Back to Journals » Infection and Drug Resistance » Volume 13
Efficacy and Safety of Nemonoxacin in Outpatients with Community-Acquired Pneumonia
Authors Zhao B, Yu X , Chen R, Zheng R
Received 2 February 2020
Accepted for publication 18 June 2020
Published 2 July 2020 Volume 2020:13 Pages 2099—2104
DOI https://doi.org/10.2147/IDR.S248092
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Suresh Antony
Bo Zhao, Xiaoxu Yu, Rui Chen, Rui Zheng
Department of Pulmonary and Critical Care Medicine, Shengjing Hospital of China Medical University, Shenyang 110004, Liaoning Province, People’s Republic of China
Correspondence: Rui Zheng
Department of Pulmonary and Critical Care Medicine, Shengjing Hospital of China Medical University, No. 36 Sanhao Street, Heping District, Shenyang 110004, Liaoning Province, People’s Republic of China
Tel +86 24-9661521211
Email [email protected]
Purpose: To evaluate the efficacy and safety of 500 mg of nemonoxacin administered orally once daily to outpatients with community-acquired pneumonia (CAP).
Patients and Methods: Patients with CAP who received nemonoxacin monotherapy were selected from outpatients who visited the Department of Pulmonary and Critical Care Medicine of Shengjing Hospital of China Medical University between July and December 2018. Their characteristics, pneumonia-related symptoms, treatment effects, and adverse reactions were recorded.
Results: In total, 337 patients with CAP were administered 500 mg of nemonoxacin orally once daily for 8.24 ± 3.73 days. Fourteen patients were lost during the follow-up period. At the end of the follow-up period, information on 323 patients (132 males and 191 females) with a median age of 52 (P25, P75: 34, 61) years was collected. On the basis of CRB-65 scores, 273 and 50 cases were classified to have low and intermediate risks, respectively. After 3 days of treatment, the symptom improvement rate was 61.3% (198 patients). Improved symptoms or cures were evident in 98.14% (317 patients) of the patients after treatment was completed. Five (1.55%) patients were hospitalized for poor treatment efficacy, and one (0.31%) patient was diagnosed with lung cancer despite improved symptoms. During oral therapy, there were three cases of skin and three cases of gastrointestinal adverse events, an incidence of 1.86%. Based on subsequent re-examinations and telephonic follow-ups, 93.50% (302 cases) of patients were satisfied with treatment effects.
Conclusion: In treating outpatients with mild-to-moderate CAP, nemonoxacin can effectively control symptoms, reducing medical costs and saving patient time. Importantly, this is a safe and effective therapeutic approach as it is well tolerated with few side effects.
Keywords: monotherapy, tolerability, CRB-65 score, symptom improvement
Introduction
Lower respiratory tract infections, including community-acquired pneumonia (CAP), were ranked fourth among the top 10 leading causes of death worldwide in 2018.1 The morbidity and mortality of patients with CAP increase with age, leading to a heavy disease burden on society.2 At present, the most prevalent pathogenic bacteria of adult CAP in the Chinese population are Mycoplasma pneumoniae and Streptococcus pneumoniae.3 Unlike in Europe and the United States, the main pathogens in China have high drug resistance.4 Thus, clinically effective and safe antibacterial drugs and empirical anti-infective treatment strategies are in a relatively high demand.5 As a novel non-fluorinated quinolone, nemonoxacin possesses a broad antibacterial spectrum and has strong efficacy.6 In previous studies, nemonoxacin has shown good tolerance and safety in patients with mild-to-moderate CAP, with few or minor reported adverse effects.7 Currently, as an antibacterial drug for adult CAP in China, it has many potential advantages but has yet to be evaluated comprehensively, as there are few cases assessing its clinical applications. Thus, in this study, we assessed the efficacy and tolerability of the use of nemonoxacin in treating outpatients with CAP.
