Back to Journals » Drug Design, Development and Therapy » Volume 10
Efficacy and safety of monoclonal antibodies targeting interleukin-17 pathway for inflammatory arthritis: a meta-analysis of randomized controlled clinical trials
Received 29 June 2015
Accepted for publication 7 April 2016
Published 9 September 2016 Volume 2016:10 Pages 2771—2777
DOI https://doi.org/10.2147/DDDT.S91374
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Min Wei,1 Dongmei Duan2
1Department of Orthopedics, 2Department of Traditional Chinese Medicine, People’s Liberation Army General Hospital, Beijing, People’s Republic of China
Abstract: T-helper 17 (Th17) pathway plays an important and distinct role in autoimmunity and inflammation. A growing body of evidence demonstrates that interleukin-17 (IL-17) is also synthesized in inflammatory arthritis tissues and exerts potent proinflammatory and joint-destructive activities. Clinical studies have been performed to evaluate the therapeutic efficacy of antibodies blocking the IL-17 signaling pathway in patients with rheumatoid arthritis (RA). In this study, we performed a meta-analysis to systematically evaluate the clinical effects of IL-17 antibodies in RA patients. By searching PubMed, five randomized, placebo-controlled randomized controlled clinical trials that tested three antibodies against IL-17A (LY2439821 and secukinumab/AIN457) and the IL-17A receptor (brodalumab) were identified. The primary outcomes that were analyzed include American College of Rheumatology (ACR) Improvement Criteria and Disease Activity Score in 28 joints (DAS28). Meanwhile, the safety and adverse effects were also systematically analyzed. The results of the meta-analysis demonstrated that IL-17 antibody is effective in ameliorating the RA symptoms. Specifically, IL-17-blocking antibody significantly reduced ACR20 and ACR50. It also dramatically reduced DAS28, an index that measures tenderness and swelling severity of joints. The side effects of and intolerance to the antibody treatment were higher than those in the placebo control. The analysis result provides evidence-based information for clinical use of these agents in the treatment of inflammatory arthritis.
Keywords: interleukin-17A, arthritis, meta-analysis, rheumatoid arthritis, clinical trials
Introduction
Rheumatoid arthritis (RA) is a chronic, inflammatory, and systemic autoimmune disease that affects ~1% of the population all over the world.1 In RA patients, the affected joints contain autoreactive T- and B-cells that produce proinflammatory cytokines, resulting in cartilage and bone damage.1 Targeting these cytokines provides a strategy for treatment, including disease-modifying antirheumatic drugs (DMARDs).2,3 such as methotrexate (MTX). However, these DMARDs only work for a small proportion of patients. New medicines are urgently in demand.
In 1995, Yao et al4 discovered that human T-cells could produce a proinflammatory cytokine, interleukin (IL)-17. These IL-17-producing cells are mainly a subset of cluster of differentiation 4 (CD4+) T-cells, a type of CD4+ “helper” lymphocytes named Th17 cells.5,6 IL-17 levels are extremely low or undetectable in normal human peripheral blood, while the levels are elevated in peripheral blood or synovial fluid in RA patients.7–10 Immunohistochemistry techniques led to the identification of a subset of IL-17-expressing T-cells in the synovium of RA patients.11,12 Moreover, the number of IL-17A-positive cells was also increased in children with juvenile inflammatory arthritis joints.13 Blocking IL-17A can reduce IL-6 expression and formation of collagen breakdown products.14
Th17 cells mediate the inflammation process by stimulating production of cytokines, chemokines, and matrix metalloproteinases.15 Many human autoimmune diseases, including RA and psoriatic arthritis, are associated with abnormal Th17 activity.16,17 Inhibition of IL-17 signaling through a ligand or its receptor could reduce inflammation and bone erosion in animal arthritis models.18 Meanwhile, clinical investigations have also been carried out to target IL-17A signaling for alleviating the symptoms of RA. This meta-analysis was undertaken to evaluate the results of clinical trials and to provide evidence-based information for using these agents in clinical treatment of inflammatory arthritis.
