Back to Journals » Neuropsychiatric Disease and Treatment » Volume 13
Efficacy and safety of Ginkgo biloba standardized extract in the treatment of vascular cognitive impairment: a randomized, double-blind, placebo-controlled clinical trial
Authors Demarin V, Bašić Kes V, Trkanjec Z, Budišić M, Bošnjak Pašić M, Črnac P, Budinčević H
Received 26 August 2016
Accepted for publication 13 December 2016
Published 16 February 2017 Volume 2017:13 Pages 483—490
DOI https://doi.org/10.2147/NDT.S120790
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Roger Pinder
Vida Demarin,1,2 Vanja Bašić Kes,1 Zlatko Trkanjec,1 Mislav Budišić,1 Marija Bošnjak Pašić,3,4 Petra Črnac,5 Hrvoje Budinčević4,5
1Department of Neurology, University Hospital Center “Sestre Milosrdnice”, 2International Institute for Brain Health, 3Department of Neurology, University Hospital Center Zagreb, Zagreb, 4Department of Neurology, School of Medicine, University Josip Juraj Strossmayer, Osijek, 5Department of Neurology, Stroke and Intensive Care Unit, University Hospital “Sveti Duh”, Zagreb, Croatia
Objectives: The aim of this randomized, double-blind, placebo-controlled trial was to determine the efficacy and safety of Ginkgo biloba extract in patients diagnosed with vascular cognitive impairment (VCI).
Methods: A total of 90 patients (aged 67.1±8.0 years; 59 women) were randomly allocated (1:1:1) to receive G. biloba 120 mg, G. biloba 60 mg, or placebo during a 6-month period. Assessment was made for efficacy indicators, including neuropsychological tests scores (Sandoz Clinical Assessment Geriatric Scale, Folstein Mini-Mental State Examination, Mattis Dementia Rating Scale, and Clinical Global Impression) and transcranial Doppler ultrasound findings. Safety indicators included laboratory findings, reported adverse reactions, and clinical examination.
Results: At the end of 6-month study period, G. biloba 120 and 60 mg showed a statistically significant positive effect in comparison with placebo only on the Clinical Global Impression score (2.6±0.8 vs 3.1±0.7 vs 2.8±0.7, respectively; P=0.038). The Clinical Global Impression score showed a significant deterioration from the baseline values in the placebo group (-0.3±0.5; P=0.021) as opposed to G. biloba groups. No significant differences were found in the transcranial Doppler ultrasound findings. Adverse reactions were significantly more common and serious in the placebo group (16 subjects) than in either of the two G. biloba extract groups (eight and nine subjects, respectively), whereas laboratory findings and clinical examinations revealed no differences between the groups receiving G. biloba extract and placebo.
Conclusion: According to our results, G. biloba seemed to slow down the cognitive deterioration in patients with VCI, but the effect was shown in only one of the four neuropsychological tests administered. However, because of this mild effect in combination with a few adverse reactions, we cannot say that it is ineffective or unsafe either. Further studies are still needed to provide unambiguous evidence on the efficacy and safety of G. biloba extract.
Keywords: Ginkgo biloba, vascular cognitive impairment, dementia
Introduction
Ginkgo biloba leaf extracts have been used in medicine for centuries. The active ingredients responsible for its beneficial effect are flavonol glycosides and terpenoids.1 G. biloba is suggested for use in Alzheimer’s dementia, vascular cognitive impairment (VCI), vertigo, and tinnitus.2–8 In symptomatic treatment of Alzheimer’s dementia, G. biloba extract shows effect over a limited period of time in some patients and has fewer adverse reactions than donepezil, galantamine, or rivastigmine.2 Clinical studies with G. biloba (EGb 761) in patients with dementia indicate that it stabilizes or slows down the decline in mental function, particularly in patients with neuropsychiatric symptoms.9–12 Cieza et al13 showed a favorable effect of G. biloba on mental health and quality of life in healthy volunteers aged >50 years.
