Back to Journals » Clinical Ophthalmology » Volume 9
Efficacy and safety of fixed-combination travoprost 0.004%/timolol 0.5% in patients transitioning from bimatoprost 0.03%/timolol 0.5% combination therapy
Authors Schnober D, Hubatsch DA, Scherzer M
Received 14 January 2015
Accepted for publication 26 February 2015
Published 7 May 2015 Volume 2015:9 Pages 825—832
DOI https://doi.org/10.2147/OPTH.S80880
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Dietmar Schnober,1 Douglas A Hubatsch,2 Maria-Luise Scherzer3
1Private Ophthalmology Practice, Werdohl, Germany; 2Alcon Laboratories, Inc., Fort Worth, TX, USA; 3Private Ophthalmology Practice, Regenstauf, Germany
Purpose: To determine the efficacy and safety of fixed-combination travoprost 0.004%/timolol 0.5% preserved with polyquaternium-1 in patients with insufficient response to bimatoprost 0.03%/timolol 0.5% preserved with benzalkonium chloride.
Patients and methods: In this open-label nonrandomized study conducted at 13 European sites, patients with primary open-angle glaucoma or ocular hypertension with insufficient intraocular pressure (IOP) reduction during bimatoprost/timolol therapy were transitioned to travoprost/timolol (DuoTrav®) administered every evening for 12 weeks. Change in IOP from baseline to week 12 was assessed in patients who transitioned from fixed-combination bimatoprost/timolol (n=57, primary endpoint). Secondary assessments included change in IOP at week 4, percentage of patients with IOP ≤18 mmHg at weeks 4 and 12, change in Ocular Surface Disease Index and ocular hyperemia scores at week 12, and patient preference. Adverse events were also reported.
Results: IOP change (mean ± SD) from baseline to week 12 was –3.8±1.9 mmHg (P<0.001); results were similar at week 4. Most patients had IOP ≤18 mmHg at weeks 4 and 12 (78.6% and 85.5%, respectively). Mean Ocular Surface Disease Index score was significantly reduced (P<0.001); no significant change in ocular hyperemia score was observed (P=0.197). Treatment-related adverse events included dysgeusia, nausea, paresthesia, myalgia, headache, and eye irritation (n=1 each). Most patients (74.5%) preferred travoprost/timolol over bimatoprost/timolol.
Conclusion: Transition to travoprost/timolol significantly reduced IOP and was well tolerated in patients who had elevated IOP despite bimatoprost/timolol therapy. Polyquaternium-1–preserved travoprost/timolol was preferred over prior treatment with benzalkonium chloride–preserved bimatoprost/timolol.
Keywords: β-blocker, glaucoma, intraocular pressure, preservative, prostaglandin analog
Introduction
Glaucoma, a chronic degenerative disease that may lead to visual impairment,1 includes a number of eye disorders, such as normal-tension glaucoma, pigmentary glaucoma, and primary open-angle glaucoma (OAG).2 Estimates of the prevalence of glaucoma in 2010 suggested a worldwide OAG prevalence of 1.96%, with the highest number of people with OAG occurring in Europe.3
Elevated intraocular pressure (IOP) is a modifiable causative factor in the development of visual impairment due to OAG.4 Commonly prescribed topical agents have been shown to be effective in lowering IOP and the associated risk for visual impairment.1,5 Agents of choice for monotherapy include prostaglandin analogs (eg, bimatoprost, travoprost), β-blockers (eg, timolol), carbonic anhydrase inhibitors (eg, brinzolamide), cholinomimetics (eg, carbachol), and α-agonists (eg, epinephrine).2,6 However, failure to achieve sufficient lowering of IOP with a given monotherapy regimen may necessitate switching treatments or adopting combination therapy, depending on the patient’s preference, the risk for local ocular adverse events (AEs), and other considerations.1,6 All prostaglandin analogs are known to effectively lower IOP,7 but individuals may respond differently to different prostaglandin analogs; a patient who does not respond to bimatoprost may respond to travoprost and vice versa.
Fixed-combination therapy allows for potentially greater bioavailability of both drugs compared with separate administration; improves safety by limiting overall daily exposure to preservatives; and perhaps most importantly, may increase adherence and thus persistence with therapy by providing more convenient administration of the combination regimen.8–10 Several studies have demonstrated the IOP-lowering efficacy of the prostaglandin analog travoprost 0.004% in fixed combination with the nonselective β-blocker timolol 0.5% and benzalkonium chloride (BAK or BAC), a preservative commonly used in ophthalmic solutions.11–14 However, long-term use of BAK-containing treatments has been shown to cause tear film instability and a shift toward more severe Ocular Surface Disease Index (OSDI) scores in patients with OAG.15,16 Therefore, topical ophthalmic solutions that are preserved with BAK alternatives and that maintain ocular surface health and improve IOP may provide a clinically beneficial option in patients with OAG.
Travoprost 0.004% and timolol 0.5% fixed-dose combination (TTFC) preserved with polyquaternium-1 (PolyQuad®; Alcon Laboratories, Inc., Fort Worth, TX, USA) is a topical ophthalmic solution that is approved in the European Union for the treatment of elevated IOP in adults with OAG or ocular hypertension (OHT) who are not sufficiently responsive to topical β-blockers or prostaglandin analogs.17 Travoprost (a prostaglandin F2α analog) reduces IOP by increasing the outflow of aqueous humor, whereas timolol (a nonselective β-blocker) reduces the production of aqueous humor in the ciliary body. A previous study demonstrated the equivalent efficacy of TTFC with or without BAK in patients with OAG or OHT.18 The objective of this open-label study was to evaluate the efficacy and tolerability of polyquad-preserved TTFC in patients with OAG or OHT who had insufficient lowering of IOP on fixed- or unfixed-combination therapy with BAK-preserved bimatoprost 0.03% and timolol 0.5%.
Patients and methods
Study design
This was a 12-week, multicenter, open-label, single-group, historical-control study conducted at 13 sites in Europe to evaluate the efficacy and safety of transition to TTFC preserved with polyquad in patients with OAG or OHT who had insufficient IOP lowering with BAK-preserved bimatoprost 0.03% and timolol 0.5% combination therapy. The protocol and study procedures were approved by an institutional review board at each study center and conducted according to principles of the Declaration of Helsinki and Good Clinical Practice. All study participants provided written informed consent before the initiation of study procedures. This study was registered on ClinicalTrials.gov as NCT01327599 on March 30, 2011.
Patient population
Inclusion criteria
Patients were recruited during routine glaucoma consultations or through solicitation if they were identified as potentially eligible based on investigator records. Patients of both sexes ≥18 years of age with a diagnosis of OHT or OAG (including pigment-dispersion OAG) in ≥1 eye were eligible for enrollment. Eligible patients must have had a best-corrected visual acuity (BCVA) of 6/60 (20/200) Snellen (equivalent to 1.0 logMAR) and received a stable IOP-lowering regimen consisting of bimatoprost 0.03% and timolol 0.5% administered concomitantly or in a fixed combination (BTFC) within 4 weeks of the screening visit. To ensure clinical stability of vision and the optic nerve throughout the study, the IOP must have been considered safe in both eyes in the opinion of the investigator. Inclusion criteria also included an IOP of 19–35 mmHg at any time of day in ≥1 eye (designated as the study eye); IOP in the non-study eye had to be controlled during the study without pharmacologic therapy or with study medication alone. Eligible patients had to discontinue use of all other ocular hypotensive medications for the entire course of the study.
Exclusion criteria
Patients were excluded if they had a history of allergy, hypersensitivity, or poor tolerance to any component of TTFC that was deemed clinically significant in the opinion of the investigator. Exclusion criteria also included any abnormality that prevented reliable applanation tonometry in either eye; corneal dystrophies; concurrent infectious or noninfectious conjunctivitis, keratitis, or uveitis in either eye; history of ocular herpes simplex or allergic rhinitis; intraocular conventional or laser surgery in either eye within 3 months of screening; risk of visual field or visual acuity worsening as a consequence of participation in the study (based on the investigator’s best judgment); progressive retinal or optic nerve disease from any cause apart from glaucoma; use of systemic medications known to affect IOP (eg, oral β-antagonists, α-adrenergic receptor modulators, angiotensin-converting enzyme [ACE] inhibitors, and calcium channel blockers) on a nonstable course within 7 days of screening or an expected change in dosage during the study; a history or risk for uveitis or cystoid macular edema; an unwillingness to risk the possibility of a darkened iris or eyelash changes; the presence of a condition that, in the opinion of the investigator, would have interfered with optimal participation in the study; or participation in any other investigational study within 30 days of the screening visit.
Intervention
Eligible patients were instructed to self-administer one drop of polyquad-preserved TTFC into the study eye(s) at 8.00 pm once daily for 12 weeks. Patients wearing contact lenses were instructed to remove them before administering study medication and to wait at least 15 minutes before reinserting the lenses. There was no washout period between the patient’s prior ocular hypotensive regimen and the start of the TTFC regimen. Patients were permitted to use systemic medications known to affect IOP (eg, oral β-antagonists, α-agonists and antagonists, ACE inhibitors, and calcium channel blockers) if their use was kept constant during the study.
Assessments
Efficacy
Clinical evaluation of IOP was performed at all study visits (ie, baseline and weeks 4 and 12).
The primary efficacy outcome was the mean change in IOP from baseline to week 12 in patients who received prior BTFC. Secondary efficacy assessments included the mean change in IOP from baseline to week 4, percentage change from baseline to week 4 and week 12, the percentage of patients who achieved target IOP (≤18 mmHg) at weeks 4 and 12, the change in OSDI from baseline to week 12 based on a Likert-type scale (0= none of the time, 1= some of the time, 2= half of the time, 3= most of the time, and 4= all of the time) that was used to calculate the overall OSDI score (0–12= normal, 13–22= mild, 23–32= moderate, and 33–100= severe), and the change in ocular hyperemia from baseline to week 12 assessed on a 4-point scale (0= none/trace, 1= mild, 2= moderate, and 3= severe). Exploratory efficacy endpoints were the change in Ocular Discomfort Scale (ODS) from baseline to week 12 and patient-reported global treatment preference and adherence questionnaire responses at week 12. Possible ODS responses ranged from 0 to 9, where 0= no discomfort and 9= significant discomfort. The possible patient-reported preference responses were either prefer study medication or prefer prior medication. Adherence questionnaire responses were based on medication preference.
Safety
Safety was assessed in all patients who received study medication. All patient-reported and investigator-assessed AEs occurring during the study, regardless of whether the event was related to the treatment, were rated as mild, moderate, or severe. Additional safety assessments included reports of serious AEs; medical history; BCVA using a Snellen visual acuity chart; slit-lamp examination of the eyelids, conjunctiva, cornea, iris, anterior chamber, and lens in both eyes at weeks 4 and 12; and ocular signs.
Statistical analysis
Demographic data and baseline characteristics were summarized in the intent-to-treat (ITT) population (ie, all patients who received study medication and had ≥1 on-therapy study visit) using descriptive statistics. Efficacy analyses were performed in the ITT subpopulation of patients who transitioned from BTFC. Per protocol, efficacy was to be evaluated in patients transitioning from unfixed bimatoprost + timolol if 45 patients were evaluable. Because only two patients met this criterion, efficacy analyses in this subpopulation were not performed, and the data presented herein represent the BTFC population unless otherwise stated. The primary efficacy endpoint was summarized using descriptive statistics and analyzed using a paired t-test. The secondary endpoint, IOP change from baseline, was summarized using descriptive statistics and analyzed using a repeated measures analysis of variance. All continuous variables and ordinal variables expressed on a numeric scale were described using a continuous variable summary calculated for each measurement time point and for changes from baseline at each postbaseline time point. The change from baseline to week 12 in the ODS was analyzed using the Wilcoxon signed rank test and patient global preference and adherence using an exact binomial test. The incidence of AEs was summarized using descriptive statistics and analyzed using the McNemar test.
For the primacy efficacy endpoint, the planned sample size of 50 patients ensured that ≥45 patients would be enrolled in the ITT population, assuming a 10% dropout rate. This sample size had 80% power to detect a difference of ≥1 mmHg between TTFC and the previous fixed-combination BTFC regimen, based on the assumption of an SD of 3 mmHg and a correlation coefficient of 0.7 for the IOP measured before and after the change to TTFC. SAS version 9.1.3 or higher (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses.
Results
Patient disposition and characteristics
A total of 60 patients were enrolled in the study, and 57 completed all three visits; one patient withdrew consent and was excluded from the ITT population (n=59) (Figure 1). Two patients in the ITT population had received prior unfixed bimatoprost + timolol therapy and were excluded from the fixed-combination population. The first patient was enrolled on August 8, 2011, and the last patient completed the study on November 19, 2012. The safety population consisted of 60 patients who received ≥1 dose of study medication.
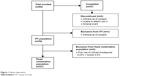  | Figure 1 Patient disposition. |
Patients in the ITT population had a mean age ± SD of 73.2±9.8 years and were mostly women (64.4%; Table 1). The majority of patients (74.6%, n=44) had a history of ocular medical and surgical events, the most frequent of which was cataracts (45 events) that occurred in 30 (50.8%) patients. Nearly two-thirds (64.4%, n=38) of the patients had a history of systemic hypertension, and approximately one-third (32.2%, n=19) had a history of metabolism and nutrition disorders, including diabetes mellitus (n=13), hypercholesterolemia (n=3), and lipid metabolism disorders (n=5); 36 of 38 women enrolled in the study were postmenopausal. Classes of concomitant medications used on a stable course for at least 7 days before the first visit included selective β-blocking agents (16 treatments) used by 16 (27.1%) patients, angiotensin II antagonists (10 treatments) used by nine (15.3%) patients, and ACE inhibitors (8 treatments) used by eight (13.6%) patients.
Efficacy
Primary endpoint
At week 12, mean IOP ± SD in the fixed-combination population (n=57) was 16.3±1.9 mmHg compared with 20.0±1.0 at baseline (Figure 2), indicating a significant reduction (mean ± SD change from baseline, −3.8±1.9 mmHg; P<0.001).
Secondary endpoints
Mean IOP ± SD was significantly reduced from baseline (20.1±1.1 mmHg) to week 4 (16.3±2.2 mmHg; mean ± SD change from baseline, −3.8±2.1; P<0.001) in patients in the fixed-combination population (Figure 2). Percentage changes from baseline to week 4 and week 12 were −18.8±10.5 and −18.9±9.1, respectively. In the fixed-combination group, 78.6% of patients had an IOP of ≤18 mmHg at week 4 and 85.5% of patients achieved an IOP of ≤18 mmHg at week 12. Results for the secondary endpoint OSDI score are summarized in Table 2. Compared with baseline, the OSDI score was significantly reduced (P<0.001) at week 12. Moreover, the percentage of patients who scored normal on the OSDI severity scale was numerically greater at week 12 (58.2% vs 44.1%) compared with baseline owing to reduced percentages of patients who scored mild, moderate, or severe on this scale (Figure 3). Also at week 12, there was a small increase in the percentage of patients who scored none/trace on the ocular hyperemia scale (74.5% vs 67.8%) that did not result in a significant change in the mean ocular hyperemia score (mean change ± SD, 0.1±0.7 units; P=0.197) compared with baseline.
Exploratory endpoints
Patients exhibited a significantly reduced (P=0.005) mean ODS score at week 12 compared with baseline (Table 2). The majority of patients (74.5%) indicated a preference for TTFC over BTFC at week 12, and the majority (64.8%) were very confident that they would adhere to the treatment regimen if prescribed their preferred medication.
Safety
Patients in the full ITT population were exposed to study medication for a mean (SD) duration of 84.8 (11.4) days, with a minimum duration of exposure of 10 days and a maximum of 113 days. Overall, six of 60 patients (10.0%) who received ≥1 dose of study medication experienced 14 AEs, the majority (57.1%) of which were unrelated to study medication. The most commonly reported ocular AEs are summarized in Table 3; one patient discontinued because of moderate eye irritation related to study medication. All but one ocular AE (OHT) resolved before the end of the study. Nonocular AEs were reported by six of 60 (10.0%) patients. Five nonocular AEs reported by two patients were considered to be treatment related, including mild dysgeusia, moderate nausea, paresthesia, myalgia, and severe headache; all but dysgeusia resolved before the end of the study.
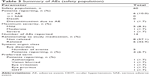  | Table 3 Summary of AEs (safety population) |
No significant changes from baseline to week 12 in BCVA (median, 6/9 and 6/8, respectively) or slit-lamp parameters were observed. No deaths or serious AEs were reported.
Discussion
This study demonstrated that polyquad-preserved TTFC administered once daily in the evening produced a significant reduction in IOP in patients with OAG and OHT who were transitioning from BAK-preserved BTFC because of insufficient lowering of IOP. Treatment with TTFC was well tolerated, and there were no reported deaths or serious AEs.
One of the main goals of treatment in patients with OAG or OHT is a reduction in IOP to a stable target pressure as a means of reducing the risk of further visual impairment.19 Hence, guidelines from the European Glaucoma Society recommend a target of at least 20% reduction from baseline IOP in patients with OAG.19 However, not all patients achieve this target IOP with prostaglandin analog and timolol combination therapy, and some may require discontinuation because of AEs.20,21 In such cases, patients may benefit from transition to another prostaglandin analog and timolol combination therapy.21–23 In the current study, patients whose IOP was uncontrolled with and patients who were unable to tolerate a BAK-preserved bimatoprost-based combination regimen showed a reduction in IOP from 20.0 to 16.3 mmHg (18.8% reduction) at 4 weeks that was maintained over 3 months of treatment with TTFC. This result demonstrates the clinical utility of transitioning patients from one prostaglandin analog/timolol combination therapy to another for enhanced IOP reduction before considering more intensive treatment options (eg, surgery).
The reduction in IOP achieved with polyquad-preserved TTFC in the current study compares favorably to reductions reported in patients with OAG or OHT receiving a BAK-preserved TTFC regimen. In these earlier studies, the baseline IOP was generally higher, ranging from approximately 23 to 27 mmHg compared with the current study, but patients had a stable reduction in IOP on the BAK-preserved regimen (mean IOP at week 12, range: 15.6–18.4 mmHg), and no clinically relevant safety concerns (eg, visual acuity, ocular signs) were raised. However, this BAK-containing regimen was associated with ocular hyperemia in 12.4%–14.3% of patients.11–14 Patients with OAG or OHT receiving polyquad-preserved TTFC in the present study showed no clinically relevant changes from baseline in ocular signs and safety, and individual eye disorders were reported by only one patient each. Thus, TTFC preserved with polyquad was well tolerated and patients achieved a reduction in IOP that was equivalent to that reported previously in patients unable to tolerate a BAK-containing IOP-lowering regimen.
Evidence suggests that TTFC preserved with polyquad lowers IOP through a combined mechanism of increased aqueous humor outflow stimulated by a prostaglandin analog (ie, Travatan) and decreased aqueous humor production caused by a nonselective β-blocker (ie, timolol).1 Clinical studies have shown that polyquad-preserved TTFC provides additional IOP-lowering effects in patients with OAG who are switched from BAK-preserved monotherapy (eg, nonselective β-blocker, prostaglandin analogs) and concurrent therapy (eg, α2-adrenergic agonist or prostaglandin analog + nonselective β-blocker).23–25 Thus, switching from BAK-preserved monotherapy or concurrent therapy to polyquad-preserved TTFC can provide an additional IOP-lowering benefit in patients with OAG or OHT. Importantly, fixed-combination medications preserved with alternatives to BAK may have practical advantages. Ocular signs and symptoms are common in patients who use BAK-preserved eyedrops, with up to 43% of patients reporting discomfort, burning or stinging, foreign body sensation, dry eyes, tearing, and itchy eyelids after instillation.26 Preparations preserved without BAK may reduce ocular surface disease symptoms, improve hyperemia severity, and increase patient acceptance, thereby increasing the likelihood that patients will adhere to their prescribed treatment regimen. In addition, medications preserved with BAK alternatives may improve ocular health by reducing the cytotoxic effects associated with BAK exposure.27,28
A potential limitation of this study is that prior BTFC therapy was preserved with BAK, whereas TTFC was preserved with polyquad, resulting in treatment differences between active agents and preservatives. Although IOP-lowering efficacy was unlikely to have been influenced by differences in preservatives, this factor likely contributed to safety, tolerability, and preference outcomes. Conclusions regarding the direct effects of active agents versus preservatives on these outcomes cannot be made based on the current study. Furthermore, this study was conducted in a small cohort of patients with OAG or OHT. Although all outcomes with the exception of safety were evaluated in patients with prior treatment with BAK-preserved BTFC, there were too few patients with prior treatment with unfixed bimatoprost + timolol to permit a subgroup analysis to be performed. Open-label studies are prone to the potential for bias; however, these results may more closely reflect clinical practice (ie, situations in which all participants know the medication is being altered) than a single- or double-blind trial.
Conclusion
Polyquad-preserved TTFC provided additional IOP lowering in patients with primary OAG or OHT who experienced insufficient IOP reduction with concomitant, BAK-preserved BTFC treatment. No serious AEs or changes in ocular signs were reported with TTFC. Thus, polyquad-preserved TTFC provides a well-tolerated and convenient, fixed-combination therapeutic option for patients whose IOP is uncontrolled with, or patients who are unable to tolerate, a BAK-preserved bimatoprost-based combination regimen.
Acknowledgments
This study was funded by Alcon Research, Ltd. Medical writing assistance was provided by Jillian Gee, PhD, CMPP, and Craig D Albright, PhD (Complete Healthcare Communications, Inc., Chadds Ford, PA, USA), and was funded by Alcon.
Disclosure
Dr Scherzer has no conflicts of interest to declare. Dr Schnober has received honoraria from Alcon Research, Ltd. and Allergan, Inc. Mr Hubatsch is an Alcon employee. The authors report no other conflicts of interest in this work.
References
Webers CA, Beckers HJ, Nuijts RM, Schouten JS. Pharmacological management of primary open-angle glaucoma: second-line options and beyond. Drugs Aging. 2008;25(9):729–759. | ||
Beidoe G, Mousa SA. Current primary open-angle glaucoma treatments and future directions. Clin Ophthalmol. 2012;6:1699–1707. | ||
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. | ||
Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009;360(11):1113–1124. | ||
Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713. | ||
van Gestel A, Webers CA, Severens JL, et al. The long-term outcomes of four alternative treatment strategies for primary open-angle glaucoma. Acta Ophthalmol. 2012;90(1):20–31. | ||
Alexander CL, Miller SJ, Abel SR. Prostaglandin analog treatment of glaucoma and ocular hypertension. Ann Pharmacother. 2002;36(3):504–511. | ||
Higginbotham EJ. Considerations in glaucoma therapy: fixed combinations versus their component medications. Clin Ophthalmol. 2010;4:1–9. | ||
Robin AL, Novack GD, Covert DW, Crockett RS, Marcic TS. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol. 2007;144(4):533–540. | ||
Chrai SS, Makoid MC, Eriksen SP, Robinson JR. Drop size and initial dosing frequency problems of topically applied ophthalmic drugs. J Pharm Sci. 1974;63(3):333–338. | ||
Barnebey HS, Orengo-Nania S, Flowers BE, et al. The safety and efficacy of travoprost 0.004%/timolol 0.5% fixed combination ophthalmic solution. Am J Ophthalmol. 2005;140(1):1–7. | ||
Schuman JS, Katz GJ, Lewis RA, et al. Efficacy and safety of a fixed combination of travoprost 0.004%/timolol 0.5% ophthalmic solution once daily for open-angle glaucoma or ocular hypertension. Am J Ophthalmol. 2005;140(2):242–250. | ||
Hughes BA, Bacharach J, Craven ER, et al. A three-month, multicenter, double-masked study of the safety and efficacy of travoprost 0.004%/timolol 0.5% ophthalmic solution compared to travoprost 0.004% ophthalmic solution and timolol 0.5% dosed concomitantly in subjects with open angle glaucoma or ocular hypertension. J Glaucoma. 2005;14(5):392–399. | ||
Gross RL, Sullivan EK, Wells DT, Mallick S, Landry TA, Bergamini MV. Pooled results of two randomized clinical trials comparing the efficacy and safety of travoprost 0.004%/timolol 0.5% in fixed combination versus concomitant travoprost 0.004% and timolol 0.5%. Clin Ophthalmol. 2007;1(3):317–322. | ||
Tomic M, Kastelan S, Metez Soldo K, Salopek-Rabatic J. Influence of BAK-preserved prostaglandin analog treatment on the ocular surface health in patients with newly diagnosed primary open-angle glaucoma. Biomed Res Int. 2013;2013:603782. | ||
Katz G, Springs CL, Craven ER, Montecchi-Palmer M. Ocular surface disease in patients with glaucoma or ocular hypertension treated with either BAK-preserved latanoprost or BAK-free travoprost. Clin Ophthalmol. 2010;4:1253–1261. | ||
DuoTrav® (travoprost 0.004%/timolol 0.5%). Full Prescribing Information. Surrey, UK: Alcon Laboratories (UK) Ltd; 2013. | ||
Kitazawa Y, Smith P, Sasaki N, Kotake S, Bae K, Iwamoto Y. Travoprost 0.004%/timolol 0.5%-fixed combination with and without benzalkonium chloride: a prospective, randomized, doubled-masked comparison of safety and efficacy. Eye (Lond). 2011;25(9):1161–1169. | ||
European Glaucoma Society. Terminology and Guidelines for Glaucoma. 3rd ed. Savona, Italy: Dogma S.r.l.; 2008. | ||
Brandt JD, Cantor LB, Katz LJ, et al. Bimatoprost/timolol fixed combination: a 3-month double-masked, randomized parallel comparison to its individual components in patients with glaucoma or ocular hypertension. J Glaucoma. 2008;17(3):211–216. | ||
Pfennigsdorf S, de Jong L, Makk S, et al. A combined analysis of five observational studies evaluating the efficacy and tolerability of bimatoprost/timolol fixed combination in patients with primary open-angle glaucoma or ocular hypertension. Clin Ophthalmol. 2013;7:1219–1225. | ||
Centofanti M, Oddone F, Gandolfi S, et al. Comparison of travoprost and bimatoprost plus timolol fixed combinations in open-angle glaucoma patients previously treated with latanoprost plus timolol fixed combination. Am J Ophthalmol. 2010;150(4):575–580. | ||
Scherzer ML, Liehneova I, Negrete FJ, Schnober D. Travoprost 0.004%/timolol 0.5% fixed combination in patients transitioning from fixed or unfixed bimatoprost 0.03%/timolol 0.5%. Adv Ther. 2011;28(8):661–670. | ||
Arend KO, Raber T. Observational study results in glaucoma patients undergoing a regimen replacement to fixed combination travoprost 0.004%/timolol 0.5% in Germany. J Ocul Pharmacol Ther. 2008;24(4): 414–420. | ||
Pfeiffer N, Scherzer ML, Maier H, et al. Safety and efficacy of changing to the travoprost/timolol maleate fixed combination (DuoTrav) from prior mono- or adjunctive therapy. Clin Ophthalmol. 2010;4:459–466. | ||
Baudouin C, Labbe A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312–334. | ||
De Saint Jean M, Brignole F, Bringuier AF, Bauchet A, Feldmann G, Baudouin C. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest Ophthalmol Vis Sci. 1999;40(3):619–630. | ||
Guenoun JM, Baudouin C, Rat P, Pauly A, Warnet JM, Brignole-Baudouin F. In vitro study of inflammatory potential and toxicity profile of latanoprost, travoprost, and bimatoprost in conjunctiva-derived epithelial cells. Invest Ophthalmol Vis Sci. 2005;46(7):2444–2450. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




