Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Efficacy and Safety of Low-Dose Rituximab in Anti-MuSK Myasthenia Gravis Patients: A Retrospective Study
Authors Meng X, Zeng Z, Wang Y, Guo S, Wang C, Wang B, Guo S
Received 18 January 2022
Accepted for publication 21 April 2022
Published 3 May 2022 Volume 2022:18 Pages 953—964
DOI https://doi.org/10.2147/NDT.S358851
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Xin Meng,1 Ziling Zeng,2 Yunda Wang,3 Shuai Guo,1 Chunjuan Wang,2 Baojie Wang,1 Shougang Guo1,2
1Department of Neurology, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, People’s Republic of China; 2Department of Neurology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, People’s Republic of China; 3Department of Neurosurgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, People’s Republic of China
Correspondence: Shougang Guo, Department of Neurology, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jing Wu Road, Huaiyin District, Jinan, Shandong, 250021, People’s Republic of China, Tel +86-13220585081, Fax +86-531-87937741, Email [email protected]
Purpose: To evaluate the efficacy and safety of low dosages of rituximab (RTX) in the treatment of MuSK-antibody-positive MG patients.
Patients and Methods: We retrospectively analyzed the data of MuSK-antibody-positive MG patients who were treated with low dosages of RTX from January 2018 to October 2021. The long-term treatment response to RTX was assessed by Myasthenia Gravis Foundation of America (MGFA) post-interventional status (PIS), Myasthenia Gravis Status and Treatment Intensity (MGSTI), dosage of steroid, MG-related activities of daily living (MG-ADL) and myasthenic muscle score (MMS) at the end of follow-up.
Results: Clinical improvement was observed in all eight patients with follow-up for 8 to 29 months after treatment. At the last visit, complete stable remission had been achieved in one patient, pharmacologic remission in three patients, minimal manifestations status in three patients and improved in one patient based on the MGFA-PIS criteria. MGSTI level 2 or better had been reached in six (75%) patients at the last visit. The steroid dosage decreased from 60 mg at baseline to 15 mg at the last follow-up (p = 0.011). The average MG-ADL score decreased from 11 (range 7 to 15) to 0 (range 0 to 3; p = 0.011), and the MMS improved from 38.5 (range 24 to 60) to 100 (range 90 to 100; p = 0.012). These differences were all statistically significant. During RTX treatment and subsequent follow-up, 1 patient reported minor post-infusion malaise.
Conclusion: Low-dose RTX is effective and safe for treating anti-MuSK antibody positive MG patients. A long-term response is observed after treatment. Larger prospective studies are required to provide further evidence.
Keywords: myasthenia gravis, rituximab, low-dose, muscle-specific kinase
Introduction
Myasthenia gravis (MG) is an acquired autoimmune disease of the postsynaptic neuromuscular junction characterized by fluctuating muscle weakness and fatigue that can involve any of the skeletal muscles.1
The pathogenic antibodies that have been discovered thus far include anti-acetylcholine receptor antibody (AChR), muscle-specific receptor tyrosine kinase antibody (MuSK), and low-density lipoprotein receptor-related protein 4 antibody (LRP4).1–3 Patients with anti-MuSK-antibody-positive myasthenia gravis (MuSK-MG) account for 5 to 8% of all patients with MG.4 Compared with patients with anti-AChR-antibody-positive, and anti-AChR- and MuSK-antibody double-negative MG, MuSK-MG patients have an earlier onset,5 and their clinical stage and condition are more serious at the time of onset.4,6
The mainstay of treatment for MuSK-MG is immunotherapy. MuSK-MG responds quickly and effectively to steroids, but frequent relapses occur during steroid dose reduction.7 When a patient’s condition deteriorates, high-dose prednisone combined with plasma exchange or intravenous immunoglobulin should be considered. Traditional immunosuppressants, including azathioprine, mycophenolate mofetil, tacrolimus, and cyclosporine, have been successfully administered as steroid-sparing agents to treat patients with MuSK-MG.7,8 However, some MuSK-MG patients still do not benefit from these traditional therapies.9 Although early clinical remission can be achieved in patients after receiving standard treatments, these patients are more likely to relapse than anti-AChR MG (AChR-MG) patients, which seriously affects their quality of life.