Methods
Database search, selection criteria, and quality assessment
Database search was performed in PubMed using the keywords “interleukin-17A” and “rheumatoid arthritis”. Eligible studies were selected based on the following criteria: 1) study design: randomized, double-blinded, placebo-controlled clinical trials (RCTs); 2) subjects: patients with RA; 3) intervention: administration of antibodies for blocking IL-17A signaling, including LY2439821 used by Genovese et al,19 a humanized anti-IL-17 monoclonal antibody; secukinumab (AIN457), a fully human monoclonal anti-IL-17A antibody used by Genovese et al,20 Hueber et al,21 and Patel et al;22 brodalumab, a human anti-IL-17 receptor monoclonal antibody used by Martin et al.23 The quality of included trials was assessed using the Jadad scale score (zero to five), with a score of ≥3 indicating high quality.24
Outcomes, data extraction, and statistical analysis
Effects of treatment were measured by the improvement in the percentage of patients achieving American College of Rheumatology (ACR) scores ACR20, ACR50, and ACR70 according to Felson’s method.25 Another measurement, Disease Activity Score in 28 joints (DAS28), was also used in the studies based on standard guidelines.26
All analyses were performed using the Review Manager, version 5.1.0 (Cochrane Collaboration, Oxford, UK). The χ2 Cochran Q-test was performed to detect heterogeneity. Random- or fixed-effects inverse variance-weighted method was used to test the difference in significance levels.27 Mean difference and the associated 95% confidence interval (CI) for ACR20/50/70 were used to assess the significance of difference.
Results
Identification of eligible studies from database search
In order to obtain eligible clinical studies, we first carried out a database search. The terms “interleukin-17A” and “rheumatoid arthritis” were used as the keywords to search the PubMed database, which resulted in a total of 32 articles being retrieved. Further stringent selections were performed by using filtering criteria with the clinical trials and RCTs. We identified five eligible studies, namely, Genevese et al,19,20 Hueber et al,21 Martin et al,23 and Patel et al22 (Figure 1). These randomized, double-blind, and placebo-controlled studies are summarized briefly in Table 1.
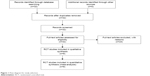  | Figure 1 Flow diagram for study selection. |
Three different antibodies were tested by three different research groups; the antibodies include LY2439821, a human anti-IL-17 monoclonal antibody used in Genovese et al,19 secukinumab (AIN457), a human anti-IL-17A antibody tested in Genovese et al,20 Hueber et al,21 and Patel et al;22 brodalumab (AMG827), a human anti-IL-17 receptor A monoclonal antibody tested in Martin et al.23
Meta-analysis of the outcomes tested
A major end point used in these studies was the ACR score, a widely used standard to measure the improvement in symptom reduction in RA patients. We collected the ACR20, ACR50, and ACR70 values from the eligible studies identified from the search, including Genovese et al,19,20 Hueber et al,21 Martin et al,23 and Patel et al.22 A meta-analysis was performed and results are presented in Figure 2, showing that treatment with the IL-17-signaling blockers had a dramatic effect on ACR20 (Figure 2A, P=0.0005) and ACR50 (Figure 2B, P=0.007). ACR70 tends to change, but the effect is not significant enough (Figure 2C, P=0.1). Of note, brodalumab, the IL-17A receptor antibody, resulted in a lesser effect than the other three IL-17A antibodies,23 suggesting that neutralizing of IL-17A ligand provides a more efficient approach for RA treatment.
