility for the scope, direction, content of, and editorial decisions relating to, the manuscript, were involved at all stages of development, and approved the submitted manuscript. The authors received no compensation related to the development of the manuscript. This work was supported by Boehringer Ingelheim Pharma GmbH and Co, KG. Medical writing assistance was provided by Claire Scofield of Complete HealthVizion, which was contracted and compensated by Boehringer Ingelheim Pharma GmbH and Co, KG.
References
Tashkin DP, Ferguson GT. Combination bronchodilator therapy in the management of chronic obstructive pulmonary disease. Respir Res. 2013;14:49. | |
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2013. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Accessed July 15, 2013. | |
Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. | |
Dahl R, Greefhorst LAPM, Nowak D, et al. Inhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):778–784. | |
Cazzola M, Santangelo G, Piccolo A, et al. Effect of salmeterol and formoterol in patients with chronic obstructive pulmonary disease. Pulm Pharmacol. 1994;7(2):103–107. | |
Cazzola M, Matera MG. Novel long-acting bronchodilators for COPD and asthma. Br J Pharmacol. 2008;155(3):291–299. | |
Toy EL, Beaulieu NU, McHale JM, et al. Treatment of COPD: relationships between daily dosing frequency, adherence, resource use, and costs. Respir Med. 2011;105(3):435–441. | |
Bouyssou T, Casarosa P, Naline E, et al. Pharmacological characterization of olodaterol, a novel inhaled β2-adrenoceptor agonist exerting a 24-hour-long duration of action in preclinical models. J Pharmacol Exp Ther. 2010;334(1):53–62. | |
Casarosa P, Kollak I, Kiechle T, et al. Functional and biochemical rationales for the 24-hour-long duration of action of olodaterol. J Pharmacol Exp Ther. 2011;337(3):600–609. | |
Bouyssou T, Hoenke C, Rudolf K, et al. Discovery of olodaterol, a novel inhaled β2-adrenoceptor agonist with a 24 h bronchodilatory efficacy. Bioorg Med Chem Lett. 2010;20(4):1410–1414. | |
O’Byrne PM, van der Linde J, Cockcroft DW, et al. Prolonged bronchoprotection against inhaled methacholine by inhaled BI 1744, a long-acting β2-agonist, in patients with mild asthma. J Allergy Clin Immunol. 2009;124(6):1217–1221. | |
O’Byrne PM, D’Urzo T, Gahlemann M, Hart L, Wang F, Beck E. Dose-finding study of once-daily treatment with olodaterol, a novel long-acting β2-agonist, in patients with asthma. Am J Respir Crit Care Med. 2012;185:A3963 (Abstract). | |
van Noord JA, Smeets JJ, Drenth BM, et al. 24-hour bronchodilation following a single dose of the novel β2-agonist olodaterol in COPD. Pulm Pharmacol Ther. 2011;24(6):666–672. | |
Joos G, Aumann JL, Coeck C, Korducki L, Hamilton AL, van Noord J. Comparison of 24-hour FEV1 profile for once-daily versus twice-daily treatment with olodaterol, a novel long-acting β2-agonist, in patients with COPD. Am J Respir Crit Care Med. 2012;185:A2930 (Abstract). | |
van Noord JA, Korducki L, Hamilton A, Koker P. Four weeks once daily treatment with BI 1744 CL, a novel long-acting β2-agonist, is effective in COPD patients. Am J Respir Crit Care Med. 2009;179:A6183 (Abstract). | |
US Department of Health and Human Services, US Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for industry. Chronic obstructive pulmonary disease: developing drugs for treatment. Draft guidance. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071575.pdf. Accessed April 26, 2012. | |
Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: a comparison of two techniques. J Clin Epidemiol. 1996;49(11):1215–1219. | |
Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. | |
European Medicines Agency. Statistical principles for clinical trials. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E9/Step4/E9_Guideline.pdf. Accessed July 24, 2013. | |
McGarvey L, Koch A, Sachs P, et al. 48-week administration of olodaterol QD via Respimat® vs placebo and formoterol BID in patients with COPD: Pooled safety analysis. Eur Respir J. 2013;42 Suppl 57:749s, P3633 (Abstract). | |
Tomlinson B, Cruickshank JM, Hayes Y, et al. Selective β-adrenoceptor partial agonist effects of pindolol and xamoterol on skeletal muscle assessed by plasma creatine kinase changes in healthy subjects. Br J Clin Pharmacol. 1990;30(5):665–672. | |
Albert P, Agusti A, Edwards L, et al. Bronchodilator responsiveness as a phenotypic characteristic of established chronic obstructive pulmonary disease. Thorax. 2012;67(8):701–708. | |
Koch A, Paggiaro P, Hamilton A, et al. Symptomatic benefit of olodaterol QD delivered via Respimat® vs placebo and formoterol BID in patients with COPD: Combined analysis from two 48-week studies. Eur Respir J. 2013;42 Suppl 57:145s, P763 (Abstract). | |
Koch A, Pizzichini E, Hamilton A, et al. Lung function efficacy of olodaterol QD delivered via Respimat® vs placebo and formoterol BID in patients with COPD: two 48-week studies. Eur Respir J. 2013;42 Suppl 57:146s, P764 (Abstract). | |
Ferguson GT, Sachs P, Hamilton A, Tetzlaff K, Korducki L, Koch A. Efficacy of olodaterol once daily (QD) via Respimat® in GOLD 2/3 COPD patients not receiving background therapy: pooled data from 48-week studies. Abstract 727A presented at CHEST, Chicago, IL, USA, October 26–31, 2013. | |
European Medicines Agency. Guideline on clinical investigation of medicinal products in the treatment of chronic obstructive pulmonary disease (COPD). Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/08/WC500130880.pdf. Accessed August 7, 2013. | |
Derom E, Westerman J, Grönke L, et al. The 24-hour lung function profile of once-daily tiotropium and olodaterol fixed-dose combination compared with placebo and monotherapies in chronic obstructive pulmonary disease (VIVACITO). Abstract D44 presented at American Thoracic Society; San Diego, CA, USA. May 16–21, 2014. | |
Lange P, Aumann J-L, Derom E, et al. The 24-h FEV1 time profile of olodaterol QD delivered via Respimat® in COPD: results from two 6-week studies. Eur Respir J. 2013;42 Suppl 57:982s, 4635 (Abstract). | |
Feldman G, Bernstein J, Hamilton A, Nivens C, Wang F, LaForce C. The 24-hour FEV1 time profile of olodaterol once daily (QD) via Respimat® and formoterol twice daily (BID) via Aerolizer® in patients with COPD: results from two 6-week studies. Abstract 749A presented at CHEST, Chicago, IL, USA, October 26–31, 2013. | |
Maltais F, Kirsten A, Hamilton A, De Sousa D, Wang F, Decramer M. Evaluation of the effects of olodaterol on exercise endurance in patients with COPD: results from two 6-week studies. Abstract 748A presented at CHEST, Chicago, IL, USA, October 26–31, 2013. |
Online supplementary tables and figures
  | Table S1 Null and alternative hypotheses |
  | Figure S2 FEV1 over time from 1 hour pre-dose to 3 hours post-dose in the combined data set at day 1 (A) and week 12 (B). |
  | Figure S3 Weekly mean morning (A) and evening (B) PEFR over 48 weeks in study 1222.11 and study 1222.12. |
  | Figure S4 Daytime (A) and nighttime (B) rescue medication use (combined data set). |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.