Rituximab (RTX) is a human-mouse chimeric monoclonal antibody that specifically binds to the CD20 antigen, which is expressed on the surface of 95% of B lymphoma cells and normal B lymphocytes.10 RTX, as a drug that can deplete B cells and their precursor cells, was initially approved to treat non-Hodgkin’s lymphoma.11 However, the administration of RTX has gradually expanded to treat other autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, immune thrombocytopenic purpura, autoimmune encephalitis and neuromyelitis optica spectrum disorder.12–16 Recently, RTX has been found to be effective in MG patients. Previous studies have confirmed the good long-term efficacy of RTX in AChR-MG, and RTX can improve the prognosis and reduce the recurrence rate.17 Several case reports and clinical series have shown that RTX is even more effective for treating Musk-MG than AChR-MG.18–21 RTX can not only improve the clinical symptoms of MuSK-MG patients but also reduce the dose of concomitant steroids or other immunosuppressants.
The current recommended dose refers to the B-cell lymphoma regimen: 375 mg/(m2·week) as continuous treatment for 4 weeks.22 However, due to the high price of RTX and the possible risks of using a high-dose, we found that low-dose RTX (375 mg/m2 for 1 or 2 infusions), can also be effective for MuSK-MG patients.
There are currently no consistent treatment plans for RTX treatment of MuSK-MG. This study explored the clinical efficacy and safety of low-dose RTX in MuSK-MG patients.
Materials and Methods
Patients
Eight MuSK-MG patients were enrolled in this study from January 2018 to October 2021 in the Department of Neurology of Shandong Provincial Hospital affiliated with Cheeloo College of Medicine, Shandong University. All the patients fulfilled the following criteria: (1) were aged 18 years or older; (2) met the criteria for myasthenia gravis based on the clinical symptoms and the positive results of the pharmacological and/or electrophysiological tests; (3) were positive for anti-MuSK antibody and negative for AChR antibody; (4) received rituximab treatment; and (5) did not have other autoimmune diseases.
This study was approved by the Research Ethics Committee of Shandong Provincial Hospital affiliated to Cheeloo College of Medicine, Shandong University and conformed to the principles of the Declaration of Helsinki.
Clinical and Laboratory Assessment
We evaluated patients before and after rituximab treatment. We collected the demographic characteristics, clinical manifestations, myasthenic muscle score (MMS), MG-related activities of daily living (MG-ADL) scores, number of relapses of the disease, daily dose of prednisone, types of immunosuppressive agents, peripheral B cell levels and antibody titers. The CD19+ B-cell and CD19+CD27+ B-cell counts were determined by flow cytometry in peripheral blood samples. The depletion of the number of B cells was defined as CD19+ B cell population being < 1% of the total lymphocytes and CD19+CD27+ memory B cells < 0.05%. Serum MuSK autoantibody titers were detected with an enzyme-linked immunosorbent assay (ELISA) or cell-based assay (CBA). The reference range for the normal value of MuSK antibody detected with ELISA was defined as ≤ 0.4 U/mL.
Evaluation of Efficacy and Safety
We defined the baseline time of follow-up based on the first day of initiating RTX. We used the Myasthenia Gravis Foundation of America (MGFA) post-interventional status (PIS), Myasthenia Gravis Status and Treatment Intensity (MGSTI), dosage of steroids and clinical scales to evaluate its long-term efficacy. All adverse events were collected and graded according to the Common Terminology Criteria for Adverse Events (CTCAE).
MGSTI is a new outcome score that combines the MGFA-PIS and the dose of immunosuppressants.21
Statistical Analysis
Statistical analysis was performed using SPSS 23.0 software. GraphPad Prism 8 software was used for graphing. All data were tested for normality. Continuous variables conforming to a normal distribution are expressed as the mean ± standard deviation (SD), and Student’s t-test was used to analyze the differences in these variables. Quantitative data without a normal distribution are presented as medians and ranges. The difference before and after RTX infusion was compared using the Wilcoxon matched-pairs signed rank test. A P-value < 0.05 was considered statistically significant.
Results
Cohort Characteristics
A total of 8 MuSK-MG patients were included, all of whom were females. The mean age of the patients at the time of onset of MG was 44.9 ± 9.7 years. The median duration of the disease prior to RTX infusion was 6 (range 1–36) months.
Among these patients, bulbar impairment was demonstrated in all the patients (7 with dysarthria, 8 with dysphagia, and 4 with masticatory difficulty). Four patients experienced respiratory muscle weakness. Extraocular muscles were also involved in six patients. Five patients had limb muscle weakness and four patients had axial muscle weakness (Figure 1). No patients experienced muscle atrophy. None of the patients presented with either thymic hyperplasia or thymomas. None of the patients had other systemic immune diseases.