Patients and Methods
Study Subjects
Patients with CAP who were administered nemonoxacin monotherapy were selected from outpatients who visited the Department of Pulmonary and Critical Care Medicine at Shengjing Hospital of China Medical University between July and December 2018. The Guidelines for the Diagnosis and Treatment of Community-Acquired Pneumonia in Adults in China formulated by the Chinese Thoracic Society in 2016 were used as a reference for establishing CAP diagnostic criteria in this study.3
Patient Characteristics
The general information and data on diagnosis, concomitant diseases, previous antibiotic exposure, and symptoms and severity of pneumonia of the patients were collected. The severity of CAP was evaluated using the CRB-65 score.3 In total, the score was derived by scoring four indices, with one point given for meeting each index: (1) unconsciousness, (2) respiratory rate ≥ 30/min, (3) systolic blood pressure < 90 mmHg or diastolic blood pressure ≤ 60 mmHg, and (4) age ≥ 65 years. A score of 0 corresponded to low risk, 1–2 to intermediate risk and ≥ 3 to high risk.
Treatment and Evaluation
Treatment regimens were determined by the attending physicians. Based on the patient’s condition, inflammatory indicators, drug sensitivity results, and lung imaging, 500 mg of oral nemonoxacin was prescribed to be administered once daily. According to a re-examination of patient records and subsequent telephonic follow-ups, clinical efficacy and medication safety were evaluated. The main indicators evaluated were symptom improvements within 3 days of medication, including stabilization of body temperature for 24 h and significant patient-perceived improvement in symptoms such as coughing, expectorating, chest pain, and dyspnea; average duration of therapy; treatment effect defined as “cured,” “improved,” “unhealed” (requiring treatment plan adjustment or changing the place of consultation), or “others;” adverse drug reactions; and each patient’s satisfaction with the treatment rated as “satisfied,” “neutral,” or “unsatisfied.”
Statistical Analyses
Data analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA). Normality was verified using the Kolmogorov–Smirnov one-sample test. P < 0.05 was considered statistically significant. Normally distributed data are expressed as means ± standard deviations, non-normally distributed data as median (P25, P75), and categorical data as percentages.
Results
A total of 337 patients with CAP were treated with nemonoxacin; of these, 14 patients were lost during the follow-up period. In total, information on 323 patients (132 males and 191 females) with a median age of 52 (P25, P75: 34, 61) years was collected (Table 1). Based on the CRB-65 scores, 273 cases were classified as low-risk and 50 as intermediate-risk. Of 133 patients who completed the mycoplasma immunoglobulin M antibody test, 19 patients (14.29%) showed weak positive or positive results; other relevant test indicators are shown in Table 2. Notably, 50 (15.48%) patients had concomitant chronic conditions, of which 9 (2.79%) patients had two or more concomitant conditions. The most common conditions were cardiovascular and cerebrovascular diseases (26, 8.05%), chronic respiratory diseases (12, 3.72%), and diabetes mellitus type 2 (10, 3.10%). Before consultation, 77 (23.82%) patients stated they used antibiotics, 53 (16.41%) patients stated they were on medications (although specific medications were unknown), and 193 (59.75%) patients denied any previous antibiotic use. Among the antibiotics used by the 77 patients, the most common antibiotics were macrolides (38, 49.35%), β-lactams (33, 42.86%), and quinolones (4, 5.19%). Among these patients, 17 (22.08%) had been treated with two or more drugs.
 |
Table 1 General Information |
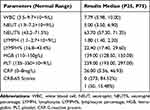 |
Table 2 Test Indicators |
Nemonoxacin was administered for an average of 8.24 ± 3.73 days. Based on re-examination of the patient records and subsequent telephonic follow-ups, the symptom improvement rate was 61.3% after 3 days of treatment. Upon treatment completion, 98.1% of the patients were cured or achieved symptom improvement, whereas 5 (1.55%) patients were hospitalized owing to poor treatment efficacy. One (0.31%) patient achieved symptom improvement but was later diagnosed with lung cancer.
During oral nemonoxacin treatment, one female patient experienced tolerable itching around the neck with no rash, and her symptoms resolved after completing the treatment. Two female patients developed systemic rashes with mild pruritus and no other discomfort, one on the 10th and the other on the 12th day of treatment. Dermatitis medicamentosa was considered after dermatological diagnosis, and the conditions improved after symptomatic treatment. Moreover, before drug administration, these two patients confirmed they were allergic to various drugs including β-lactams (penicillin and cephalosporins), sulfonamides (trimethoprim/sulfamethoxazole), macrolides (erythromycin and azithromycin), and quinolones (moxifloxacin and levofloxacin). Both the patients were satisfied with the anti-infective effects of nemonoxacin. Moreover, they understood and recognized the adverse reactions and felt that the adverse event onset was later and milder than with other drugs. Three instances of gastrointestinal symptoms occurred in two male patients; these mainly experienced mild tolerable symptoms of nausea without vomiting or diarrhea. After 2 days of treatment, one female patient who was mainly suffering from intolerable diarrhea was hospitalized, after which her treatment was altered. Neurological conditions and palpitations were not seen. The overall incidence of adverse reactions was 1.86% (6 patients). Based on re-examination of the patient records and follow-ups via telephone, 302 (93.50%) patients were satisfied with the treatment (Table 3).