Mechanisms of the action of G. biloba are complex, and its pharmacological effects include antioxidant activity as a free radical-scavenger, capillary fragility reduction,14,15 antagonization of the platelet activating factor,16 and modification of energy metabolism, particularly during hypoxia.17 Moreover, G. biloba enhances cerebral and vestibular blood flow and protects neurons from oxidative damage.18 A recent preclinical study showed the effect of G. biloba extract on the structure of the cornu ammonis in aged rats, thus providing a neuroanatomical basis for memory improvement.19
VCI is a broad spectrum of syndromes ranging from mild cognitive impairment to dementia, which are influenced by risk factors for cerebrovascular disease.20 Cerebrovascular aging can lead to the loss of the integrity of the blood–brain barrier, eventually resulting in cognitive and sensorimotor decline. Two major types of cognitive dysfunction due to chronic cerebral hypoperfusion are VCI and dementia.21 Also, the term VCI has sometimes been used in literature to denote a mild cognitive impairment due to cerebrovascular insufficiently severe to be diagnosed as dementia.22
The aim of our study was to determine the efficacy and safety of G. biloba in comparison with placebo in patients with VCI.
Patients and methods
Study design
This was a 6-month, randomized, double-blind, placebo-controlled, parallel-group clinical trial conducted in a single center in Zagreb, Croatia. The study protocol was approved by the Local Ethics Committee of Sestre Milosrdnice University Hospital, and the study was registered with ClinicalTrials.gov (NCT00446485) before the start of patient enrollment.
Patients
The study included 90 patients presenting with VCI symptoms at the Outpatient Clinic of the Department of Neurology of “Sestre Milosrdnice” University Hospital from May 2007 to April 2010. The inclusion criteria were as follows: age ≥50 years; fulfilled VCI criteria based on cerebrovascular insufficiency and Folstein Mini-Mental State Examination (MMSE) score of >20 and ≤28; completed initial screening; for women, postmenopause or use of contraception; and signed written informed consent was received from all participants before participation.
The exclusion criteria included symptoms caused by psychiatric, metabolic, endocrine, nutritional, or heart disease; epilepsy, liver or kidney failure, uncontrolled diabetes mellitus, or hypertension; malignancy; history of myocardial infarction in the previous 6 months; alcohol or drug abuse; use of the following drugs: ticlopidine, clopidogrel, pentoxifylline, acetylsalicylic acid, dihydroergotamine, warfarin, and/or etibiskumacetate; and a positive pregnancy test in women.
The subjects were withdrawn from the study if they developed newly diagnosed psychiatric disease, acute myocardial infarction, resistant hypertension or diabetes mellitus, liver or kidney failure, alcohol or drug abuse, or serious adverse reaction during the course of the study. They could also be withdrawn at their own request or at the investigator’s or sponsor’s request (eg, noncompliance, lost to follow-up, consent withdrawal, and so on).
Screening
At the beginning of the study, the subjects underwent an initial screening assessment. The physician recorded patient demographic data, the presence or absence of inclusion/exclusion criteria, blood pressure, heart rate, respiratory rate, body temperature, weight and height, smoking habit, physical examination findings and concomitant diseases, symptoms and signs, and concomitant medications. The subjects also underwent a brain computed tomography scan, routine laboratory testing (erythrocyte sedimentation rate [ESR], complete blood cell count [CBC], blood glucose, urea, creatinine, transaminases, bilirubin, sodium, potassium, chlorides, high-density lipoproteins [HDL], low-density lipoproteins [LDL] triglycerides, activated partial thromboplastin time [APTT]), and prothrombin time, electrocardiogram (ECG), carotid and vertebral arteries ultrasound (CAVA), transcranial duplex ultrasound (TCD), and a neuropsychological test battery consisting of Sandoz Clinical Assessment Geriatric Scale (SCAG), MMSE, Mattis Dementia Rating Scale (MDRS), and Clinical Global Impression Scale (CGI).
Intervention
The study drug was a G. biloba extract in the form of a tablet containing 60 mg of dry extract of G. biloba leaf (G. biloba L, folium), that is, a standardized G. biloba extract EGb 761 that contains 24% flavonol glycosides and 6% terpens.
The subjects were randomly assigned to receive either two G. biloba tablets daily (a total of 120 mg of G. biloba extract), one placebo tablet and one G. biloba tablet daily (a total of 60 mg of G. biloba extract), or two placebo tablets daily for 6 months. The placebo tablet was identical to the active tablet in size, color, and shape (white and round tablet). The odor and taste of tablets were masked. Patients were instructed to swallow the tablets whole, without breaking them, after a meal every day.
Randomization, blinding, and study protocol
The block randomization was used to randomize subjects into three groups with equal sample sizes. Each box carried a unique drug number (code) that was generated and assigned to the active drug and placebo. The randomization codes were stored in a locked compartment at the sponsors’ office. The sealed envelopes were also given to the principal investigator and were inaccessible to other staff involved in the trial. The block length was not disclosed to the investigators or clinical staff. This procedure ensured both double-blinding and concealment of allocation sequence. The principal investigator was instructed to open the sealed envelope only in case of a serious adverse event. In that case, the principal investigator was required to complete a special case report form. The envelopes were returned to the sponsor at the end of the study.
The subjects were invited to attend a total of seven study visits in 30-day intervals during the 6-month study period. Laboratory testing, TCD, and neuropsychological tests (MDRS, CGI, SCAG, and MMSE) were repeated every 90 days (visits 1, 4, and 7). ECG and CAVA were performed at the first and final visits, that is, 180 days apart. The duration of the treatment period was 180 days.
The primary outcome measure was a change in MDRS, CGI, SCAG, and MMSE scores from the baseline values. The secondary outcome measures were changes in the results of the laboratory tests, TCD, and CAVA from the baseline values. Reported adverse reactions, physical and neurological examination findings, blood pressure, pulse, respiration rate, body temperature, and changes in concomitant therapy were recorded at each study visit, visits 2–7. Adverse reactions were reported to the study clinical monitor and study steering committee. Treatment adherence was checked for all subjects by inquiry and by counting the returned tablets on visits 3, 5, and 7.
Statistical analysis
Data for categorical variables were presented as frequencies and proportion (%), and data for continuous variables were expressed as mean and standard deviation (mean ± SD). The comparison between the groups that finished the study per protocol and the ones that discontinued the study prematurely was done using either chi-square test or Fisher’s exact test for categorical variables and Student’s t-test or Mann–Whitney U test (depending on the type of distribution) for continuous variables. The difference between the treatment groups and placebo group in the primary and secondary outcome measures was evaluated using a repeated-measures analysis of variance (ANOVA) and, for categorical variables, using Friedman ANOVA. For multiple comparisons, Bonferroni correction was used. The treatment success rate was evaluated as a favorable therapeutic outcome defined as a global improvement on a 0–7 scale and efficiency index on a 1–16 scale. The incidences of adverse reactions, concomitant diseases, and medications were shown as frequencies. Data were analyzed using the software package Statistica for Windows, version 6.0 (StatSoft Inc., Tulsa, OK, USA). All tests were two-sided with P<0.05 considered as statistically significant.
Results
Subject characteristics
The study was completed according to the protocol by 58 of a total of 90 subjects included in the study (Figure 1).
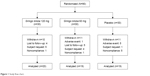  | Figure 1 Study flow chart. |
A total of 32 patients discontinued the study before the end of the treatment period, with equal representation among groups (P=0.953). Adverse reactions as a reason for withdrawal were most frequent in the placebo group (P=0.009). Nine patients were lost to follow-up. Ten patients withdrew their consent. Seven subjects were excluded from the study because of inadequate compliance (P=0.015 for all reasons among groups).
There was no statistically significant difference for any baseline parameter in subjects who discontinued the study regarding their random distribution across groups and basic demographic parameters (P>0.05 for all). A statistically significant difference between the subjects who completed and those who discontinued the study was found only in the red blood cell count (P=0.032), platelet count (P=0.049), TCD of the left anterior cerebral artery (P=0.003), and TCD of the basilar artery (P=0.04) (Table 1).
Efficacy evaluation
No statistically significant difference between the G. biloba and placebo groups was found in MDRS, SCAG, and MMSE scores (Table 2). However, there was a statistically significant difference between the G. biloba and placebo groups in CGI scores (P=0.038).
The differences between the G. biloba and placebo groups in TCD measurements were not statistically significant for any of the left- or right-side arteries (P>0.10 for all). The baseline CAVA measurements showed a wide range of different values in the carotid arteries, ranging from normal (unremarkable) findings to bilateral carotid artery stenosis. The statistical significance of the observed internal carotid artery differences was not evaluated because of a wide range of recorded differences and no further categorization predicted.
Satisfactory compliance regarding the medication use (>80% of the dose taken) was determined in all subjects who completed the study in all the three groups at every visit.
Safety evaluation
At the end of the study, no statistically significant difference was found between the G. biloba and placebo groups in the secondary outcome indicators (ESR, CBC, glucose, urea, creatinine, transaminases, bilirubin, sodium, potassium, chlorides, HDL, LDL, triglycerides, APTT, and PV; P>0.05 for all). No statistically significant difference was found between the G. biloba and placebo groups in physical and neurological findings (P>0.05 for all). Also, there was no statistically significant difference between the G. biloba and placebo groups in compliance and premature withdrawal from the study. As for concomitant therapy, medications for cardiovascular conditions, neurological conditions, and nonsteroidal anti-inflammatory drugs (except acetylsalicylic acid) were most frequently used among the subjects.
Adverse reactions
A total of 33 adverse reactions was reported, 12 of which were serious (Table 3). The greatest number of adverse reactions was reported in the placebo group (nine serious), followed by the G. biloba 60 mg group (three serious) and the G. biloba 120 mg group (no serious adverse reactions; χ2=7,342; df=2; P=0.026). The most commonly reported adverse reaction was nausea, followed by vomiting and vertigo. These adverse reactions were most commonly reported in the placebo group. Six subjects terminated the study prematurely because of adverse reactions. For one subject in the placebo group, the randomization code had to be revealed because of a serious adverse reaction.
  | Table 3 ARs reported by subjects taking Ginkgo biloba and placebo during the 6-month study period |
Discussion
Our study results for G. biloba efficacy in the treatment of patients with VCI showed a statistically significant positive effect of G. biloba extract only on the CGI as the primary outcome indicator.
Several studies about the effect of G. biloba on cognition have been published, majority reporting a positive effect.23 However, a randomized placebo-controlled trial by Vellas et al24 showed that the long-term use of standardized G. biloba extract did not reduce the risk of progression to Alzheimer’s disease compared with placebo. Two recently conducted large randomized trials, the GEM (Ginkgo Evaluation of Memory) study and the GuidAge study, showed no benefit of G. biloba for age-related cognitive decline.25,26 However, a meta-analysis by Weinmann et al27 showed G. biloba to be more effective than placebo in the treatment of dementia, although with a moderate effect. Meta-analyses by Tan et al11 and Wang et al28 suggest that G. biloba is able to slow down or stabilize cognitive decline in patients with dementia. A recent study by Amieva et al29 showed that G. biloba EGb 761 significantly slowed down the cognitive decline in comparison to piracetam. The possible stabilizing effect on cognitive decline and clinical presentation measured with CGI was also shown in our study.
Unfortunately, we did not find any improvement in cognitive decline measured by MMSE, SCAG, and MDRS or blood flow velocity increase in the cerebral arteries. Recently, Zhang and Xue4 showed that G. biloba with any platelet therapy increases blood flow velocities in the middle and anterior cerebral arteries in VCI patients with cognitive ability improvement measured by the Montreal Cognitive Assessment after 3 months.
Lower cognitive decline was reported in nondemented elderly subjects who used G. biloba extract.29 Some studies showed the improvement of cognitive and neuropsychiatric symptoms and functional ability in dementia.10,30 The results reported by Brondino et al31 suggest improved cognitive function and activities of daily living in patients with dementia treated with G. biloba. A systematic review and meta-analysis by Yang et al32 found that G. biloba is potentially beneficial for the improvement of cognitive function, activities of daily living, and global clinical assessment in patients with mild cognitive impairment or Alzheimer’s disease. However, Mazza et al33 reported no evidence of relevant differences in the efficacy of G. biloba and donepezil in the treatment of mild-to-moderate Alzheimer’s dementia. Kruntoradova et al34 results suggest that EGb 761® represents a cost-saving intervention with more quality-adjusted life years/life years gained, that is, dominant therapy compared to no pharmacotherapy in the treatment of mild dementia in a 10-year horizon, as EGb 761 showed very similar results (slightly cheaper and less effective) in comparison to iAchE (eg, donepezil). Still, due to limited sample sizes, and inconsistent findings and methodological quality of included trials, more research is warranted to confirm the effectiveness and safety of G. biloba in the treatment of mild cognitive impairment and Alzheimer’s disease.32
Despite no statistically significant differences for safety outcomes regarding laboratory and clinical findings in our study, adverse reactions were significantly more common and serious in the placebo group. G. biloba seemed to be safe, with no serious adverse reactions compared to placebo. G. biloba extract is widely used in the treatment of acute ischemic stroke in the People’s Republic of China.35 A Cochrane Collaboration systematic review showed that high-quality, large-scale randomized trials are necessary to confirm the beneficial effect of G. biloba on recovery after acute stroke.36 Recent studies on G. biloba extract have proven G. biloba to be beneficial and safe in the treatment of acute cerebral infarction, improving the outcome and showing neuroprotective effect.37,38 However, previous investigations on G. biloba in prophylactic therapy for ischemic stroke did not show that daily intake was able to prevent stroke or other cardiovascular or cerebrovascular events.12,39–41 Although G. biloba has not been found effective in dementia prevention, clinical evidence for the use of G. biloba to slow its progression is promising and warrants further clinical investigation.24
Similar to other clinical trials, our study had several limitations. The CGI rating scale to measure symptoms severity, treatment response, and efficacy of treatment is considered to be somewhat subjective, and the follow-up period was relatively short, with a high dropout rate. One more limitation was that the patients were enrolled at only one study center.
Conclusion
According to our results, G. biloba seemed to slow down the cognitive deterioration in patients with VCI, but the effect was shown in only one of the four neuropsychologic tests administered. However, because of this mild effect in combination with a few adverse reactions, we cannot say that it is ineffective or unsafe either. Further studies are still needed to provide unambiguous evidence on the efficacy and safety of G. biloba extract.
Acknowledgments
The study was sponsored by Milsing d.o.o. The Ginkgo biloba standardized extract and placebo were donated by a company Milsing d.o.o. Linguistic help with the preparation of the manuscript was provided by Aleksandra Misak, MD.
Author contributions
All authors were involved in either the design of the study or data analysis and interpretation. All authors contributed to the drafting of the manuscript or critical revision of the manuscript for important intellectual content.
Disclosure
The authors report no conflicts of interest in this work.
References
Ude C, Schubert-Zsilavecz M, Wurglics M. Ginkgo biloba extracts: a review of the pharmacokinetics of the active ingredients. Clin Pharmacokinet. 2013;52(9):727–749. | ||
Ihl R, Frolich L, Winblad B, Schneider L, Burns A, Moller HJ; WFSBP Task Force on Treatment Guidelines for Alzheimer’s Disease and other Dementias. World federation of societies of biological psychiatry (WFSBP) guidelines for the biological treatment of Alzheimer’s disease and other dementias. World J Biol Psychiatry. 2011;12(1):2–32. | ||
Yancheva S, Ihl R, Nikolova G, Panayotov P, Schlaefke S, Hoerr R; GINDON Study Group. Ginkgo biloba extract EGb 761®, donepezil or both combined in the treatment of Alzheimer’s disease with neuropsychiatric features: a randomised, double-blind, exploratory trial. Aging Ment Health. 2009;13(2):183–190. | ||
Zhang SJ, Xue ZY. Effect of Western medicine therapy assisted by Ginkgo biloba tablet on vascular cognitive impairment of none dementia. Asian Pac J Trop Med. 2012;5(8):661–664. | ||
Orendorz-Fraczkowska K, Pospiech L, Gawron W. [Results of combined treatment for vestibular receptor impairment with physical therapy and Ginkgo biloba extract (Egb 761)]. Otolaryngol Pol. 2002;56(1):83–88. Polish. | ||
Sokolova L, Hoerr R, Mishchenko T. Treatment of vertigo: a randomized, double-blind trial comparing efficacy and safety of Ginkgo biloba extract EGb 761 and betahistine. Int J Otolaryngol. 2014;2014:682439. | ||
Morgenstern C, Biermann E. The efficacy of Ginkgo special extract EGb 761 in patients with tinnitus. Int J Clin Pharmacol Ther. 2002;40(5):188–197. | ||
von Boetticher A. Ginkgo biloba extract in the treatment of tinnitus: a systematic review. Neuropsychiatr Dis Treat. 2011;7:441–447. | ||
Gavrilova SI, Preuss UW, Wong JW, Hoerr R, Kaschel R, Bachinskaya N; GIMCIPlus Study Group. Efficacy and safety of Ginkgo biloba extract EGb 761 in mild cognitive impairment with neuropsychiatric symptoms: a randomized, placebo-controlled, double-blind, multi-center trial. Int J Geriatr Psychiatry. 2014;29(10):1087–1095. | ||
Ihl R, Tribanek M, Bachinskaya N; GOTADAY Study Group. Efficacy and tolerability of a once daily formulation of Ginkgo biloba extract EGb 761® in Alzheimer’s disease and vascular dementia: results from a randomised controlled trial. Pharmacopsychiatry. 2012;45(2):41–46. | ||
Tan MS, Yu JT, Tan CC, et al. Efficacy and adverse effects of Ginkgo biloba for cognitive impairment and dementia: a systematic review and meta-analysis. J Alzheimers Dis. 2015;43(2):589–603. | ||
Nash KM, Shah ZA. Current perspectives on the beneficial role of Ginkgo biloba in neurological and cerebrovascular disorders. Integr Med Insights. 2015;10:1–9. | ||
Cieza A, Maier P, Poppel E. Effects of Ginkgo biloba on mental functioning in healthy volunteers. Arch Med Res. 2003;34(5):373–381. | ||
Pincemail J, Dupuis M, Nasr C, et al. Superoxide anion scavenging effect and superoxide dismutase activity of Ginkgo biloba extract. Experientia. 1989;45(8):708–712. | ||
Clostre F. [Ginkgo biloba extract (EGb 761). State of knowledge in the dawn of the year 2000]. Ann Pharm Fr. 1999;57(Suppl 1):1S8–1S88. French. | ||
Chung KF, Dent G, McCusker M, Guinot P, Page CP, Barnes PJ. Effect of a ginkgolide mixture (BN 52063) in antagonising skin and platelet responses to platelet activating factor in man. Lancet. 1987;1(8527):248–251. | ||
Hofferberth B. [The effect of Ginkgo biloba extract on neurophysiological and psychometric measurement results in patients with psychotic organic brain syndrome. A double-blind study against placebo]. Arzneimittelforschung. 1989;39(8):918–922. German. | ||
Abdel-Wahab BA, Abd El-Aziz SM. Ginkgo biloba protects against intermittent hypoxia-induced memory deficits and hippocampal DNA damage in rats. Phytomedicine. 2012;19(5):444–450. | ||
Hosseini-Sharifabad M, Anvari M. Effects of Ginkgo biloba extract on the structure of Cornu Ammonis in aged rat: a morphometric study. Iran J Basic Med Sci. 2015;18(9):932–937. | ||