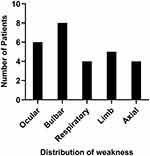 |
Figure 1 Distribution of weaknesses among the eight MuSK myasthenia gravis patients at baseline. Abbreviation: MuSK, muscle-specific tyrosine kinase. |
Before RTX, one patient received one course of intravenous immunoglobulin infusion, and all the patients received steroid therapy. To avoid the temporary aggravation of myasthenic symptoms caused by the use of glucocorticoids and the possibility of triggering myasthenic crisis, all the patients were treated with 80–240 mg/d methylprednisolone according to the severity of the patient’s condition, and the dose was gradually reduced after 3 to 5 days of intravenous infusion. The initial oral dose of prednisone acetate was 1 mg/kg. The dose was tapered after 1 month of maintenance with dose tapering not exceeding 10 mg per month. When reducing the dose to 40 mg, the reduction did not exceed 5 mg per month. Five patients (62.5%) were taking pyridostigmine (60–180 mg/d). None of the patients were taking other oral immunosuppressive agents. None of the patients underwent thymectomy.
The demographic and clinical characteristics of the patients are shown in Table 1.
 |
Table 1 Patient Characteristics of Enrolled MuSK Myasthenia Gravis Patient |
Dosages
Five patients (62.5%) received protocol A (375 mg/m2 rituximab intravenously twice at an interval of two weeks between the infusions), and three patients (37.5%) received protocol B (only 375 mg/m2 as a single infusion).
Six patients (75%) received 1 cycle (4 for protocol A, 2 for protocol B) of rituximab, and one patient (patient no. 5, 12.5%) received 2 cycles of protocol A for clinical relapse. While one patient (patient no. 2, 12.5%) received 4 cycles of protocol B, who received the second cycle of RTX due to a relapse and repeated another 2 cycles every 6 months.
Rituximab Efficacy
MGFA-PIS, Daily Dose of Prednisone and MGSTI
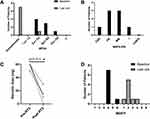
Eight female patients were followed for a median period of 25.5 (range 8–29) months. All 8 patients showed clinical improvement at the final follow-up after low-dose RTX treatment. At the last follow-up, patient no. 2 reached a MGFA score of 2a, and the other 7 patients became asymptomatic (Figure 2A). One (12.5%) of the patients achieved a complete stable remission (CSR), three (37.5%) patients achieved a pharmacologic remission (PR), three (37.5%) patients had minimal manifestations (MM) and the remaining one (12.5%) patient had a functional improvement but still had ongoing symptoms of myasthenia (Figure 2B).
All eight patients were taking steroids at the beginning of this study. The mean dose of prednisolone had decreased from 60 mg at baseline to 15 mg at the last follow-up (p=0.011). Only one patient (12.5%) was able to discontinue prednisone entirely during the study. Three patients (37.5%) had reduced their daily dose of prednisone to 10 mg or less (Figure 2C).
The MGSTI scores were level 4 in seven (87.5%) patients and level 6 in one (12.5%) patient at baseline. However, six (75%) patients reached a MGSTI level of 2 or better at the last visit (Figure 2D).
The MG-ADL and MMS Scores
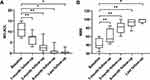
In all patients, the MG-ADL and MMS scores improved significantly after starting RTX treatment (Figure 3). The average MG-ADL and MMS of the 8 patients were 11 (range 7 to 15) and 38.5 (range 24 to 60), respectively, at baseline. At follow-up, the MG-ADL had declined significantly to 7.5 (range 3 to 11; p<0.001) at 1 month after-RTX, 2.5 (range 0 to 9; p<0.001) at 3 months, 1 (range 0 to 5; p=0.011) at 6 months, 0 (range 0 to 3; p=0.011) at the time of last visit. There was a significant improvement in the MMS to 54.5 (range 42 to 85; p=0.001) at 1 month, 82.5 (range 60 to 100; p<0.001) at 3 months, 94 (range 73 to 100; p<0.001) at 6 months, and 100 (range 90 to 100; p=0.012) at the last follow-up.