 |
Table 3 Treatment Effect |
Discussion
Despite the continuous increase in the number and strength of antibiotics, the incidence of CAP increases annually, and the mortality rate of critical patients remains high, especially in the elderly and those with concomitant diseases.8
At present, M. pneumoniae is the most common CAP pathogen in China that has a high drug resistance rate in Asia. M. pneumoniae infection is not associated with specific clinical symptoms; moreover, no rapid and effective diagnostic methods exist for the same.9 Empiric atypical coverage is associated with a considerable reduction in clinical failure in hospitalized adults with CAP.10 We found that many of our study patients were not tested for mycoplasma antibodies before their consultation, and no dynamic re-examinations are typically performed if an antibody test provides negative results. These factors, along with the insignificant relief obtained with macrolides, lead the primary clinician to rule out the possibility of M. pneumoniae infection. Consequently, its diagnosis and treatment are delayed, resulting in greater economic loss and social burden. For these reasons, we believe that there are significant clinical benefits of covering atypical bacteria.
According to China Antimicrobial Surveillance statistics, the resistance rate of S. pneumoniae to macrolides is as high as 63.2–75.4%, and the resistance rates of S. pneumoniae to oral penicillin and second-generation cephalosporins are also relatively high: 24.5–36.6% and 39.9–75.40%, respectively.3 In the presence of such severe drug resistance, medications such as fluoroquinolones have a stronger antibacterial effect and broader antibacterial spectrum. However, some fluoroquinolones are cardiotoxic and hepatotoxic. When used clinically, rashes, psychiatric symptoms, and digestive system-related side effects are relatively common, especially in the elderly and patients with certain underlying diseases; hence, the application of fluoroquinolones is restricted.11,12 Moreover, with the broadening clinical usage of this class of drugs, the issue of drug resistance has gradually become more prominent. Therefore, the development of novel drugs that can overcome the shortcomings of fluoroquinolones is urgent.13
China’s CAP guidelines recommend outpatient treatment and oral medication for mild and moderate cases. Nevertheless, most patients prefer intravenous medication and can receive infusion treatments from health institutions of different levels. However, the varying efficacies of drugs not only wastes medical resources but also results in the misuse of antibiotics, increasing the risk of drug resistance. Many currently available antibiotics are administered more than once daily and require good compliance to achieve effective results. Moreover, macrolides and quinolones are concentration-dependent drugs. Although once daily administration is more convenient, the volume of intravenous liquid is usually 250 mL. For elderly patients with heart failure, this volume will exacerbate fluid burden and can lead to acute left-side heart failure in severe cases.
Nemonoxacin, a non-fluorinated quinolone drug, is extremely effective when administered orally once daily and has a broad antibacterial spectrum against Gram-positive cocci, atypical pathogens, and most Gram-negative bacteria. For treating methicillin-resistant Staphylococcus aureus, penicillin-resistant S. pneumoniae, and Enterococcus faecium, nemonoxacin has a higher antibacterial efficacy than other quinolones.14 Its site of action differs from that of fluoroquinolones, and no cross-resistance has been observed.15,16
For outpatients, it is difficult to obtain drug sensitivity results of an infection in a timely and accurate manner, and nearly half of patients are exposed to antibiotics before this information is available. In our investigation, 77 (23.82%) patients stated clearly that they used antibiotics before consultation, the most common being macrolides (49.35%), β-lactams (42.86%), and quinolones (5.19%). Among these patients, 17 (22.08%) patients had been treated with two or more drugs. Also, 53 (16.41%) patients confirmed medication use prior to consultation, though the specific medications were unknown. Most of these patients sought treatment owing to the poor efficacy or adverse reactions of their previous treatment. As a drug with a broad antibacterial spectrum, strong antibacterial efficacy, and less tendency to being resistant, nemonoxacin is a new treatment option for clinicians and patients.