  | Figure 2 Forest plot of meta-analysis. |
Another readout, DAS28, was derived from tender 28-joint and swollen 28-joint counts.26 All three studies using IL-17-neutralizing antibodies found marked reduction in the joint swollen points relative to the placebo control treatment, supporting the efficacy of these antibodies in alleviating the RA symptoms. In Genovese et al,19 greater decrease in DAS28 was observed after treatment with the antibody LY2439821 (P<0.05): −2.3, −2.4, and −2.3 in 0.2 mg/kg, 2.0 mg/kg, and all-combined groups, respectively, compared to that in the placebo control group (−1.7). Similar findings were achieved by antibody treatment using secukinumab (AIN457) in Genovese et al,20 Hueber et al,21 and Patel et al.22
Safety and adverse effects
Adverse effects were investigated in these studies, and we performed a meta-analysis for the same. The median doses of IL-17A antibodies were chosen for analysis: 75 mg secukinumab in Genovese et al20 (doses tested ranged from 25 mg to 300 mg) and 0.6 mg/kg LY249821 in Genovese et al19 (doses tested ranged from 0.6 mg/kg to 2.0 mg/kg). Meta-analysis results demonstrated the total events of adverse effects were significantly higher in IL-17A-treated patients than in placebo control-treated patients (odds ratio [OR] =2.03; 95% CI: 1.24–3.33; P=0.005; Figure 3). Hueber et al21 reported one severe adverse effect (SAE) (laryngeal abscess due to RA of the cricoarytenoid joint) in AIN457-treated patients. Two SAEs (interstitial lung disease and brachial plexopathy) were recorded in the placebo-treated group. AIN457 treatment slightly increased the overall AE incidence. Headache, diarrhea, leukemia, and vertigo were reported in patients treated with LY2439821. However, these side effects occurred equally in placebo-treated patients.
Assessment for publication bias
In order to assess the publication bias, Begg’s funnel plots test28 was performed. As shown in Figure 4, the symmetrical patterns of these plots indicate that there is no publication bias in the studies selected for meta-analysis of ACR20 (Figure 4A), ACR50 (Figure 4B), and ACR70 (Figure 4C), There is also no publication bias in the studies selected for meta-analysis of AEs (Figure 4D).
Discussion
In this study, we performed a meta-analysis on five clinical trials investigating therapeutic antibodies blocking the IL-17A signaling pathway of RA. The results demonstrated the beneficial role of IL-17A antibody in the alleviation of RA symptoms, as measured by ACR20/50/70 and DAS28, two widely used outcomes for assessment of recovery from this disease. The reduced tender joint count, swollen joint count, and pain after the antibody treatment might have resulted from the blockage of some step in the immune response pathway, such as production of cytokines or chemokines and neutrophil recruitment.7
IL-17A signaling plays a key role in this inflammatory disease.29–33 In the mouse model, IL-17 overexpression gave rise to joint inflammation, as well as bone and cartilage damage.34 By contrast, disruption of IL-17 signaling in the mouse can protect the bone cartilage from arthritis induction.35–37 Murine Th17 cells play critical roles in chronic, erosive diseases,38,39 with Toll-like receptor 4 having been proven to be critical in Th17-mediated inflammatory arthritis.40,41
Moreover, IL-17A antibody has also beneficial effects on other immune-mediated diseases, such as psoriasis, a skin autoimmune disease. Psoriatic arthritis also affects many people in the world. The IL-17A antibody secukinumab has also been shown to have beneficial effects on patients with this disease. IL-17A expression is increased in patients with psoriatic arthritis,42–44 along with increased IL-17A receptor levels,42 in these patients.
One of these antibodies, brodalumab, has little effect on RA. It is a human immunoglobulin G2 monoclonal antibody against IL-17 receptor A and blocks the biological activity of IL-17A and IL-17F, as well as A/F heterodimer signaling. This antibody was tested and found to be capable of suppressing the inflammatory process in psoriasis.16 This might be due to the multiple ligands involved in RA.
Tumor necrosis factor (TNF) can regulate IL-17A signaling; an anti-TNF antibody, infliximab, was tested in terms of its ability to reduce the number of IL-17-producing cells and decrease the disease activity.45 Other TNF-blocking treatments had a similar effect in RA patients.46,47 Yue et al demonstrated a decrease in peripheral Th17 cells in RA patients treated with adalimumab/MTX.48 Therefore, targeting Th17 cells provides a tool to treat inflammatory diseases such as RA.
However, antibodies targeting Th17 may have potential side effects that would adversely affect their clinical application. It has been reported that neutralization of IL-17 in mice exacerbated Candida-induced dermatitis in skin.49 Moreover, it is suspected that neutralization of IL-17RA might be linked to suicidal thoughts.50 However, increased plasma concentration of proinflammatory cytokines, especially IL-17a, after myocardial infarction plays an important role in the stress reaction that predisposes to depression during the next 6 months.51 A recent study also demonstrated a higher rate of injection site reaction and allergy.52 The exact roles of IL-17a and IL-17-neutralizing antibodies in this process remain to be elucidated, and comprehensive evaluation of these agents for clinical application will be needed.
Acknowledgment
This work was supported by the grants of National Natural Science Foundation of China (No 81473710 and No 81473710) and Beijing Natural Science Foundation (No 7152134).
Disclosure
The authors report no conflicts of interest in this work.
References
McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–2219. | ||
Daikh DI, St Clair EW. Updated recommendations for the treatment of rheumatoid arthritis: another step on a long road. Arthritis Care Res (Hoboken). 2012;64(5):648–651. | ||
Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64(5):625–639. | ||
Yao Z, Painter SL, Fanslow WC, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155(12):5483–5486. | ||
Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol. 2006;18(6):670–675. | ||
Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888–898. | ||
Ziolkowska M, Koc A, Luszczykiewicz G, et al. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol. 2000;164(5):2832–2838. | ||
Cho ML, Yoon CH, Hwang SY, et al. Effector function of type II collagen-stimulated T cells from rheumatoid arthritis patients: cross-talk between T cells and synovial fibroblasts. Arthritis Rheum. 2004;50(3):776–784. | ||
Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103(9):1345–1352. | ||
Raza K, Falciani F, Curnow SJ, et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther. 2005;7(4):R784–R795. | ||
Chabaud M, Durand JM, Buchs N, et al. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42(5):963–970. | ||
Joosten LA, Radstake TR, Lubberts E, et al. Association of interleukin-18 expression with enhanced levels of both interleukin-1beta and tumor necrosis factor alpha in knee synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 2003;48(2):339–347. | ||
Nistala K, Moncrieffe H, Newton KR, Varsani H, Hunter P, Wedderburn LR. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum. 2008;58(3):875–887. | ||
Chabaud M, Fossiez F, Taupin JL, Miossec P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol. 1998;161(1):409–414. | ||
Gaffen SL. Recent advances in the IL-17 cytokine family. Curr Opin Immunol. 2011;23(5):613–619. | ||
Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366(13):1181–1189. | ||
Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763–776. | ||
Lubberts E. IL-17/Th17 targeting: on the road to prevent chronic destructive arthritis? Cytokine. 2008;41(2):84–91. | ||
Genovese MC, Van den Bosch F, Roberson SA, et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 2010;62(4):929–939. | ||
Genovese MC, Durez P, Richards HB, et al. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann Rheum Dis. 2013;72(6):863–869. | ||
Hueber W, Patel DD, Dryja T, et al; Rheumatoid Arthritis Study Group, Uveitis Study Group. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2(52):52ra72. | ||
Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann Rheum Dis. 2013;72(Suppl 2):ii116–ii123. | ||
Martin DA, Churchill M, Flores-Suarez L, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013;15(5):R164. | ||
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. | ||
Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The committee on outcome measures in rheumatoid arthritis clinical trials. Arthritis Rheum. 1993;36(6):729–740. | ||
Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. | ||
Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester West Sussex, UK: John Wiley & Sons Ltd.; 2011. | ||
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. | ||
Kryczek I, Bruce AT, Gudjonsson JE, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181(7):4733–4741. | ||
Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129(9):2175–2183. | ||
Zaba LC, Suárez-Fariñas M, Fuentes-Duculan J, et al. Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J Allergy Clin Immunol. 2009;124(5):1022–10.e1–395. | ||
Lin AM, Rubin CJ, Khandpur R, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187(1):490–500. | ||
Cai Y, Shen X, Ding C, et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity. 2011;35(4):596–610. | ||
Lubberts E, Joosten LA, Oppers B, et al. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J Immunol. 2001;167(2):1004–1013. | ||
Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171(11):6173–6177. | ||
Bush KA, Farmer KM, Walker JS, Kirkham BW. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum. 2002;46(3):802–805. | ||
Lubberts E, Koenders MI, Oppers-Walgreen B, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50(2):650–659. | ||
Koenders MI, Lubberts E, Oppers-Walgreen B, et al. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am J Pathol. 2005;167(1):141–149. | ||
Abdollahi-Roodsaz S, Joosten LA, Helsen MM, et al. Shift from toll-like receptor 2 (TLR-2) toward TLR-4 dependency in the erosive stage of chronic streptococcal cell wall arthritis coincident with TLR-4-mediated interleukin-17 production. Arthritis Rheum. 2008;58(12):3753–3764. | ||
van Lent PL, Grevers LC, Blom AB, et al. Stimulation of chondrocyte-mediated cartilage destruction by S100A8 in experimental murine arthritis. Arthritis Rheum. 2008;58(12):3776–3787. | ||
Abdollahi-Roodsaz S, Joosten LA, Roelofs MF, et al. Inhibition of toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 2007;56(9):2957–2967. | ||
Raychaudhuri SP, Raychaudhuri SK, Genovese MC. IL-17 receptor and its functional significance in psoriatic arthritis. Mol Cell Biochem. 2012;359(1–2):419–429. | ||
Jandus C, Bioley G, Rivals JP, Dudler J, Speiser D, Romero P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58(8):2307–2317. | ||
Noordenbos T, Yeremenko N, Gofita I, et al. Interleukin-17-positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum. 2012;64(1):99–109. | ||
Shen H, Xia L, Lu J, Xiao W. Infliximab reduces the frequency of interleukin 17-producing cells and the amounts of interleukin 17 in patients with rheumatoid arthritis. J Investig Med. 2010;58(7):905–908. | ||
Kageyama Y, Kobayashi H, Kato N. Infliximab treatment reduces the serum levels of interleukin-23 in patients with rheumatoid arthritis. Mod Rheumatol. 2009;19(6):657–662. | ||
Kageyama Y, Kobayashi H, Kato N, Shimazu M. Etanercept reduces the serum levels of macrophage chemotactic protein-1 in patients with rheumatoid arthritis. Mod Rheumatol. 2009;19(4):372–378. | ||
Yue C, You X, Zhao L, et al. The effects of adalimumab and methotrexate treatment on peripheral Th17 cells and IL-17/IL-6 secretion in rheumatoid arthritis patients. Rheumatol Int. 2010;30(12):1553–1557. | ||
Zhang H, Li H, Li Y, et al. IL-17 plays a central role in initiating experimental Candida albicans infection in mouse corneas. Eur J Immunol. 2013;43(10):2671–2682. | ||
Schmidt C. Suicidal thoughts end Amgen’s blockbuster aspirations for psoriasis drug. Nat Biotechnol. 2015;33(9):894–895. | ||
Wilkowska A, Pikuła M, Rynkiewicz A, Wdowczyk-Szulc J, Trzonkowski P, Landowski J. Increased plasma pro-inflammatory cytokine concentrations after myocardial infarction and the presence of depression during next 6-months. Psychiatr Pol. 2015;49(3):455–464. | ||
Genovese MC, Greenwald M, Cho CS, et al. A phase II randomized study of subcutaneous ixekizumab, an anti-interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheumatol. 2014;66(7):1693–1704. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