B Lymphocyte Subsets and Serum Autoantibody Levels
B lymphocyte subsets were detected at baseline and 1, 3, and 6 months after RTX treatment (Figure 4). Six patients underwent tests of CD19+ B cells and the CD19+CD27+ B cells counts were regularly measured in five patients. A remarkable decrease in the percentage of CD19+ B cells was observed after the RTX infusions. Six (100%) patients achieved CD19+ B-cell depletion at 1 month, and continuously reached CD19+ B cell counts of < 1% at 3 months and 6 months. The median CD19+ B-cell counts decreased to 0.300% (0.020–0.570%) at 6 months compared with 19.135% (10.360–30.430%) at baseline (p=0.001). We observed similar results in the CD19+CD27+ B-cell counts. Four of five (80%) patients had reduced CD19+CD27+ B cells < 0.05% at 1 month and all became 0% at 3 months. However, the CD19+CD27+ B cells reappeared in 3 patients at 6 months. There was also a significant decline in CD19+CD27+ B cells from 5.88 (4.00–16.48%) at baseline to 0.11% (0.00–0.22%) at 6 months. However, there were three patients with CD19+CD27+ B-cell counts of >0.05%, but CD19+ B-cell counts <1% at 6 months. They did not have worse clinical symptoms.
The MuSK antibody levels (using ELISA) were >12 U/mL in five patients, and 4.59 in one patient. Using cell-based assays, they were 1:10 in two patients. In the six patients tested positive for MuSK-Ab by ELISA, the MuSK antibody levels were still >0.4 U/mL at 1 month. The antibody levels of the two patients decreased to 0.09 and 0.19 U/mL progressively at 6 months. However, the MuSK antibody level of patient no. 5 increased from 5.45 U/mL at 3 months to 16.92 U/mL at 6 months, and a clinical relapse occurred in this patient at 9 months. Patient no. 6 also had an increase in antibody levels (0.42 at 3 months vs 2.27 at 6 months), but continuous clinical improvement was observed in this patient. In the two patients tested positive for MuSK-Ab by CBA, the MuSK antibody levels became negative at 6 months.
Follow-Up and Retreatment
One patient (patient no. 2) suffered from respiratory muscle weakness 3 days after the first cycle of RTX and finally developed a MG crisis. Then, patient no. 2 was rescued by an intravenous immunoglobulin infusion. Two patients had 3 relapses in total during the whole follow-up period (Figure 5). Patient no. 2 had relapses at 5 and 7 months after the first cycle and received IVIg as a rescue treatment. Since the patient did not come to our hospital for the first relapse, the levels of CD19+ B cells and CD19+CD27+ B cells in the serum at the time of relapse were not detected. The CD19+ B-cell count of total lymphocytes in peripheral blood at the second relapse was 0.32%, and the CD19+CD27+ B cell count was not detected. The second cycle of RTX was infused after the second relapse, and then patient no. 2 received two cycles of RTX every six months. Patient no. 5 had a relapse at 9 months and improved again after repeating a cycle of rituximab treatment. The CD19+ B-cell and CD19+CD27+ B-cell counts were 0.38% and 0%, respectively. The MGFA-PIS at these two patients’ most recent follow-up showed improvement and pharmacologic remission.
Safety of RTX
RTX was well tolerated, with mild post-infusion malaise reported by one patient, which fully resolved after one week. None of the patients had allergic reactions. There were no serious side effects during the follow-up period.
Discussion
In this retrospective observational study, we observed clinical improvements in all eight patients after receiving low-dose RTX. At the last follow-up, minimal manifestation status or better was achieved in seven patients, MGSTI level 2 or higher in six patients, and significant improvements in the MG-ADL and MMS scores in all the patients. Our results supported that low-dose RTX was effective for MuSK-MG, and could not only relieve clinical symptoms in the short term, but also improve the long-term prognosis.
In a previous meta-analysis, which included a total of 168 MG patients in 37 studies from January 2000 to January 2014, 83.9% of patients had a good response to RTX.18 Another systematic review showed that clinical improvement after RTX treatment was achieved in 68% of AChR-MG patients and that minimal manifestation status was reached in 54% of AChR-MG patients.24 This may be due to bias in patient selection. RTX also has beneficial effects in refractory MG.25 In a prospective study by Fatehi et al, 34 patients with refractory MG had significant decreases in Myasthenia Gravis Composite (MGC) and MG-ADL scores after treatment with Zytux, an RTX biosimilar.26 In a retrospective study with a 10-year follow-up, sustained clinical remission was achieved in all 4 patients with refractory MG after RTX therapy.27 These results demonstrate that RTX is effective not only as an induction therapy, but also for sustained remission in patients with MG. Some authors have suggested that the efficacy of RTX may vary due to different types of antibodies.23,25 In a recent study, the minimum disease state or better was achieved in 72% of MuSK-MG patients after RTX treatment, while this outcome was achieved in only 30% of AChR-MG patients.23 This finding indicates that RTX may be more effective for MuSK-MG patients than AChR-MG patients. This may be because AChR antibodies are mainly of the IgG type, dominated by IgG1 to IgG3, while the main MuSK-antibody subtype is IgG4, which is produced by short-lived plasma cells.28 RTX eliminates B lymphocytes and short-lived plasma cells, but has no effect on long-lived plasma cells. Thus, long-lived CD20-plasma cells continue to secrete autoantibodies.29 In our study, we observed that most patients could benefit from RTX treatment. Even when two patients experienced relapse during the follow-up in our study, RTX was still effective for relapse. However, we could not compare the efficacy of RTX among MG patients with different pathogenic antibodies.
There is no consistent regimen for the use of RTX in MG patients. The most commonly used regimen is similar to that used in the treatment of non-Hodgkin’s lymphoma (375 mg/m2 per week as a continuous infusion for 4 weeks).22 In previous studies, the standard-dose RTX treatment was effective for MG, but the cost of rituximab is a burden for many patients and their families in China. Therefore, in our study, we explored the effectiveness of low-dose rituximab in MuSK-MG patients. Eight patients were infused with a dose of 375 mg/m2×1-2 rituximab, and all the patients had clinical improvement at the end of the follow-up. In a retrospective multicenter study, 11 patients were treated with protocol 4 + 2 (375 mg/m2 every week for four consecutive weeks and then monthly for the next 2 months), 5 were treated with protocol 1 + 1 (two 1 g doses separated by 2 weeks), and 9 were treated with protocol 4 (375 mg/m2 every week for four consecutive weeks). At a mean follow-up of 5 years, the relapse rates were 18.2%, 80%, and 33.3%, respectively. In our study, 25% patients relapsed with a median follow-up time of 25.5 months.30 The patients in our study appeared to have a lower recurrence rate than those treated with protocol 1 + 1 and 4. Our explanation for this finding is that the patient’s condition and follow-up period vary between different studies. A long-term prospective comparison of these different protocols is necessary.
The number of rituximab treatment cycles required to achieve disease remission is still unclear. In our cohort, six of eight patients received a single cycle of RTX, and they all achieved good clinical outcomes at the end of follow-up. A previous study reported long-term benefits in all six MuSK-MG patients after one cycle during a mean follow-up period of 31 months, and no reinfusions were needed again.20 However, some doctors thought it was necessary to repeat RTX infusions to prolong the B cell depletion.22 In our study, only two patients relapsed. This suggests that repeated RTX infusions may not be necessary in some patients due to the fact that it increases the cost of treatment and the risk of adverse reactions.
Our study confirmed that the daily dose of prednisone was reduced after RTX treatment, which is consistent with previous studies. Our patients only received steroids after RTX treatment. At the last visit, the doses of steroids were significantly decreased or completely tapered off in all patients, and these patients did not require additional immunosuppressive agents. Similar to a previous report, 27 patients with MG (13 anti-MuSK+, 10 anti-AChR+, and 4 double seronegative patients) were treated with RTX, many of whom stopped taking oral steroid-sparing agents upon the initiation of RTX therapy. The daily dose of prednisone in the anti-AChR+ group decreased from 19.6 mg to 10.0 mg, from 20.5 mg to 9.2 mg in the anti-MuSK+ group, and from 18.8 to 14 mg in the double seronegative group. During a median follow-up of 33 months, no patients were worse or had to take oral steroid-sparing agents again.31 This demonstrates that it is safe to stop all oral immunosuppressive agents except steroids when starting RTX treatment. In addition, the dose of steroids after RTX was significantly tapered, and other adjuvant therapy was rarely needed, which was extremely cost-effective for the patients.
The pathogenesis of MG and the mechanism by which RTX exerts this effect in the treatment of MG have not been fully elucidated. However, it is clear that autoactivated B lymphocytes play a key role in the pathophysiological process of this autoimmune disease.32 In our cohort, the levels of CD19+ and CD27+ B-cell counts decreased significantly after RTX treatment in all six patients in whom B-lymphocyte subgroup levels were detected. However, the counts of CD27+ B cells began to rise at 6 months in our study. Nevertheless, the CD19+ B-cell counts were still <1%. We observed that only one patient relapsed among three patients whose CD27+ B-cell counts were >0.05% at 6 months. The remaining two patients did not relapse with no changes in treatment plans. In our study, the return of B lymphocytes in patients may not be associated with the recurrence of clinical symptoms. Additionally, two patients had clinical relapses even though B cells were depleted. Similar results were observed in a previous study, where only 4 of 10 relapses were predicted by a CD19/CD20 recovery.33 These results suggest that the treatment of MG patients with RTX should not rely solely on laboratory indicators, and the clinical status of patients still needs to be closely monitored. The relationship between B-lymphocyte count and clinical disease status needs to be further explored.
Two assays, CBA and ELISA, were used in our study to detect MuSK antibody titres. A previous study showed that the overall concordance between BIOCHIP mosaic-based indirect immunofluorescence and ELISA for anti-MuSK reactivity was 84%.34 However, in our study, the same serum sample was not tested by both methods at the same time, and the number of samples was small, so we could not compare the sensitivities of the two methods. Therefore, more studies with larger numbers of samples are needed to compare the role of different detection methods for detecting antibodies in the diagnosis of MG. Recent studies have shown that the reduction of serum MuSK antibody titers is in good agreement with clinical improvement,35 but the differences in the testing methods of patients’ samples limited us to drawing this conclusion.
In our study, RTX was well-tolerated, and only one patient reported mild malaise. This rate is significantly lower than the adverse reaction rate of other studies,23 and this may be due to the use of relatively low RTX doses and anti-allergic drugs to prevent side effects in advance. The common adverse reactions related to RTX include infusion-related reactions, respiratory and urinary tract infections, unspecified mental disorders and chronic pain syndromes.23 Severe adverse events include haematologic derangements, hypogammaglobulinemia, posterior reversible encephalopathy syndrome (PRES), and progressive multifocal leukoencephalopathy (PML). It has been reported that two MG patients developed progressive multifocal leukoencephalopathy after receiving RTX treatment.36,37 High-dose RTX predisposes patients to opportunistic infections, thereby increasing the risk of myasthenic exacerbation.29,38 However, in our study, we did not observe any severe adverse reactions during the follow-up. Therefore, it appears to be safer to use low-dose RTX, especially in the context of pandemics. We must closely monitor the symptoms and changes in the patients’ conditions to prevent serious adverse reactions.
There are certain limitations in our study. First, the sample size of the participants was small because of the rarity of cases. Second, due to the retrospective design, the anti-MuSK antibody titers and the B-lymphocyte subsets were not available for every patient. Third, our study only evaluated the efficacy of RTX and did not compare it with other immunosuppressive agents. Finally, the dose of RTX was inconsistent among all of the patients, and we cannot determine the optimal regimen for MG based on this study alone.
Conclusion
In conclusion, our study showed that low-dose rituximab is effective and safe in MuSK-MG patients. However, a larger, multicenter and randomized controlled study is needed to assess the long-term safety and efficacy of low-dose RTX in the treatment of anti-MuSK myasthenia gravis.
Abbreviations
MG, Myasthenia gravis; RTX, rituximab; MuSK, muscle-specific tyrosine kinase; AChR, acetylcholine receptor; LRP4, low-density lipoprotein receptor-related protein 4; MGFA, Myasthenia Gravis Foundation of America; PIS, postinterventional status; MGSTI, Myasthenia Gravis Status and Treatment Intensity; CSR, complete stable remission; PR, pharmacological remission; MM, minimal manifestation; MMS, myasthenic muscle score; MG-ADL, MG-related activities of daily living.
Ethics Approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Biomedical Research Ethic Committee of Shandong Provincial Hospital and individual consent for this retrospective analysis was waived. This study was retrospective and did not interfere with the entire medical process of the patients. The medical records or biological specimens used in this study were obtained from past practices. For the included patients, nonidentifiable data were used, and no identifiable information was involved, so the informed consent of the included patients was exempted.
Privacy and Confidentiality Statement
The authors were committed to protecting the privacy and confidentiality of participants’ personal information. All data collected by the research project were anonymized or maintained with confidentiality.
Consent for Publication
Written informed consent for publication of their details was obtained from all the patients.
Acknowledgments
We would like to thank all of the patients who participated in this study.
Author Contributions
All authors made significant contributions to conception, study design, execution, data acquisition, analysis and interpretation. All authors drafted or substantially revised or critically reviewed the article. All authors agreed to submit to the journal, agreed on all versions of the article and are accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Phillips WD, Vincent A. Pathogenesis of myasthenia gravis: update on disease types, models, and mechanisms. F1000Res. 2016;5:1513. doi:10.12688/f1000research.8206.1
2. Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. 2001;7(3):365–368. doi:10.1038/85520
3. Pevzner A, Schoser B, Peters K, et al. Anti-LRP4 autoantibodies in AChR- and MuSK-antibody-negative myasthenia gravis. J Neurol. 2012;259(3):427–435. doi:10.1007/s00415-011-6194-7
4. Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren JJGM. Myasthenia gravis. Nat Rev Dis Primers. 2019;5(1):30. doi:10.1038/s41572-019-0079-y
5. Pasnoor M, Wolfe GI, Nations S, et al. Clinical findings in MuSK-antibody positive myasthenia gravis: a US experience. Muscle Nerve. 2010;41(3):370–374. doi:10.1002/mus.21533
6. Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. 2016;87(4):419–425. doi:10.1212/WNL.0000000000002790
7. Rodolico C, Bonanno C, Toscano A, Vita G. MuSK-associated myasthenia gravis: clinical features and management. Front Neurol. 2020;11:660. doi:10.3389/fneur.2020.00660
8. Evoli A, Alboini PE, Damato V, et al. Myasthenia gravis with antibodies to MuSK: an update. Ann N Y Acad Sci. 2018;1412(1):82–89. doi:10.1111/nyas.13518
9. Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14(10):1023–1036. doi:10.1016/S1474-4422(15)00145-3
10. Salles G, Barrett M, Foà R, et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017;34(10):2232–2273. doi:10.1007/s12325-017-0612-x
11. Plosker GL, Figgitt DP. Rituximab: a review of its use in non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia. Drugs. 2003;63(8):803–843. doi:10.2165/00003495-200363080-00005
12. Grace RF, Shimano KA, Bhat R, et al. Second-line treatments in children with immune thrombocytopenia: effect on platelet count and patient-centered outcomes. Am J Hematol. 2019;94(7):741–750. doi:10.1002/ajh.25479
13. Einarsson JT, Evert M, Geborek P, Saxne T, Lundgren M, Kapetanovic MC. Rituximab in clinical practice: dosage, drug adherence, Ig levels, infections, and drug antibodies. Clin Rheumatol. 2017;36(12):2743–2750. doi:10.1007/s10067-017-3848-6
14. Hui-Yuen JS, Nguyen SC, Askanase AD. Targeted B cell therapies in the treatment of adult and pediatric systemic lupus erythematosus. Lupus. 2016;25(10):1086–1096. doi:10.1177/0961203316652491
15. Collongues N, de Seze J. An update on the evidence for the efficacy and safety of rituximab in the management of neuromyelitis optica. Ther Adv Neurol Disord. 2016;9(3):180–188. doi:10.1177/1756285616632653
16. Ishiura H, Matsuda S, Higashihara M, et al. Response of anti-NMDA receptor encephalitis without tumor to immunotherapy including rituximab. Neurology. 2008;71(23):1921–1923. doi:10.1212/01.wnl.0000336648.43562.59
17. Robeson KR, Kumar A, Keung B, et al. Durability of the rituximab response in acetylcholine receptor autoantibody-positive myasthenia gravis. JAMA Neurol. 2017;74(1):60–66. doi:10.1001/jamaneurol.2016.4190
18. Iorio R, Damato V, Alboini PE, Evoli A. Efficacy and safety of rituximab for myasthenia gravis: a systematic review and meta-analysis. J Neurol. 2015;262(5):1115–1119. doi:10.1007/s00415-014-7532-3
19. Zhou Y, Yan C, Gu X, et al. Short-term effect of low-dose rituximab on myasthenia gravis with muscle-specific tyrosine kinase antibody. Muscle Nerve. 2021;63(6):824–830. doi:10.1002/mus.27233
20. Díaz-Manera J, Martínez-Hernández E, Querol L, et al. Long-lasting treatment effect of rituximab in MuSK myasthenia. Neurology. 2012;78(3):189–193. doi:10.1212/WNL.0b013e3182407982
21. Hehir MK, Hobson-Webb LD, Benatar M, et al. Rituximab as treatment for anti-MuSK myasthenia gravis: multicenter blinded prospective review. Neurology. 2017;89(10):1069–1077. doi:10.1212/WNL.0000000000004341
22. Keung B, Robeson KR, DiCapua DB, et al. Long-term benefit of rituximab in MuSK autoantibody myasthenia gravis patients. J Neurol Neurosurg Psychiatry. 2013;84(12):1407–1409. doi:10.1136/jnnp-2012-303664
23. Tandan R, Hehir MK
24. Di Stefano V, Lupica A, Rispoli MG, Di Muzio A, Brighina F, Rodolico C. Rituximab in AChR subtype of myasthenia gravis: systematic review. J Neurol Neurosurg Psychiatry. 2020;91(4):392–395. doi:10.1136/jnnp-2019-322606
25. Bastakoti S, Kunwar S, Poudel S, et al. Rituximab in the management of refractory myasthenia gravis and variability of its efficacy in anti-MuSK positive and anti-AChR positive myasthenia gravis. Cureus. 2021;13(11):e19416. doi:10.7759/cureus.19416
26. Fatehi F, Moradi K, Okhovat AA, et al. Zytux in refractory myasthenia gravis: a multicenter, open-labeled, Clinical Trial Study of effectiveness and safety of a rituximab biosimilar. Front Neurol. 2021;12:682622. doi:10.3389/fneur.2021.682622
27. Stieglbauer K, Pichler R, Topakian R. 10-year-outcomes after rituximab for myasthenia gravis: efficacy, safety, costs of inhospital care, and impact on childbearing potential. J Neurol Sci. 2017;375:241–244.
28. Takata K, Stathopoulos P, Cao M, et al. Characterization of pathogenic monoclonal autoantibodies derived from muscle-specific kinase myasthenia gravis patients. JCI Insight. 2019;4(12). doi:10.1172/jci.insight.127167
29. Landon-Cardinal O, Friedman D, Guiguet M, et al. Efficacy of rituximab in refractory generalized anti-AChR myasthenia gravis. J Neuromuscul Dis. 2018;5(2):241–249. doi:10.3233/JND-180300
30. Cortés-Vicente E, Rojas-Garcia R, Díaz-Manera J. The impact of rituximab infusion protocol on the long-term outcome in anti-MuSK myasthenia gravis. Ann Clin Transl Neurol. 2018;5(6):710–716. doi:10.1002/acn3.564
31. Roda RH, Doherty L, Corse AM. Stopping oral steroid-sparing agents at initiation of rituximab in myasthenia gravis. Neuromuscul Disord. 2019;29(7):554–561. doi:10.1016/j.nmd.2019.06.002
32. Stathopoulos P, Kumar A, Heiden JAV, Pascual-Goñi E, Nowak RJ, O’Connor KC. Mechanisms underlying B cell immune dysregulation and autoantibody production in MuSK myasthenia gravis. Ann N Y Acad Sci. 2018;1412(1):154–165. doi:10.1111/nyas.13535
33. Beecher G, Anderson D, Siddiqi ZA. Rituximab in refractory myasthenia gravis: extended prospective study results. Muscle Nerve. 2018;58(3):452–455. doi:10.1002/mus.26156
34. Gambino CM, Agnello L, Lo Sasso B, et al. Comparative analysis of BIOCHIP mosaic-based indirect immunofluorescence with enzyme-linked immunosorbent assay for diagnosing myasthenia gravis. Diagnostics. 2021;11(11):2098. doi:10.3390/diagnostics11112098
35. Zhao S, Zhang K, Ren K, et al. Clinical features, treatment and prognosis of MuSK antibody-associated myasthenia gravis in Northwest China: a single-centre retrospective cohort study. BMC Neurol. 2021;21(1):428. doi:10.1186/s12883-021-02439-7
36. Afanasiev V, Demeret S, Bolgert F, Eymard B, Laforêt P, Benveniste O. Resistant myasthenia gravis and rituximab: a monocentric retrospective study of 28 patients. Neuromuscul Disord. 2017;27(3):251–258. doi:10.1016/j.nmd.2016.12.004
37. Kanth KM, Solorzano GE, Goldman MD. PML in a patient with myasthenia gravis treated with multiple immunosuppressing agents. Neurol Clin Pract. 2016;6(2):e17–e19. doi:10.1212/CPJ.0000000000000202
38. Dos Santos A, Noury JB, Genestet S, et al. Efficacy and safety of rituximab in myasthenia gravis: a French multicentre real-life study. Eur j Neurol. 2020;27(11):2277–2285. doi:10.1111/ene.14391
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.