In a prospective randomized controlled study, when compared with once daily administration of 500 mg levofloxacin, a 7-day course of once daily 750- or 500-mg oral nemonoxacin had equally good efficacy and tolerability for treating mild or moderate pneumonia.17 Additional research showed that the peak plasma level of nemonoxacin was attained within 1–2 h of oral administration and decreased gradually over 72 h, showing its rapid onset of action and proper concentration distribution in tissues.18
In the current investigation, the mean duration of therapy was 8.24 ± 3.73 days, the symptom improvement rate was 61.3% (198 patients) after 3 days of treatment, and 98.14% (317 patients) of the cases were cured or achieved symptom improvement at the end of therapy. Only 5 (1.55%) cases were hospitalized owing to poor treatment efficacy. Moreover, 50 (15.48%) patients had at least one other concomitant chronic condition and 14 (2.79%) had at least 2 concomitant chronic conditions. The most common of these conditions were cardiovascular and cerebrovascular diseases (26, 8.05%), chronic respiratory diseases (12, 3.72%), and type 2 diabetes (10, 3.10%). The concomitant conditions of these patients did not worsen during the oral treatment, and no adverse reactions such as neurological symptoms or palpitations occurred. Three patients developed dermatological symptoms and 3 patients developed gastrointestinal symptoms. The overall incidence of adverse reactions was 1.86% (6 patients). Thus, nemonoxacin showed excellent tolerability, which is consistent with the conclusions drawn from previous clinical trials conducted in China. For example, healthy subjects in China showed no serious adverse reactions after treatment with different oral doses of nemonoxacin (125–1000 mg). The main adverse reactions were mild nausea and mild rash with or without pruritus. Most abnormal laboratory findings were also mild and transient, and the subjects recovered without treatment.18,19 Compared with other quinolones, nemonoxacin has relatively weak antibacterial activity against Mycobacterium tuberculosis.20 In tuberculosis endemic areas, the initial use of fluoroquinolones for CAP is associated with a risk of delaying tuberculosis diagnosis, but using nemonoxacin can reduce this risk.21 It is also particularly suitable for outpatients and patients who cannot be further identified or screened for tuberculosis.
The current study has some limitations. First, retrospective studies are associated with some bias. Second, the study lacked a comparison between nemonoxacin and other antibacterial drugs. In the future, large-scale prospective studies are required to further document the efficacy and safety of nemonoxacin.
Conclusion
As nemonoxacin has been in the market since a short time, the number of cases treated with this drug is relatively limited. Nevertheless, based on our retrospective study, we conclude that in the current situation of severe drug resistance, nemonoxacin is an emerging option for the treatment of CAP. Specifically, in outpatients with mild-to-moderate CAP, it can be used to effectively control the disease without the need for intravenous administration or inpatient treatment. This can reduce medical costs and save patients’ time. Moreover, as it is associated with relatively few adverse reactions and is well tolerated, it can be safely and effectively used for treating CAP.
Ethics Approval and Informed Consent
Our study was approved by the medical ethics committee of the Shengjing Hospital of China Medical University (ethical batch number 2020ps096k). It was a retrospective study, without direct intervention. All patient data are anonymous. Subjects’ information and privacy are fully protected. Therefore, the institutional review board waived the need for written informed consent provided by participants. The study was in accordance with the Declaration of Helsinki.
Disclosure
The authors report no conflicts of interest in this work.
References
1. WHO. The top 10 causes of death[EB/OL]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death.
2. Welte T, Kohnlein T. Global and local epidemiology of community-acquired pneumonia: the experience of the CAPNETZ network. Semin Respir Crit Care Med. 2009;30(2):127–135. doi:10.1055/s-0029-1202941
3. Society CT. Guidelines for the diagnosis and treatment of community-acquired pneumonia in adults in China. Zhonghua Jiehe He Huxi Zazhi. 2016;39(4):253–279.
4. Liu YF, Gao Y, Chen MF, et al. Etiological analysis and predictive diagnostic model building of community-acquired pneumonia in adult outpatients in Beijing, China. BMC Infect Dis. 2013;13:309. doi:10.1186/1471-2334-13-309
5. Kaya S, Telci CO, Oguz A. Community-acquired pneumonia requiring hospitalization. N Engl J Med. 2015;373(24):2381.
6. Adam HJ, Laing NM, King CR, et al. In vitro activity of nemonoxacin, a novel nonfluorinated quinolone, against 2440 clinical isolates. Antimicrob Agents Chemother. 2009;53(11):4915–4920. doi:10.1128/AAC.00078-09
7. Lauderdale TL, Shiau YR, Lai JF, et al. Comparative in vitro activities of nemonoxacin (TG-873870), a novel nonfluorinated quinolone, and other quinolones against clinical isolates. Antimicrob Agents Chemother. 2010;54(3):1338–1342. doi:10.1128/AAC.01197-09
8. Torres A, Chalmers JD, Dela CC, et al. Challenges in severe community-acquired pneumonia: a point-of-view review. Intensive Care Med. 2019;45(2):159–171. doi:10.1007/s00134-019-05519-y
9. Sun HSH. Research progress on direct injury of Mycoplasma pneumoniae and its immunological mechanism. Zhonghua Weishengwuxue He Mianyixue Zazhi. 2015;35(1):65–68.
10. Eljaaly K, Alshehri S, Aljabri A, et al. Clinical failure with and without empiric atypical bacteria coverage in hospitalized adults with community-acquired pneumonia: a systematic review and meta-analysis. BMC Infect Dis. 2017;17(1):385. doi:10.1186/s12879-017-2495-5
11. Kocsis B, Domokos J, Szabo D. Chemical structure and pharmacokinetics of novel quinolone agents represented by avarofloxacin, delafloxacin, finafloxacin, zabofloxacin and nemonoxacin. Ann Clin Microbiol Antimicrob. 2016;15(1):34. doi:10.1186/s12941-016-0150-4
12. Eljaaly K, Alkhalaf A, Alhifany AA, et al. Photosensitivity induced by lomefloxacin versus other fluoroquinolones: A meta-analysis. J Infect Chemother. 2020;26(6):535–539. doi:10.1016/j.jiac.2020.01.005
13. Lin YC, Huang YT, Tsai PJ, et al. Antimicrobial susceptibilities and molecular epidemiology of clinical isolates of Clostridium difficile in taiwan. Antimicrob Agents Chemother. 2011;55(4):1701–1705. doi:10.1128/AAC.01440-10
14. Qin X, Huang H. Review of nemonoxacin with special focus on clinical development. Drug Des Devel Ther. 2014;8:765–774. doi:10.2147/DDDT.S63581
15. Roychoudhury S, Makin K, Twinem T, et al. In vitro resistance development to nemonoxacin in Streptococcus pneumoniae: a unique profile for a novel nonfluorinated Quinolone. Microb Drug Resist. 2016;22(7):578–584. doi:10.1089/mdr.2016.0021
16. Gajdacs M. The concept of an ideal antibiotic: implications for drug design. Molecules. 2019;24(5):892. doi:10.3390/molecules24050892
17. van Rensburg DJ, Perng RP, Mitha IH, et al. Efficacy and safety of nemonoxacin versus levofloxacin for community-acquired pneumonia. Antimicrob Agents Chemother. 2010;54(10):4098–4106. doi:10.1128/AAC.00295-10
18. Guo B, Wu X, Zhang Y, et al. Safety and clinical pharmacokinetics of nemonoxacin, a novel non-fluorinated quinolone, in healthy Chinese volunteers following single and multiple oral doses. Clin Drug Investig. 2012;32(7):475–486. doi:10.2165/11632780-000000000-00000
19. Lai CC, Lee KY, Lin SW, et al. Nemonoxacin (TG-873870) for treatment of community-acquired pneumonia. Expert Rev Anti Infect Ther. 2014;12(4):401–417. doi:10.1586/14787210.2014.894881
20. Tan CK, Lai CC, Liao CH, et al. Comparative in vitro activities of the new quinolone nemonoxacin (TG-873870), gemifloxacin and other quinolones against clinical isolates of Mycobacterium tuberculosis. J Antimicrob Chemother. 2009;64(2):428–429. doi:10.1093/jac/dkp174
21. Dooley KE, Golub J, Goes FS, et al. Empiric treatment of community-acquired pneumonia with fluoroquinolones, and delays in the treatment of tuberculosis. Clin Infect Dis. 2002;34(12):1607–1612. doi:10.1086/340618
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