Battistin L, Cagnin A. Vascular cognitive disorder. A biological and clinical overview. Neurochem Res. 2010;35(12):1933–1938. | ||
Yang T, Sun Y, Lu Z, Leak RK, Zhang F. The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res Rev. 2017;34:15–29. | ||
Sachdev P, Kalaria R, O’Brien J, et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord. 2014;28(3):206–218. | ||
Kleijnen J, Knipschild P. Ginkgo biloba for cerebral insufficiency. Br J Clin Pharmacol. 1992;34(4):352–358. | ||
Vellas B, Coley N, Ousset PJ, et al; GuidAge Study Group. Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): a randomised placebo-controlled trial. Lancet Neurol. 2012;11(10):851–859. | ||
Snitz BE, O’Meara ES, Carlson MC, et al; Ginkgo Evaluation of Memory (GEM) Study Investigators. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA. 2009;302(24):2663–2670. | ||
Schneider LS. Ginkgo and AD: key negatives and lessons from GuidAge. Lancet Neurol. 2012;11(10):836–837. | ||
Weinmann S, Roll S, Schwarzbach C, Vauth C, Willich SN. Effects of Ginkgo biloba in dementia: systematic review and meta-analysis. BMC Geriatr. 2010;10:14. | ||
Wang BS, Wang H, Song YY, et al. Effectiveness of standardized Ginkgo biloba extract on cognitive symptoms of dementia with a six-month treatment: a bivariate random effect meta-analysis. Pharmacopsychiatry. 2010;43(3):86–91. | ||
Amieva H, Meillon C, Helmer C, Barberger-Gateau P, Dartigues JF. Ginkgo biloba extract and long-term cognitive decline: a 20-year follow-up population-based study. PLoS One. 2013;8(1):e52755. | ||
Ihl R. Effects of Ginkgo biloba extract EGb 761®in dementia with neuropsychiatric features: review of recently completed randomised, controlled trials. Int J Psychiatry Clin Pract. 2013;17(Suppl 1):8–14. | ||
Brondino N, De Silvestri A, Re S, et al. A systematic review and meta-analysis of Ginkgo biloba in neuropsychiatric disorders: from ancient tradition to modern-day medicine. Evid Based Complement Alternat Med. 2013;2013:915691. | ||
Yang G, Wang Y, Sun J, Zhang K, Liu J. Ginkgo biloba for mild cognitive impairment and alzheimer’s disease a systematic review and meta-analysis of randomized controlled trials. Curr Top Med Chem. 2016;16(5):520–528. | ||
Mazza M, Capuano A, Bria P, Mazza S. Ginkgo biloba and donepezil: a comparison in the treatment of Alzheimer’s dementia in a randomized placebo-controlled double-blind study. Eur J Neurol. 2006;13(9):981–985. | ||
Kruntoradova K, Mandelikova M, Mlcoch T, Dolezal T. Cost-effectiveness analysis of Ginkgo biloba extract (Egb761® – Tanakan®) for the treatment of dementia in the Czech Republic. Value Health. 2015;18(7):A756. | ||
Zeng X, Liu M, Yang Y, Li Y, Asplund K. Ginkgo biloba for acute ischaemic stroke. Cochrane Database Syst Rev. 2005;(4):CD003691. | ||
Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev. 2009;(1):CD003120. | ||
Oskouei DS, Rikhtegar R, Hashemilar M, et al. The effect of Ginkgo biloba on functional outcome of patients with acute ischemic stroke: a double-blind, placebo-controlled, randomized clinical trial. J Stroke Cerebrovasc Dis. 2013;22(8):e557–e563. | ||
Wang L, Zhang T, Bai K. [System evaluation on Ginkgo biloba extract in the treatment of acute cerebral infarction]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2015;40(10):1096–1102. Chinese. | ||
Gardner CD, Zehnder JL, Rigby AJ, Nicholus JR, Farquhar JW. Effect of Ginkgo biloba (EGb 761) and aspirin on platelet aggregation and platelet function analysis among older adults at risk of cardiovascular disease: a randomized clinical trial. Blood Coagul Fibrinolysis. 2007;18(8):787–793. | ||
Hong JM, Shin DH, Lim YA, Lee JS, Joo IS. Ticlopidine with Ginkgo biloba extract: a feasible combination for patients with acute cerebral ischemia. Thromb Res. 2013;131(4):e147–e153. | ||
Kuller LH, Ives DG, Fitzpatrick AL, et al. Does Ginkgo biloba reduce the risk of cardiovascular events? Circ Cardiovasc Qual Outcomes. 2010;3(1):41–47. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


