Back to Journals » Journal of Pain Research » Volume 16
Efficacy and Safety of Erector Spinae Plane Block for Perioperative Pain Management in Lumbar Spinal Surgery: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Authors Fu MY , Hao J, Ye LH, Jiang W, Lv YW, Shen JL, Fu T
Received 29 December 2022
Accepted for publication 15 April 2023
Published 3 May 2023 Volume 2023:16 Pages 1453—1475
DOI https://doi.org/10.2147/JPR.S402931
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ellen M Soffin
Meng-Yu Fu,1 Jie Hao,2 Lun-Hui Ye,3 Wei Jiang,2 Ying-Wen Lv,1 Jie-Liang Shen,2 Tao Fu1
1Department of Orthopaedics, The Thirteenth People’s Hospital of Chongqing (The Geriatric Hospital of Chongqing), Chongqing, 400053, People’s Republic of China; 2Department of Orthopaedics, The First Affiliated Hospital of Chongqing Medical University, Chongqing, 400016, People’s Republic of China; 3Department of Anesthesiology, The Thirteenth People’s Hospital of Chongqing (The Geriatric Hospital of Chongqing), Chongqing, 400053, People’s Republic of China
Correspondence: Jie-Liang Shen; Tao Fu, Email [email protected]; [email protected]
Background: Since the application of ultrasound-guided erector spinae plane block (ESPB) in 2016, the approach has been gradually applied to perioperative analgesia in various surgeries. In recent years, more and more studies have focused on the effect of ESPB in perioperative analgesia of lumbar spinal surgery, but its clinical effect remains controversial.
Objective: This systematic review and meta-analysis was designed to explore the efficacy and safety of ESPB used for perioperative pain management in lumbar spinal surgery.
Methods: The Pubmed, Web of Science, Cochrane Library, and EMBASE databases were comprehensively searched for relevant articles from inception to March 2022. Randomized controlled trials (RCTs) comparing ESPB with placebo or without ESPB in lumbar spinal surgery were included. The Review Manager 5.3 software was employed for this meta-analysis.
Results: Nineteen RCTs with 1381 participants were included for final analysis. ESPB group exhibited lower intraoperative consumption of sufentanil and remifentanil, lower total opioid consumption within 24 h and 48 h after surgery, lower incidence of rescue analgesia, longer time to first rescue analgesic and lower number of PCA button presses compared to the control group (P< 0.05). Moreover, the ESPB group had significantly lower pain scores at rest and on movement within 48 h after surgery compared with the control group (P< 0.05). In terms of opioid-related adverse reactions, ESPB reduced the incidence of postoperative nausea, vomitting, somnolence and itching in comparison to the control group (P< 0.05). ESPB-related serious complications were not reported in included studies.
Conclusion: This meta-analysis demonstrated that ESPB used in lumbar spinal surgery was effective in relieving postoperative pain, decreasing the perioperative consumption of opioids, as well as decreasing the incidence of postoperative opioid-related adverse reactions.
Keywords: erector spinae plane block, lumbar spinal surgery, perioperative analgesia, randomized controlled trial, meta-analysis
Introduction
Posterior lumbar spinal surgery is the gold standard for the majority of lumbar spinal diseases. As is well documented, acute severe pain in the surgical area often occurs after lumbar spinal surgery, and the pain lasts for at least 3 days.1 Not effectively managing postoperative pain leads to a decline in the patient’s activity, impacts postoperative rehabilitation, and eventually increases the incidence of various complications such as deep vein thrombosis, pulmonary embolism, and pulmonary infection, thereby prolonging hospital stay.2,3 Indeed, effective postoperative pain management helps to achieve favorable surgical results.4 Opioid analgesics are generally used to manage acute postoperative pain. However, although they exert strong analgesic effects, they are often associated with adverse reactions such as nausea and vomiting, skin itching, dizziness, lethargy, and urinary retention, and may even lead to respiratory depression and drug dependence in some cases.5 Notably, a traditional single analgesic drug or method can not achieve an ideal analgesic effect, and hence, the implementation of multimodal analgesia (MMA) can effectively reduce the incidence of postoperative adverse reactions while enhancing the postoperative analgesic effect.6 Consequently, regional analgesia has become an essential part of multimodal analgesia, but yet its application in lumbar spinal surgery is still limited.7
In recent years, with the popularization and development of ultrasound-guided technology, regional nerve block has been extensively used in clinical practice. Erector spinae plane block (ESPB) is a novel trunk nerve block technology whereby local anesthetics are injected between the deep of the erector spinae muscle and the transverse process and was first reported by Forero et al8 in 2016 regarding the treatment of severe neuropathic pain in the thorax-back. ESPB functions by local anesthetics diffusing in the thoracolumbar fascia, which blocks the dorsal and ventral branches of spinal nerves in the corresponding areas and alleviates nociceptive pain and visceral pain at the same time.8 With the increasing demand of clinicians for visualization and accuracy and the in-depth study of the anatomy of the posterior branch of the spinal nerve, the theoretical basis of ultrasound-guided ESPB has been gradually clarified.9 ESPB is a simple procedure that possesses advantages such as a high safety profile and exerting an extensive analgesic effect9 and has been progressively applied to perioperative analgesia in thoracic, abdominal, breast, and orthopedic surgeries.10 Although more and more randomized controlled trials (RCTs) are exploring its role in perioperative analgesia in lumbar spinal surgery, its efficacy and safety remain controversial. Therefore, this study aimed to systematically evaluate the efficacy and safety of ESPB in perioperative pain management for lumbar spinal surgery so as to provide a reference for clinical decision-making.
Materials and Methods
Search Strategy
This study was designed and conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.11 The PubMed, Web of Science, EMBASE, and Cochrane Library databases were comprehensively searched for relevant articles from inception to March 2022. MeSH terms and free-text words were used for the search, including “erector spinae plane block”, “erector spinae block”, “ESP block”, “ESPB”, “lumbar”, “spine”, “spinal”, “lumbar spine surgery”, “lumbar spinal surgery”, “lumbar surgery”, “spine surgery” and “spinal surgery” (The search terms and strategy are available in Supplement 1). The search was restricted to the English language. Finally, the systematic review and meta-analysis was registered on the PROSPERO website under the registration number CRD42022371256.
Inclusion and Exclusion Criteria
Inclusion criteria: (1) RCTs; (2) Patients aged more than 18 years old; (3) Patients underwent spinal surgery with general anesthesia; (4) The intervention was ESPB in the experimental group, with no block or sham block in the control group; (5) The studies included at least one outcome that could be used in this meta-analysis. Exclusion criteria: (1) Ongoing RCTs; (2) non-RCTs; (3) Animal experiments or cadaveric studies; (4) Duplicate publications; (5) Reviews, case reports, comments, and conference abstracts; (6) Articles without complete results; (7) Full-text not available.
Articles Selection and Data Extraction
Two researchers (MYF, JLS) independently screened the eligible literature according to the inclusion and exclusion criteria, then two researchers (WJ and LHY) extracted the data and cross-checked. Disagreements between the researchers were resolved by reaching a consensus with a third reviewer (TF). After the retrieved literature was imported into the NoteExpress software for automatic duplicate screening, the title and abstract of each literature were manually examined. After excluding articles that did not meet the inclusion criteria, the full-text of each literature was independently screened to determine whether the literature was eligible to be included in the meta-analysis. If the information provided in included literature was incomplete, the author was contacted to acquire the required information. The following data were extracted from the studies: (1) Characteristics of studies: author, country, year of publication, age of participants, study design, sample size, type of operation and intervention; (2) Outcomes: intraoperative consumption of opioids, postoperative consumption of opioids, rescue analgesia, time to first rescue analgesic, number of patient-controlled intravenous analgesia (PCIA) button presses, post-operative pain scores at rest and on movement, opioid-related adverse reactions, and ESPB-related complications. Given that the pain score of visual analog scale (VAS) and numerical rating scale (NRS) are both rated on a scale of 0 to 10 points, the assessment of pain was considered equivalent;12 (3) Information related to the risk of bias assessment and quality assessment. The mean and standard deviation of continuous variables and the event number and total number of discontinuous variables were extracted from the studies. If continuous variables were represented by median, interquartile range, and range, then the approach utilized by Luo et al13 and Wan et al14 was used to convert these variables into mean and standard deviation. Notably, the types of opioids used for postoperative analgesia were different in different studies. Thus, in order to standardize outcome measures, postoperative opioid doses were converted to intravenous morphine milligram equivalents (MMA).15
Risk of Bias Assessment and Quality Assessment
Two researchers (MYF and JH) and independently evaluated the risk of bias in the included studies, and the results were cross-checked. Discrepancies were resolved by reaching a consensus with a third researcher (YWL). The assessment indicators included 6 aspects and 7 items in total: selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting) and other biases. Finally, each item was evaluated as “low risk”, “high risk”, or “unclear risk”.16 The risk of bias diagram was drawn using the RevMan 5.3 software.
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system was employed to assess the quality of evidence for outcomes by two researchers (MYF and JLS).17 There were five factors that may lower the quality of evidence: risk of bias, inconsistency, imprecision, indirectness, and publication bias. The GRADEpro software was utilized to assess the aforementioned five factors and divide the quality of evidence into the following four levels: high, moderate, low, and very low. The level of evidence represents the strength of evidence.
Statistical Analysis
RevMan 5.3 software provided by Cochrane collaboration was used for this meta-analysis. Continuous variables were represented by mean difference (MD), while discontinuous variables were expressed as risk ratio (RR), and 95% confidence interval (CI) were calculated for each outcome. Heterogeneity among the studies was assessed using the I2 value and chi-square test. I2≤50% and P>0.1 indicated no significant heterogeneity among the studies, then the fixed-effects model was used. In contrast, I2>50% and P≤0.1 indicated significant heterogeneity among the studies, and further analysis was conducted to determine the source of heterogeneity, such as subgroup analysis or sensitivity analysis was subsequently carried out. If the source of heterogeneity was not identified, the random-effects model was used for analysis. P≤0.05 was considered statistically significant. Publication bias was investigated when the number of included studies in an outcome≥10. The RevMan 5.3 software was used to construct the funnel plot, while Stata 12 software was used to draw the Egger’s regression chart; publication bias was then assessed according to the symmetry of the funnel chart and Egger’s test.18 If the result of the Egger’s test was P<0.05, the Duval and Tweedie trim and fill method was used to evaluate the impact of publication bias on the results of the meta-analysis using the Stata 12 software.
Results
Literature Search and Characteristics
A total of 597 studies were identified by the word search on the four databases, and the NoteExpress software was used to eliminate 249 duplicated studies. After reading the titles and abstracts of the remaining 348 studies, 317 studies that did not meet the inclusion criteria were excluded. Finally, after reading the full-text of the remaining 31 studies, 19 RCTs19–37 were included in this meta-analysis. The flowchart of the literature screening process is illustrated in Figure 1. A total of 1381 patients were included in this analysis, of which 691 patients were in the ESPB group, and 690 patients were in the control group. The characteristics of the included studies are listed in Table 1.
 |
Table 1 Characteristics of Included Studies |
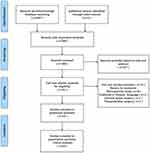 |
Figure 1 Flowchart of study selection strategy. |
Risk of Bias Assessment
The risk of bias of the included 19 RCTs is presented in Table 2 and Figure 2. In terms of random sequence generation, 4 studies only mentioned randomization without providing the randomization process;21–23,28 the remaining 15 studies reported the randomization method, including random number tables and computer-generated randomization.19,20,24–27,29–37 Regarding allocation concealment, 8 studies used sealed opaque envelopes,20–23,27,33,35,37 whereas 11 studies did not report allocation concealment.19,24–26,28–32,34,36 Concerning blinding of participants and personnel, 3 studies explicitly reported that blinding was not performed,19,24,25 10 studies mentioned the blinding method,20,21,28–30,32–35,37 while blinding was not mentioned in the remaining 6 studies.22,23,25,27,31,36 Only 16 out of the 19 included studies reported the implementation of blinding for outcome assessment.19–21,23–25,27–30,32–37 Complete data were available in 8 studies,21–26,28,35 while the remaining 11 studies exist certain participants failed to be followed up.19,20,27,29–34,36,37 Selective reporting was identified in one study,35 given that the study excluded unsatisfied outcomes. Other biases were not unclear in the included studies.
 |
Table 2 Author’s Judgements About Each Risk of Bias for Each Included Study Based on Cochrane Risk of Bias Assessment Items |
 |
Figure 2 Risk of bias summary of the included studies. |
Meta-Analysis Results
Intraoperative Sufentanil Consumption (mg)
4 RCTs reported on intraoperative sufentanil consumption.21,27,31,33 The pooled effect size of the 4 RCTs revealed that heterogeneity was significantly high (I2= 96%, P<0.00001). Then, sensitivity analysis was performed, and the result showed that heterogeneity was still high. Thus, the random-effect model was used for the analysis. The result showed that intraoperative sufentanil consumption was significantly lower in the ESPB group compared with the control group (MD=−10.88, 95% CI [−17.14, −4.63], P=0.0006, Figure 3).
 |
Figure 3 Forest plot for comparison of intraoperative sufentanil consumption between the ESPB group and control group. |
Intraoperative Remifentanil Consumption (μg)
5 RCTs reported on intraoperative remifentanil consumption.24,28,31,32,35 The pooled effect size of the 5 RCTs showed that heterogeneity was significantly high (I2= 98%, P<0.00001). Further sensitivity analysis found that heterogeneity was still high; hence, the random-effect model was used for this analysis. The result exposed that intraoperative remifentanil consumption was significantly lower in the ESPB group compared to the control group (MD=−286.59, 95% CI [−386.94, −186.25], P<0.00001, Figure 4).
 |
Figure 4 Forest plot for comparison of intraoperative remifentanil consumption between the ESPB group and control group. |
Total Opioid Consumption Within 24 h After Surgery (mg)
13 RCTs reported on total opioid consumption within 24 h after surgery,19–21,24–27,29,30,32,35–37 of which morphine was administered in 8 RCTs20,21,25,27,29,30,32,37 and fentanyl was used in 2 RCTs.24,36 Likewise, sufentanil was given in 2 RCTs,26,35 and tramadol in 1 RCT.19 The pooled effect size of the 13 RCTs showed that heterogeneity was significantly high (I2= 97%, P<0.00001) and remained high after performing sensitivity and subgroup analyses. Therefore, the random-effect model was used for the analysis. The result demonstrated that total opioid consumption within 24 h after surgery was significantly lower in the ESPB group in comparison to the control group (MD=−9.81, 95% CI [−12.64, −6.97], P<0.00001, Figure 5).
 |
Figure 5 Forest plot for comparison of total opioid consumption within 24 h after surgery between the ESPB group and control group. |
Total Opioid Consumption Within 48 h After Surgery (mg)
6 RCTs reported on total opioid consumption within 48 h after surgery,26–28,31,33,35 of which 2 RCTs used morphine,27,33 3 RCTs utilized sufentanil26,31,35 and 1 RCT used oxycodone.27 The pooled effect size of the 6 RCTs revealed that heterogeneity was significantly high (I2= 97%, P<0.00001) and remained high after sensitivity and subgroup analyses were performed. Thus, the random-effect model was used for the analysis. The analysis found that total opioid consumption within 48 h after surgery was significantly lower in the ESPB group compared to the control group (MD=−16.58, 95% CI [−28.99, −4.16], P=0.009, Figure 6).
 |
Figure 6 Forest plot for comparison of total opioid consumption within 48 h after surgery between the ESPB group and control group. |
Rescue Analgesia
A total of 10 RCTs reported on the use of rescue analgesia.19,22–25,28–31,35 The pooled effect size of the 10 RCTs showed that heterogeneity was significantly high (I2= 95%, P=0.61). Sensitivity analysis was subsequently conducted, showing that the sources of heterogeneity were predominantly from the studies conducted by Siam et al22 and Zhang et al.35 Then, there was no evidence of heterogeneity after excluding the two studies (Siam et al22 and Zhang et al35) (I2= 0%, P=0.61). Therefore, the fixed-effect model was used for this analysis, and the result showed that the incidence of rescue analgesia was significantly lower in the ESPB group compared to the control group (RR=−0.33, 95% CI [0.25, 0.43], P<0.00001, Figure 7).
 |
Figure 7 Forest plot for comparison of rescue analgesia between the ESPB group and control group. |
Time to First Rescue Analgesic (min)
A total of 9 RCTs reported the time to first rescue analgesic.19–23,25,27,29,32 The pooled effect size of the 9 RCTs showed that heterogeneity was significantly high (I2=100%, P<0.00001), and sensitivity analysis was thus performed. Considering that heterogeneity remained high following sensitivity analysis, the random-effect model was used for the analysis. The result exhibited that the time to first rescue analgesic was significantly longer in the ESPB group compared to the control group (MD=373.04, 95% CI [175.76, 570.32], P=0.0002, Figure 8).
 |
Figure 8 Forest plot for comparison of time to first rescue analgesic between the ESPB group and control group. |
PCIA Button Pressing Number (Times)
A total of 5 RCTs reported the times of PCIA button presses.21,26,29,33,37 The pooled effect size of the 5 RCTs found that heterogeneity was significantly high (I2=98%, P<0.00001) and remained high after the sensitivity analysis. Thus, the random-effect model was used for this analysis. The result showed the times of PCIA button presses in the ESPB group was significantly lower compared with the control group (MD=−13.98, 95% CI [−23.49, −4.48], P=0.004, Figure 9).
 |
Figure 9 Forest plot for comparison of PCA button pressing number between the ESPB group and control group. |
Postoperative Pain Scores (VAS/NRS) at Rest
A total of 13 RCTs reported on postoperative pain scores at rest,19,21,23–30,34,36,37 of which 6 RCTs utilized VAS19,23,24,28,29,36 while the remaining 7 RCTs used NRS.21,25–27,30,34,37 The analysis determined that the postoperative pain scores at rest were significantly lower in the ESPB group in comparison to the control group at 0 h postoperatively (MD=−2.84, 95% CI [−3.06, −2.63], P<0.00001; I2=0%, P=0.63), 2 h postoperatively (MD=−1.43, 95% CI [−2.23, −0.63], P=0.0005; I2=94%, P<0.00001), 4 h postoperatively (MD=−1.14, 95% CI [−2.05, −0.24], P=0.01; I2=95%, P<0.0001), 8 h postoperatively (MD=−1.90, 95% CI [−2.21, −1.59], P<0.00001; I2=0%, P=1.00), 12 h postoperatively (MD=−0.46, 95% CI [−0.75, −0.18], P=0.002; I2=35%, P=0.17), 24 h postoperatively (MD=−0.59, 95% CI [−0.73, −0.46], P<0.00001; I2=0%, P=0.51) and 48 h postoperatively (MD=−0.30, 95% CI [−0.54, −0.06], P=0.02; I2=51%, P=0.09) (Figure 10).
 |
Figure 10 Forest plot for comparison of postoperative pain scores at rest between the ESPB group and control group. |
Postoperative Pain Scores (VAS/NRS) on Movement
A total of 7 RCTs described postoperative pain scores on movement,19,21,24,26–28,36 among which 4 RCTs used VAS19,24,28,36 and 3 RCTs used NRS.21,26,27 The result showed that the postoperative pain scores on movement in the ESPB group were significantly lower in comparison to the control group at 0 h postoperatively (MD=−2.79, 95% CI [−3.20, −2.37], P<0.00001; I2=0%, P=0.62), 2 h postoperatively (MD=−2.28, 95% CI [−2.84, −1.72], P<0.00001; I2=73%, P=0.05), 4 h postoperatively (MD=−1.62, 95% CI [−2.86, −0.39], P=0.010; I2=94%, P<0.0001), 8 h postoperatively (MD=−1.71, 95% CI [−2.90, −0.53], P=0.005; I2=95%, P<0.0001), 12 h postoperatively (MD=−1.07, 95% CI [−1.96, −0.19], P=0.002; I2=88%, P=0.0003), 24 h postoperatively (MD=−0.72, 95% CI [−0.95, −0.49], P<0.00001; I2=0%, P=0.83) and 48 h postoperatively (MD=−1.19, 95% CI [−1.64, −0.73], P<0.00001; I2=0%, P=0.44) (Figure 11).
 |
Figure 11 Forest plot for comparison of postoperative pain scores on movement between the ESPB group and control group. |
Opioid-Related Adverse Reactions
A total of 13 RCTs reported postoperative opioid-related adverse reactions,20,21,23,24,26,28,30–35,37 of which 7 RCTs reported nausea,20,24,26,30,32,33,37 8 RCTs reported vomiting,20,21,24,26,30,32,33,37 3 RCTs reported dizziness,28,33,35 3 RCTs reported somnolence,20,31,33 and 6 RCTs reported itching.23,24,26,31,34,37 The result showed that there was no significant difference between the ESPB group and control group in the incidence of postoperative dizziness (RR=0.61, 95% CI [0.20, 1.85], P=0.38; I2=0%, P=0.94). In contrast, the result revealed that the ESPB group was associated with a lower incidence of nausea (RR=0.40, 95% CI [0.27, 0.60], P<0.00001; I2=0%, P=0.79), vomiting (RR=0.43, 95% CI[0.26, 0.71], P=0.001; I2=3%, P=0.41), somnolence (RR=0.28, 95% CI [0.11, 0.74], P=0.01; I2=0%, P=0.55) and itching (RR=0.46, 95% CI [0.27, 0.77], P=0.003; I2=36%, P=0.16) (Figure 12).
 |
Figure 12 Forest plot for comparison of postoperative complications between the ESPB group and control group. |
ESPB-Related Complications
There were no ESPB-related complications reported in all included studies, such as hematoma or infection at the puncture site, injury of the vertebral nerves, and toxicity of local anesthetic.
Sensitivity and Subgroup Analyses
The time to first rescue analgesic was associated with high heterogeneity (I2=100%, P<0.00001); sensitivity analysis was performed by excluding one study at a time from the meta-analysis to explore the source of heterogeneity. Heterogeneity was still high, indicating that the meta-analysis results were relatively reliable. Afterward, subgroup analysis was conducted on 7 studies using either bupivacaine or levobupivacaine in order to exclude the influence of the type of local anesthetics.19,20,22,23,25,29,32 Heterogeneity was still high (I2=100%, P<0.00001), which may arise from differences in drug concentration, block level, type of operation, and study area. Finally, conducting a subgroup analysis on the remaining 2 studies21,27 using ropivacaine revealed that there was no evidence of heterogeneity (I2=0%, P=0.91). The pooled effect size of the 5 studies21,23,26,27,34 with regard to post-operative pain score at rest at 48 h showed that the pain score in the ESPB group was significantly lower than that in the control group (MD=−0.30, 95% CI [−0.54, −0.06], P=0.02; I2=51%, P=0.09). However, when subgroup analysis was conducted according to the type of local anesthetic, the pooled effect size of 3 studies23,26,34 that used bupivacaine showed that there was no significant difference between the ESPB and control groups (MD=−0.13, 95% CI [−0.31, −0.04], P=0.14; I2=0%, P=0.86). However, the pooled effect size of the remaining 2 studies21,27 that used ropivacaine showed that the postoperative pain score at rest at 48 h in the ESPB group was significantly lower than that in the control group (MD=−0.65, 95% CI [−0.97, −0.33], P<0.001; I2=0%, P=0.81).
Similarly, sensitivity and subgroup analyses were conducted for intraoperative sufentanil consumption, intraoperative remifentanil consumption, total opioid consumption within 24 h after surgery, total opioid consumption within 48 h after surgery, number of PCIA button presses, postoperative pain scores at rest at 2 h and 4 h, postoperative pain score on movement at 12 h, and heterogeneity was still high, implying that the meta-analysis results were reliable. When sensitivity analysis was performed on rescue analgesia, postoperative pain scores at rest at 0 h, 8 h, 12 h, and 24 h, there was no significant heterogeneity when re-performing the meta-analysis of the remaining studies after excluding the studies one by one, and this did not significantly affect the pooled effect; the model still favored ESPB over control.
Publication Bias Assessment
Funnel plots and Egger’s regression charts were constructed for two outcomes, namely total opioid consumption within 24 h after surgery and post-operative pain score at rest at 24 h, while the Egger’s test was used to detect publication bias. The funnel plot of total opioid consumption within 24 h after surgery was asymmetric (Figure 13A) and combined with the result of the Egger’s test (t= −8.48, P=0.000), indicated possible publication bias (Figure 14A). The Duvaland Tweedie trim and fill method was used to address publication bias by re-computing the pooled effect; the corrected pooled effect was statistically significant [MD= −2.167, 95% CI (−3.049, −1.284), P=0.00], signaling that the result was robust and publication bias had a marginal influence on total opioid consumption within 24 h after surgery. The funnel plot of post-operative pain score at rest at 24 h was relatively symmetrical (Figure 13B) and, in conjunction with the result of the Egger’s test (t=0.04, P=0.967), demonstrated that there was a low likelihood of publication bias (Figure 14B).
 |
Figure 13 Funnel plot for publication bias assessment of total opioid consumption within 24h after surgery (A) and postoperative pain score at rest at 24h (B). |
 |
Figure 14 Egger’s publication bias plot of total opioid consumption within 24h after surgery (A) and postoperative pain score at rest at 24h (B). |
Grade Assessment
The GRADE system was used to evaluate the quality of evidence, results showed that the quality of evidence for all results was every low to moderate. The summary result of the outcomes and quality of evidence were shown in Table 3.
 |
Table 3 Summary Result of Outcomes and Quality of Evidence |
Discussion
In recent years, the concept of enhanced recovery after surgery (ERAS) has been extensively applied in various surgical modalities, aiming to minimize postoperative complications, shorten hospitalization times, reduce hospitalization costs, improve satisfaction, and accelerate postoperative rehabilitation.1 Perioperative pain management plays a paramount role in ERAS. The principal source of postoperative pain caused by posterior lumbar spinal surgery is structural damage in the surgical area, such as decollement of paravertebral muscles, discectomy and destruction of bony structures, which generates a large amount of inflammatory mediators in the local area or plasma, and continuously stimulates peripheral receptors, leading to the exacerbation of pain.38 In addition, central pain is induced by pulling and irritating nerve roots during operation.38 Numerous drugs and approaches have been used clinically for postoperative analgesia in lumbar spinal surgery, such as non steroidal anti-inflammatory drugs (NSAIDs), opioids, patient-controlled intravenous analgesia, epidural analgesia, and local infiltration analgesia, but they are all associated with adverse reactions.6 Multiple pain-causing mechanisms work synergistically to cause severe, lasting pain following posterior lumbar spinal surgery. However, the implementation of multimodal analgesia can better manage posterior pain and lower the incidence of adverse reactions.6,39 As previously mentioned, ESPB has become an integral part of multimodal analgesia, and more and more clinical studies have been conducted on the analgesic effect of ESPB in posterior spinal surgery.19–37
The posterior branch of the spinal nerve runs backward through the transverse processes of adjacent vertebrae, throwing out branches to innervate the corresponding muscles, bones, ligaments, and other posterior structures,9 thereby providing an anatomical basis for the application of ESPB in postoperative analgesia for lumbar spinal surgery. Ivanusic et al40 reported that 20 mL dye was injected between the T5 transverse process and erector spinae muscle, and autopsies revealed that the dye primarily diffused along the deep surface of the erector spinae muscle to the tail side and cranium side and that 25%~70% of the posterior branches of the spinal nerve were stained on the T3-T6 plane. The biggest advantages of ESPB are its simplicity, convenience, and high safety profile. Compared with intraspinal and paravertebral blocks, the target injection point is superficial and far from vital organs and blood vessels, with a low risk of pneumothorax, nerve injury, hematoma, and other complications.41 Besides, the transverse process and erector spinae muscle are easily identifiable under ultrasound guidance, which is convenient for puncturing and injecting local anesthetics.42 Herein, none of the 19 included studies reported complications related to ESPB, indicating that it is safe for perioperative analgesia in lumbar spinal surgery.
A total of 19 RCTs were included in this meta-analysis with the purpose of assessing the effectiveness and safety of ESPB for perioperative pain management in lumbar spinal surgery by summarizing the evidence of clinical studies and by describing both the quality of evidence and the strength of recommendations, so as to provide evidence-based medicine for clinical application. This meta-analysis revealed that intraoperative consumption of sufentanil and remifentanil was lower in the ESPB group than that in the control group, suggesting that ESPB for preventive analgesia prior to skin incision in lumbar spinal surgery could block the nociceptive nerve reflex during the surgery, reduce the pain stress reaction, and thus decrease the intraoperative consumption of sufentanil and remifentanil. Pain score is an instrumental index for quantifying postoperative analgesic effects. Our study found that ESPB significantly reduced the pain scores at rest and on movement at every time point within 48 h after lumbar spinal surgery compared with sham block or no block. Previous meta-analyses3,10,15,43–45 have already established that ESPB can effectively alleviate postoperative pain within 24 hours of spinal surgery, but its efficacy in relieving postoperative pain 48 hours after surgery remains controversial. On the one hand, a meta-analysis conducted by Xiao et al44 demonstrated that the pain scores at rest and on movement at 48 hours were significantly lower than those in the control group. On the other hand, the meta-analysis performed by Duan et al45 showed no significant difference in pain scores at rest and on movement at 48 hours between the ESPB and control group. In the present meta-analysis, although no significant differences were noted in pain scores at rest at 48 hours between the ESPB group and control group, the pooled effect was associated with significant heterogeneity. Then, subgroup analysis was conducted according to the type of local anesthetic, and the results showed that ESPB using ropivacaine can prolong the pain relief time to 48 hours after the operation, but there was no significant difference in pain score at 48 hours between the bupivacaine ESPB group and control group. Ropivacaine is a novel type of long-acting amide local anesthetic and is the propyl analog of bupivacaine; it possesses benefits such as lower toxic of cardiovascular and central nervous system compared with bupivacaine.46 Ropivacaine has the characteristics of separation block in sensory and motor nerves when used at low concentration, chiefly blocking sensory nerve fibers, but has almost no blocking effect on motor nerve fibers; thus, it is convenient for early rehabilitation exercise in orthopedic patients.47 More importantly, the combination of local anesthetics and adjuvants can further prolong the duration of action of ESPB. De Cassai et al48 reported that adrenaline constricts local blood vessels so as to reduce the systemic absorption of local anesthetics, thus prolonging the analgesic time of ESPB. Dexmedetomidine is a highly selective α2 adrenergic receptor agonist that activates the α2 adrenergic receptor. It is abundantly distributed in the central nervous and peripheral nerves, thus exerting analgesic, sedative, and anxiolytic effects. Notably, it does not induce respiratory depression.49 When dexmedetomidine is used locally, it can promote local vasoconstriction and delay the absorption of local anesthetics, thus prolonging its effect.50 At the same time, it can reduce the production of inflammatory factors and exerts anti-inflammatory effects.51 Yi-Han et al52 demonstrated that the combination of dexmedetomidine and ropivacaine for ESPB following posterior lumbar spine surgery could extend the sensory block and enhance the analgesic effect in a recent randomized controlled study.
Considering that ESPB can effectively relieve pain after lumbar spinal surgery, our meta-analysis also determined that ESPB can effectively decrease the postoperative consumption of opioids, number of PCA button presses, and the rate of rescue analgesia as well as extend the time to the first rescue analgesic drug. The main issues regarding the use of opioids after surgery are related to opioid-related adverse reactions, including postoperative nausea and vomiting, pruritus, somnolence, dizziness, constipation, urinary retention, respiratory depression, and so on.5 Our meta-analysis demonstrated that ESPB can reduce the incidence of postoperative nausea, vomiting, dizziness, and itching after lumbar spinal surgery, but there was no significant difference in the incidence of postoperative somnolence between the ESPB group and the control group. The occurrence of opioid-related adverse reactions is theorized to be dose-dependent.43 This study did not explore the impact of ESPB on recovery after surgery, and its role in the recovery of patients following spinal surgery is unclear. A meta-analysis showed no significant difference in the length of hospital stay and time to first ambulation between the ESPB group and control group,43 but another meta-analysis described that ESPB can effectively shorten hospital stay.10 There are many factors that affect the rapid recovery of patients after surgery, and these involve preoperative, perioperative, and postoperative management, as well as multidisciplinary cooperation. However, the majority of studies focused on the impact of pain on rapid recovery after surgery.
Different transverse processes can be selected for ESPB according to the surgical requirements to block posterior branch of the spinal nerve in corresponding ranges. Chin et al53 performed ESPB at the T7 vertebra level, and the sensory block level could reach to L2 or L3. In addition, Alici et al54 reported that a high volume (40 mL) single injection lumbar ESPB at L3 vertebra level, analgesia distribution was observed from T10 to S2 with the pin-prick test. Currently, we had not searched for a comparative study on the analgesic effects of thoracic spinal ESPB and lumbar spinal ESPB in postoperative analgesia. Lumbar spinal ESPB is different from thoracic spinal ESPB in that local anesthetics can diffuse along the front of the transverse process to enter the psoas major muscle space and intervertebral foramen region, producing a similar effect of lumbar plexus block. Therefore, Julgar S and Balaban O55 proposed an improved ESPB, which involves injecting a portion of local anesthetic into the deep surface of the erector spinae muscle and then passing the needle tip beyond the transverse process to inject the remaining local anesthetic between the psoas major muscle and transverse process. Our meta-analysis did not explore whether there were differences between thoracic spinal ESPB and lumbar spinal ESPB in perioperative pain management of lumbar spine surgery, as there were many confounding factors. Therefore, more high-quality randomized controlled trials are needed to verify this in future researches.
This study has the following limitations: To begin, because it is challenging to fully implement randomized control and blinding methods in studies, some of the studies did not report the allocation concealment and blinding methods. Thus, selection bias may have occurred during the selection and allocation of patients, thereby affecting the quality of the included literature. Secondly, most outcomes were associated with significant heterogeneity. Common sources of heterogeneity were comorbidities, surgical segment, surgeon, location of ESPB, and local anesthetic concentration and dose, but the sources of heterogeneity were not identifiable despite performing sensitivity and subgroup analyses. Thirdly, the sample sizes of the included studies were small, which led to a small number of included studies for some outcomes in the meta-analysis, thereby affecting the robustness of the results. Finally, the above reasons led to low evidence of GRADE for each outcome. Thus, the results of this meta-analysis should be interpreted with caution. Therefore, more high-quality, multi-center, large-sample randomized controlled trials are warranted to improve the quality of evidence.
Conclusion
In short, our meta-analysis demonstrated that ESPB used in lumbar spinal surgery is effective in relieving postoperative pain, reducing the intraoperative and postoperative consumption of opioids, reducing the rate of rescue analgesia, reducing the number of PCIA button presses, prolonging the time to first rescue analgesic, and reducing the opioid-related adverse reactions. However, there was insufficient evidence to validate the benefits of ESPB in rapid recovery after lumbar spinal surgery.
Abbreviations
ESPB, erector spinae plane block; RCTs, randomized controlled trials; PCIA, patient controlled intravenous analgesia; MD, mean difference; RR, risk ratio; CI, confidence interval; MMA, multimodal analgesia; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; VAS, visual analogue scale; NRS, numerical rating scale; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; ERAS, enhanced recovery after surgery; NSAIDs, non-steroidal anti-inflammatory drugs.
Data Sharing Statement
True and reliable.
Ethics Approval and Consent to Participate
Ethical approval or patient consent was not required since the present study was a review of previously published literature.
Disclosure
The authors declare that they have no competing interests.
References
1. Cozowicz C, Bekeris J, Poeran J, et al. Multimodal pain management and postoperative outcomes in lumbar spine fusion surgery: a population-based cohort study. Spine. 2020;45(9):580–589. doi:10.1097/BRS.0000000000003320
2. Mathiesen O, Dahl B, Thomsen BA, et al. A comprehensive multimodal pain treatment reduces opioid consumption after multilevel spine surgery. Eur Spine J. 2013;22(9):2089–2096. doi:10.1007/s00586-013-2826-1
3. Liu M-J, Zhou X-Y, Yao Y-B, et al. Postoperative analgesic efficacy of erector spinae plane block in patients undergoing lumbar spinal surgery: a systematic review and meta-analysis. Pain Ther. 2021;10(1):333–347. doi:10.1007/s40122-021-00256-x
4. Gottschalk A, Durieux ME, Nemergut EC. Intraoperative methadone improves postoperative pain control in patients undergoing complex spine surgery. Anesth Analg. 2011;112(1):218–223. doi:10.1213/ANE.0b013e3181d8a095
5. Wheeler M, Oderda GM, Ashburn MA, et al. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3(3):159–180. doi:10.1054/jpai.2002.123652
6. Ntalouka MP, Brotis AG, Bareka MV, et al. Multimodal analgesia in spine surgery: an umbrella review. World Neurosurg. 2021;149:129–139. doi:10.1016/j.wneu.2021.02.040
7. Puvanesarajah V, Liauw JA, Lo S-F, et al. Analgesic therapy for major spine surgery. Neurosurg Rev. 2015;38(3):407–418. doi:10.1007/s10143-015-0605-7
8. Forero M, Adhikary SD, Lopez H, et al. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41(5):621–627. doi:10.1097/AAP.0000000000000451
9. Saito T, Steinke H, Miyaki T, et al. Analysis of the posterior ramus of the lumbar spinal nerve: the structure of the posterior ramus of the spinal nerve. Anesthesiology. 2013;118(1):88–94. doi:10.1097/ALN.0b013e318272f40a
10. Ma J, Bi Y, Zhang Y, et al. Erector spinae plane block for postoperative analgesia in spine surgery: a systematic review and meta-analysis. Eur Spine J. 2021;30(11):3137–3149. doi:10.1007/s00586-021-06853-w
11. Moher D, Shamseer L, Clarke M, et al; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi:10.1186/2046-4053-4-1
12. Kollltveit J, Osaland M, Reimers M, et al. A comparison of pain registration by visual analog scale and numeric rating scale - a cross-sectional study of primary triage registration. medRxiv. 2020:1–7. doi:10.1101/2020.11.03.20225367
13. Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805. doi:10.1177/0962280216669183
14. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. doi:10.1186/1471-2288-14-135
15. Viderman D, Aubakirova M, Umbetzhanov Y, et al. Ultrasound-guided erector spinae plane block in thoracolumbar spinal surgery: a systematic review and meta-analysis. Front Med. 2022;9:932101. doi:10.3389/fmed.2022.932101
16. Higgins JP, Altman DG, Gøtzsche PC, et al; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928. doi:10.1136/bmj.d5928
17. Atkins D, Best D, Briss PA, et al; GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi:10.1136/bmj.328.7454.1490
18. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74(3):785–794. doi:10.1111/biom.12817
19. Yayik AM, Cesur S, Ozturk F, et al. Postoperative analgesic efficacy of the ultrasound-guided erector spinae plane block in patients undergoing lumbar spinal decompression surgery: a randomized controlled study. World Neurosurg. 2019;126:e779–e785. doi:10.1016/j.wneu.2019.02.149
20. Ghamry ME, Elgebaly AS, Anwar AG, et al. Ultrasound-guided erector spinae plane block for acute pain management in patients undergoing posterior lumbar interbody fusion under general anaesthesia. South Afr J Anaesth Analg. 2020;25(6):26–31. doi:10.36303/SAJAA.2019.25.6.A4
21. Zhang TJ, Zhang JJ, Qu ZY, et al. Bilateral erector spinae plane blocks for open posterior lumbar surgery. J Pain Res. 2020;13:709–717. doi:10.2147/JPR.S248171
22. Siam EM, Aliaa DM, Elmedany S, et al. Erector spinae plane block combined with general anaesthesia versus conventional general anaesthesia in lumbar spine surgery. Egypt J Anaesth. 2020;36(1):201–226. doi:10.1080/11101849.2020.1821501
23. Eskin MB, Ceylan A, Özhan MÖ, et al. Ultrasound-guided erector spinae block versus mid-transverse process to pleura block for postoperative analgesia in lumbar spinal surgery. Anaesthesist. 2020;69(10):742–750. doi:10.1007/s00101-020-00848-w
24. Ciftci B, Ekinci M, Celik EC, et al. Ultrasound-guided erector spinae plane block versus modified-thoracolumbar interfascial plane block for lumbar discectomy surgery: a randomized, controlled study. World Neurosurg. 2020;144:e849–e855. doi:10.1016/j.wneu.2020.09.077
25. Singh S, Choudhary NK, Lalin D, et al. Bilateral ultrasound-guided erector spinae plane block for postoperative analgesia in lumbar spine surgery: a randomized control trial. J Neurosurg Anesthesiol. 2020;32(4):330–334. doi:10.1097/ANA.0000000000000603
26. Yu Y, Wang M, Ying H, et al. The analgesic efficacy of erector spinae plane blocks in patients undergoing posterior lumbar spinal surgery for lumbar fracture. World Neurosurg. 2021;147:e1–e7. doi:10.1016/j.wneu.2020.10.175
27. Zhang -J-J, Zhang T-J, Qu Z-Y, et al. Erector spinae plane block at lower thoracic level for analgesia in lumbar spine surgery: a randomized controlled trial. World J Clin Cases. 2021;9(19):5126–5134. doi:10.12998/wjcc.v9.i19.5126
28. Zhu L, Wang M, Wang X, et al. Changes of opioid consumption after lumbar fusion using ultrasound-guided lumbar erector spinae plane block: a randomized controlled trial. Pain Physician. 2021;24(2):E161–E168.
29. Yeşiltaş S, Abdallah A, Uysal Ö, et al. The efficacy of intraoperative freehand erector spinae plane block in lumbar spondylolisthesis: a randomized controlled study. Spine. 2021;46(17):E902–E910. doi:10.1097/BRS.0000000000003966
30. Yörükoğlu HU, Içli D, Aksu C, et al. Erector spinae block for postoperative pain management in lumbar disc hernia repair. J Anesth. 2021;35(3):420–425. doi:10.1007/s00540-021-02920-0
31. Wang L, Wu Y, Dou L, et al. Comparison of two ultrasound-guided plane blocks for pain and postoperative opioid requirement in lumbar spine fusion surgery: a prospective, randomized, and controlled clinical trial. Pain Ther. 2021;10(2):1331–1341. doi:10.1007/s40122-021-00295-4
32. Wahdan AS, Radwan TA, Mohammed MM, et al. Effect of bilateral ultrasound-guided erector spinae blocks on postoperative pain and opioid use after lumbar spine surgery: a prospective randomized controlled trial. Egypt J Anaesth. 2021;37(1):100–106. doi:10.1080/11101849.2021.1893984
33. Jin Y, Zhao S, Cai J, et al. Erector spinae plane block for perioperative pain control and short-term outcomes in lumbar laminoplasty: a randomized clinical trial. J Pain Res. 2021;14:2717–2727. doi:10.2147/JPR.S321514
34. Goel VK, Chandramohan M, Murugan C, et al. Clinical efficacy of ultrasound guided bilateral erector spinae block for single-level lumbar fusion surgery: a prospective, randomized, case-control study. Spine J. 2021;21(11):1873–1880. doi:10.1016/j.spinee.2021.06.015
35. Zhang Q, Wu Y, Ren F, et al. Bilateral ultrasound-guided erector spinae plane block in patients undergoing lumbar spinal fusion: a randomized controlled trial. J Clin Anesth. 2021;68:110090. doi:10.1016/j.jclinane.2020.110090
36. Taşkaldıran Y. Is opioid-free anesthesia possible by using erector spinae plane block in spinal surgery? Cureus. 2021;13(10):e18666. doi:10.7759/cureus.18666
37. Asar S, Sarı S, Altinpulluk EY, et al. Efficacy of erector spinae plane block on postoperative pain in patients undergoing lumbar spine surgery. Eur Spine J. 2022;31(1):197–204. doi:10.1007/s00586-021-07056-z
38. Ohba T, Ebata S, Haro H. