Back to Journals » Neuropsychiatric Disease and Treatment » Volume 11
Efficacy and safety of generic escitalopram (Lexacure®) in patients with major depressive disorder: a 6-week multicenter, randomized, rater-blinded, escitalopram-comparative, non-inferiority study
Authors Jeong J, Bahk W, Woo YS, Lee K, Kim DH, Kim M, Kim W, Yang J, Lee KH
Received 19 June 2015
Accepted for publication 8 September 2015
Published 7 October 2015 Volume 2015:11 Pages 2557—2564
DOI https://doi.org/10.2147/NDT.S90796
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Wai Kwong Tang
Jong-Hyun Jeong,1 Won-Myong Bahk,1 Young Sup Woo,1 Kyung-Uk Lee,1 Do Hoon Kim,2 Moon-Doo Kim,3 Won Kim,4 Jong-Chul Yang,5 Kwang Heun Lee6
1Department of Psychiatry, Yeouido St Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, 2Department of Psychiatry, Chuncheon Sacred Heart Hospital, Hallym University, Chuncheon, 3Department of Psychiatry, Jeju National University Hospital, Jeju, 4Department of Psychiatry, Stress Research Institute, Seoul Paik Hospital, College of Medicine, Inje University, Seoul, 5Department of Psychiatry, Chonbuk National University Medical School, Jeonju, 6Department of Psychiatry, College of Medicine, Dongguk University, Gyeongju, South Korea
Objectives: The primary aim of this non-inferiority study was to investigate the clinical effectiveness and safety of generic escitalopram (Lexacure®) versus branded escitalopram (Lexapro®) for patients with major depressive disorder (MDD).
Methods: The present study included 158 patients, who were randomized (1:1) to receive a flexible dose of generic escitalopram (n=78) or branded escitalopram (n=80) over a 6-week single-blind treatment period. The clinical benefits in the two groups were evaluated using the Montgomery–Åsberg Depression Rating Scale (MADRS), the 17-item Hamilton Depression Rating Scale (HDRS), the Clinical Global Impressions-Severity scale (CGI-S), and the Clinical Global Impressions-Improvement scale (CGI-I) at baseline, week 1, week 2, week 4, and week 6. The frequency of adverse events (AEs) was also assessed to determine safety at each follow-up visit.
Results: During the 6-week study period, 30 patients (38.5%) from the generic escitalopram group and 28 patients (30.0%) from the branded escitalopram group dropped out of the study (P=0.727). The MADRS, HDRS, CGI-S, and CGI-I scores significantly decreased in both groups, and there were no significant differences between the groups. At week 6, 28 patients (57.1%) in the generic escitalopram group and 35 patients (67.3%) in the branded escitalopram group had responded to treatment (as indicated by a ≥50% decrease from the baseline MADRS score; P=0.126), and the remission rates (MADRS score: ≤10) were 42.9% (n=21) in generic escitalopram group and 53.8% (n=28) in the branded escitalopram group (P=0.135). The most frequently reported AEs were nausea (17.9%), sleepiness/somnolence (7.7%), weight gain (3.8%), and dry mouth (2.6%) in the generic escitalopram group and nausea (20.0%), sleepiness/somnolence (3.8%), weight gain (2.5%), and dry mouth (2.5%) in the branded escitalopram group.
Conclusion: The present non-inferiority study demonstrated that generic escitalopram is a safe and an effective initial treatment for patients with MDD and may also be considered as an additional therapeutic option for this population.
Keywords: depression, branded escitalopram, Lexapro®, generic escitalopram, Lexacure®
Introduction
The lifetime prevalence of major depressive disorder (MDD) has been reported to range from 4.4% to 30%.1 However, The National Comorbidity Study in the USA found that lifetime prevalence rates of MDD were 15%–17%; these results are widely considered to be an accurate estimate of the incidence of this disorder.2,3 MDD is a disabling disorder that is responsible for the greatest nonfatal burden of disease worldwide, as it accounts for approximately 12% of years lost to disability.4 However, patients with MDD do not respond well to the treatment, and they frequently progress to recurrent episodes of mood disorders, which make treatment difficult. Thus, the treatment of MDD represents a challenge in terms of reducing individual suffering as well as lowering the social costs associated with the disorder.5
Selective serotonin reuptake inhibitors (SSRIs) are a representative first-line treatment option for MDD in many countries. Although there is a controversy regarding the efficacy and early symptom improvement associated with SSRIs, these drugs exhibit favorable efficacies and tolerabilities.6 Escitalopram is a (S)-stereoisomer (S-enantiomer) of racemate citalopram (R-citalopram), which is the most selective SSRI, but the majority of antidepressive action exhibited by citalopram is thought to be due to escitalopram. The inhibition of serotonin reuptake by the S-enantiomer is reported to be 150 times greater than that by R-citalopram.7,8 Escitalopram is widely used for the treatment of depressive disorders, and its treatment efficacies and safety profiles have been evaluated by many studies and clinical trials.9–12 After the expiration of the patent for escitalopram, a number of generic forms of the drug were released.
In Korea, Lexacure® (Dong-A ST Pharma, Ltd., Seoul, South Korea) is a widely used generic formulation of escitalopram approved by the Korean Food and Drug Administration in 2008; it has good bioequivalence relative to escitalopram.13 A majority of generic drugs are released following the performance of bioequivalence tests demonstrating similar pharmacokinetics to the original drug. Accordingly, it is expected that the effects of the generic drug will be analogous to those of the original drug. However, relatively few clinical trials have been conducted to determine whether generic drugs show the same clinical efficacy and safety profile as the original drugs. Therefore, the present study was conducted to evaluate the therapeutic efficacy and safety of generic escitalopram (Lexacure®) in patients with MDD.
Methods
Study design
The present study was a multicentered, randomized, rater-blinded, and prospective, a 6-week investigation that included 158 patients who were diagnosed with MDD according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition-Text Revision (DSM-IV-TR).14 Of these, 78 patients were assigned to the generic escitalopram group, and 80 patients to the branded escitalopram group. This study was conducted at ten university hospital centers in Korea and included inpatients and outpatients who were ≥20 years of age, who had a Montgomery–Åsberg Depression Rating Scale (MADRS)15 score ≥20 at screening, and who voluntarily participated without receiving any monetary incentive. The exclusion criteria were as follows: pregnant or lactating women, medical conditions that could interfere with the activities of daily living, the presence of psychotic features, a current primary DSM-IV-TR axis I diagnosis other than MDD, and/or those who were at serious risk of suicide. Eligible patients were randomized to one of two treatment arms (at a 1:1 ratio) on the screening day using a computer-generated randomization list. The treatments were administered in a single-blinded fashion such that only the personnel who performed the MADRS rating evaluations were blind to the study treatment of each participant.
The doses of generic escitalopram and branded escitalopram were adjusted based on clinical considerations as determined by site investigators; however, it was recommended that ongoing nonpsychiatric treatments were not being modified. The participants were not allowed to take other antidepressants, mood stabilizers, antipsychotics, buspirone, psychostimulants, or anticonvulsants during the study. Benzodiazepines (<4 mg/day of lorazepam or <2 mg/day of alprazolam) and hypnotics were allowed. The total study period was 6 weeks, and each patient was examined at baseline, week 1, week 2, week 4, and week 6.
Ethics
The present study was conducted according to the Declaration of Helsinki and the guidelines for good clinical practice. Written informed consent was obtained from all subjects following an extensive explanation of the nature and procedures of the study. The study protocol was approved by the institutional review board or ethics committees at each study site.
Efficacy, safety, and compliance assessments
Efficacy was evaluated using the MADRS, the 17-item Hamilton Depression Rating Scale (HDRS),16 the Clinical Global Impressions-Severity scale (CGI-S),17 and the Clinical Global Impressions-Improvement scale (CGI-I).17 The primary efficacy outcome was the mean change in MADRS score from baseline to week 6, and additional efficacy outcome measures included the mean changes in the HDRS and CGI-S scores, and the response and remission rates at week 6. Response to treatment was defined as a ≥50% decrease in the MADRS or HDRS score, and the remission was defined as an absolute MADRS score ≤10, an HDRS score ≤7, or a CGI-I score ≤2. Tolerability and safety were determined based on the frequency and severity of adverse events (AEs). To evaluate therapeutic compliance, the subjective levels of compliance were determined at 1, 2, 4, and 6 week after the initiation of drug administration. Subjective therapeutic compliance was assessed by a question such as “How did you take the prescribed medication?” The patients were divided by the compliance rate as follows: ≥70%, 30%–69%, and <30%.
Statistical analyses
Efficacy and safety were analyzed using intention-to-treat analysis, and the last-observation-carried-forward method was applied for the endpoint analysis. All patients who received at least one dose of the study medication were included in the safety analysis.
The data are presented as mean ± standard deviation (SD) for quantitative variables and frequencies (percentage) for categorical variables. The quantitative data were analyzed with Student’s t-test, and the categorical data were analyzed with either chi-squared or Fisher’s exact test. A repeated measures analysis of variance adjusted for time was used to determine changes in each group, and a repeated measures analysis of a covariance with baseline score as a covariate was used to assess differences between the two groups. All analyses were performed using SAS 9.2 (SAS Institute, Inc., Cary, NC, USA) and a one-tailed P-value <0.05 was considered to indicate statistical significance.
Results
Patients and medication
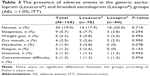
The baseline demographic and clinical characteristics of the 158 patients included in the present study are provided in Table 1. The mean dose of generic escitalopram was 11.4±3.5 mg/day at baseline, 13.4±4.8 mg/day at week 1, 14.5±4.9 mg/day at week 2, 16.2±4.8 mg/day at week 4, and 16.6±4.7 mg/day at week 6. The mean dose of branded escitalopram was 11.3±3.3 mg/day at baseline, 12.9±4.6 mg/day at week 1, 13.6±4.8 mg/day at week 2, 15.3±5.0 mg/day at week 4, and 15.4±4.9 mg/day at week 6.
There were no significant differences between the generic escitalopram and branded escitalopram groups in terms of age, sex, marital state, inpatient rate, illness duration, comorbid medical illness, mean dose of escitalopram during the entire study period, concomitant psychiatric medications at baseline, or baseline MADRS, HDRS, and CGI scores. The total dropout rate was 36.7% (n=58), and the dropout rate of the generic escitalopram group (38.5%, n=30) did not significantly differ from that of the branded escitalopram group (35.0%, n=28; P=0.727). The intention-to-treat analyses initially included 158 patients, and at the end of the trial, 101 patients (63.9%) remained enrolled in the study.
Efficacy
In the generic escitalopram group, the mean MADRS score at baseline was 30.7±5.9, and this significantly decreased to 24.2±8.2, 19.4±8.4, 15.8±8.8, and 13.5±8.8 at week 1, 2, 4, and 6, respectively (P<0.001). In the branded escitalopram group, the mean MADRS score at baseline was 30.8±4.9, and this significantly decreased to 23.4±8.0, 18.1±6.6, 14.0±6.9, and 11.3±7.3 at week 1, 2, 4, and 6, respectively (P<0.001; Table 2; Figure 1). In the generic escitalopram group, the mean HDRS score at baseline was 24.4±5.6, and this significantly decreased to 19.0±7.0, 15.0±7.2, 12.6±6.6, and 10.6±6.5 at week 1, 2, 4, and 6, respectively (P<0.001). In the branded escitalopram group, the mean HDRS score at baseline was 24.2±5.1, and this significantly decreased to 18.4±6.5, 14.7±5.8, 11.1±5.7, and 9.1±5.9 at week 1, 2, 4, and 6, respectively (P<0.001; Figure 1).
In the generic escitalopram group, the mean CGI-S score at baseline was 5.0±0.8, and it significantly decreased to 4.2±0.7, 3.7±1.1, 3.2±1.0, and 2.8±1.1 at week 1, 2, 4, and 6, respectively. In the branded escitalopram group, the mean CGI-S score at baseline was 5.0±0.7, and it significantly decreased to 4.1±0.9, 3.6±0.9, 3.0±0.9, and 2.5±0.9 at week 1, 2, 4, and 6, respectively (P<0.001; Figure 1). In the generic escitalopram group, the mean CGI-I score was 3.1±1.0, 2.6±1.3, 2.2±1.0, and 2.1±1.0 at week 1, 2, 4, and 6, respectively (P<0.001). In the branded escitalopram group, the mean CGI-I score was 2.9±1.0, 2.4±1.0, 2.0±0.9, and 1.8±0.8 at week 1, 2, 4, and 6, respectively (P<0.001). There were no significant differences in mean MADRS, HDRS, CGI-S, or CGI-I scores between the two groups (Table 2; Figure 1).
Based on the MADRS score at week 6 (≥50% decrease from baseline scores), the percentages of patients who showed a response to treatment were 57.1% (n=28) in the generic and 67.3% (n=35) in the branded escitalopram groups (P=0.126). Based on the HDRS score at week 6, the response rates (≥50% decrease from baseline scores) were 57.1% (n=28) in the generic and 67.3% (n=35) in the branded escitalopram groups. There were no significant differences between the two groups (P=0.146; Figure 2). Based on the MADRS score at week 6 (≤10), the remission rates were 42.9% (n=21) in the generic and 53.8% (n=28) in the branded escitalopram groups (P=0.135). Based on the HDRS and CGI-S scores (≤7 and ≤2, respectively), the remission rates were 34.7% (n=17) and 38.8% (n=19; P=0.060) in the generic escitalopram group, respectively, and 50.0% (n=26) and 48.1% (n=25; P=0.173) in the branded escitalopram group, respectively. There were no significant differences in remission rates between the two groups based on the MADRS, HDRS, and CGI-S scores (Figure 2).
Adverse events and safety
Both generic and branded escitalopram were well tolerated by the patients with MDD throughout the duration of the 6-week study period. However, 58 (36.7%) participants dropped out during the study: 29 (18.4%) were lost to follow-up, eleven (19.0%) withdrew their consent due to a change of mind during the first week of the study, nine (9.7%) had low compliance, four (2.5%) withdrew from the study due to AEs (primarily nausea), four (2.5%) violated the protocol, and one (0.6%) withdrew for personal reasons unrelated to the study. The dropout rates between the generic (38.5%, n=30) and branded escitalopram (35.0%, n=28) groups did not significantly differ (P=0.727).
A total of 64 AEs (33 in the generic escitalopram group and 31 in the branded escitalopram group) were reported by 47 patients (21 patients [29.7%] in the generic and 26 patients [32.5%] in the branded escitalopram groups; P=0.357) during the 6-week duration of the study. Nausea was the most frequently reported AE (19%, n=30), followed by sleepiness/daytime somnolence (5.7%, n=9), weight gain (3.2%, n=5), dry mouth (2.5%, n=4), and headache (1.9%, n=3; Table 3).
At week 1, 2, 4, and 6, compliance rates ≥70% were 85.7%, 89.8%, 87.8%, and 89.8%, respectively, in generic escitalopram group. And in the branded escitalopram group, compliance rates ≥70% were 78.8%, 90.4%, 90.4%, and 92.3%, respectively, at week 1, 2, 4, and 6. There were no significant differences between two groups (P=0.892).
Discussion
The present findings indicate that results for generic escitalopram were similar to those for branded escitalopram in the treatment of patients with MDD. Six-week treatment with generic escitalopram was associated with significant changes in the MADRS, HDRS, CGI-S, and CGI-I scores (Table 2; Figures 1 and 2), and the antidepressive effects of the generic drug were similar to those of the branded drug. These results support the findings of previous investigations of escitalopram.5,9–12
In the present study, the response and remission rates did not significantly differ between the generic escitalopram and branded escitalopram groups. According to the MADRS scores, the response rates were 57.1% in the generic escitalopram and 67.3% in the branded escitalopram group, and the remission rates were 42.9% and 53.8%, respectively. Although the response and remission rates of the generic escitalopram group were lower than those of the branded escitalopram group, these differences were not statistically significant. The therapeutic efficacy and safety of escitalopram are well known, and it is used worldwide.5,9–12 A meta-analysis of ten randomized double-blind clinical trials observed a beneficial effect of escitalopram versus placebo or an active comparator in patients with MDD, with reported response and remission rates of 58.1% and 53.8%, respectively.18 These results support the findings of the present study and indicate that escitalopram is effective for the treatment of MDD.
Furthermore, the safety profile of the generic escitalopram group was similar to that of the branded escitalopram group in the present study. Common AEs in both groups included nausea, sleepiness/somnolence, weight gain, dry mouth, headache, fatigue, insomnia, and difficulty in concentrating, which are similar to the AEs reported in the previous trials of escitalopram.5,9–12 Although a previous multicenter, double-blinded, randomized, controlled study did not find enough evidence to approve the clinical efficacy or safety profile of generic escitalopram, the authors of that study reported that this drug had similar clinical efficacy and safety profile to those of branded escitalopram in patients with MDD.19 However, because of the various formulations of generic escitalopram produced by different manufacturers typically exhibit a variety of therapeutic efficacies and safety profiles, the findings regarding one specific type of generic escitalopram should not be regarded as representative of all its formulations.
In 2003, the United States Medicaid Program spent over US$2.3 billion across three categories of antidepressant drugs including SSRIs, tricyclic antidepressants, and others. Many countries have their own set of cost-containment policies with respect to antidepressants and other drugs; these include preferred drug lists, prior authorization policies, copay systems, and drug utilization reviews.20 However, many cost-containment policies have still not been proven effective. Of course, many of these cost-containment strategies substitute a generic SSRI for a branded SSRI for the treatment of patients with MDD.
Recent studies have suggested that important differences exist in the safety, efficacy, and cost-effectiveness profiles of different SSRIs.21,22 Thus, patients who respond to and are stable during treatment with one drug may lose their responsiveness and even suffer unnecessary adverse reactions if they are switched to another drug.23,24 Under these circumstances, it is likely that the benefits of switching a patient’s current pharmacotherapy for a nonmedical reason to save drug costs may be offset by the increase in total expenditures that would stem from additional hospitalizations and/or emergency department visits and the need for other medical services. Compared with non-switchers, switchers have higher risks for all-cause mental health-related and MDD-related risk for hospitalizations and/or emergency department visits (odds ratio [OR]: 1.15, 1.34, and 1.54, respectively; all P<0.01) as well as higher risk-adjusted mental health-related and MDD-related medical costs (US$219 and US$222, respectively; both P<0.05).25
Nonetheless, some have argued that generic SSRIs and serotonin/norepinephrine reuptake inhibitors do not appear to be associated with a higher probability of therapy interruption or significant reductions in health costs.26 In addition, a large-scale study27 using the administrative databases of five local health care units (347,073 patients) reported that the clinical outcomes of the study (hospitalizations, mortality, and other health costs) did not significantly differ between generic medicine users and brand medicine users. These findings suggest that generic medicines represent a viable therapy option.
The comparative dissolution tests of generic escitalopram (Lexacure®) conducted in the present study revealed that the dissolution of the drug at 2 hours was 96% at a pH of 1.2, 97.7% at a pH of 4.0, and 90.8% at a pH of 6.8. In addition, a bioequivalence study comparing the generic and branded forms of escitalopram found that the mean total area under the curve and maximum concentration (Cmax) values of generic escitalopram and branded escitalopram were similar.13 Thus, generic escitalopram (Lexacure®) has a similar bioequivalence to branded escitalopram (Lexapro®), and the generic form of escitalopram is likely to have a similar mechanism of action to branded escitalopram.
Because the total economic burden of MDD, including personal productivity and social costs, is increasing, it is necessary for clinicians and governments to actively manage the treatment of patients with MDD. Not all patients are compelled to use generic medicines, but the availability of generic options is required in some situations. Therefore, well-made and thoroughly studied forms of generic escitalopram could be a viable additional therapeutic option for the treatment of patients with MDD.
Limitations
The present findings should be interpreted with caution due to several important limitations.
First, the sample size and statistical powers were not enough in the study. Non-inferiority study usually requires a larger sample size and higher powers than a comparator study, and this may have affected the results. Second, the analysis of the difference between groups on the MADRS demonstrated 1.8 point difference in favor of branded compared with generic escitalopram at both week 4 and 6. This might be suggested that the branded escitalopram would have been able to demonstrate an advantage in large and long-term study; however, further researches are needed. Third, the methodological limitations of the present study include the use of a rater-blinded design; this might have allowed a bias by the patients in favor of the branded product that would have influenced the results. In addition, without the use of a placebo, it is impossible to separate the true effects of escitalopram from any placebo effects. However, the clinical efficacy and safety profile of escitalopram are well known at this point, and there was no ethical rationale for the use of a placebo in the present study. Moreover, the flexible adjustment of the escitalopram dose and the concomitant use of benzodiazepines and hypnotics were permitted, making the control of variables contributing to the improvement of MDD symptoms difficult. Finally, the present study specifically investigated one generic form of escitalopram (Lexacure®), and as a result, the present findings cannot be generalized to other generic forms of the drug because every formulation exhibits different therapeutic efficacies and safety profiles.
Acknowledgment
This study was supported and funded by Dong-A ST Pharma.
Disclosure
The authors report no conflicts of interest in this work.
References
Kendler KS, Kessler RC, Neale MC, Heath AC, Eaves LJ. The prediction of major depression in women: toward an integrated etiologic model. Am J Psychiatry. 1993;150(8):1139–1148. | ||
Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095–3105. | ||
American Psychiatric Association. The American Psychiatric Publishing Textbook of Psychiatry. 6th ed. Arlington, VA: American Psychiatric Publishing; 2014. | ||
Ustun TB, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJ. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 2004;184:386–392. | ||
Colonna L, Andersen HF, Reines EH. A randomized, double-blind, 24-week study of escitalopram (10 mg/day) versus citalopram (20 mg/day) in primary care patients with major depressive disorder. Curr Med Res Opin. 2005;21(10):1659–1668. | ||
Norman TR, Olver JS. New formulations of existing antidepressants: advantages in the management of depression. CNS Drugs. 2004;18(8):505–520. | ||
Hyttel J, Bogeso KP, Perregaard J, Sanchez C. The pharmacological effect of citalopram residues in the (S)-(+)-enantiomer. J Neural Transm Gen Sect. 1992;88(2):157–160. | ||
Sanchez C, Bergqvist PB, Brennum LT, et al. Escitalopram, the S-(+)-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with potent effects in animal models predictive of antidepressant and anxiolytic activities. Psychopharmacology. 2003;167(4):353–362. | ||
Burke WJ, Gergel I, Bose A. Fixed-dose trial of the single isomer SSRI escitalopram in depressed outpatients. J Clin Psychiatry. 2002;63(4):331–336. | ||
Wade A, Michael Lemming O, Bang Hedegaard K. Escitalopram 10 mg/day is effective and well tolerated in a placebo-controlled study in depression in primary care. Int Clin Psychopharmacol. 2002;17(3):95–102. | ||
Lepola UM, Loft H, Reines EH. Escitalopram (10–20 mg/day) is effective and well tolerated in a placebo-controlled study in depression in primary care. Int Clin Psychopharmacol. 2003;18(4):211–217. | ||
Moore N, Verdoux H, Fantino B. Prospective, multicentre, randomized, double-blind study of the efficacy of escitalopram versus citalopram in outpatient treatment of major depressive disorder. Int Clin Psychopharmacol. 2005;20(3):131–137. | ||
Biosuntek. Bioequivalent Study of Escitalopram Oxalate 12.77 mg (Lexacure 10 mg). Seoul: Biosuntek; 2008. Korean. | ||
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2010. | ||
Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. | ||
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. | ||
Guy W. Clinical global impression scale. In: ECDEU Assessment of Manual for Psychopharmacology–Revised. Rockville, MD: National Institute of Mental Health; 1976:218–222. | ||
Kennedy SH, Andersen HF, Lam RW. Efficacy of escitalopram in the treatment of major depressive disorder compared with conventional selective serotonin reuptake inhibitors and venlafaxine XR: a meta-analysis. J Psychiatry Neurosci. 2006;31(2):122–131. | ||
Yu Y, Li H, Wang B, et al. Efficacy and safety of generic escitalopram versus Lexapro in the treatment of major depression: a multicenter double-blinded randomized controlled trial. Shanghai Arch Psychiatry. 2013;25(2):107–115. | ||
Kelton CM, Rebelein RP, Heaton PC, Ferrand Y, Guo JJ. Differences in the cost of antidepressants across state Medicaid programs. J Mental Health Policy Econ. 2008;11(1):33–47. | ||
Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746–758. | ||
Sullivan PW, Valuck R, Saseen J, MacFall HM. A comparison of the direct costs and cost effectiveness of serotonin reuptake inhibitors and associated adverse drug reactions. CNS Drugs. 2004;18(13):911–932. | ||
Hodgkin D, Horgan CM, Garnick DW, Len Merrick E, Volpe-Vartanian J. Management of access to branded psychotropic medications in private health plans. Clin Ther. 2007;29(2):371–380. | ||
Panzer PE, Regan TS, Chiao E, Sarnes MW. Implications of an SSRI generic step therapy pharmacy benefit design: an economic model in anxiety disorders. Am J Manag Care. 2005;11(12 Suppl):S370–S379. | ||
Wu EQ, Yu AP, Lauzon V, et al. Economic impact of therapeutic substitution of a brand selective serotonin reuptake inhibitor with an alternative generic selective serotonin reuptake inhibitor in patients with major depressive disorder. Ann Pharmacother. 2011;45(4):441–451. | ||
Vlahiotis A, Devine ST, Eichholz J, Kautzner A. Discontinuation rates and health care costs in adult patients starting generic versus brand SSRI or SNRI antidepressants in commercial health plans. J Manag Care Pharm. 2011;17(2):123–132. | ||
Colombo GL, Agabiti-Rosei E, Margonato A, Mencacci C, Montecucco CM, Trevisan R. Off-patent generic medicines vs. off-patent brand medicines for six reference drugs: a retrospective claims data study from five local healthcare units in the Lombardy Region of Italy. PloS One. 2013;8(12):e82990. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.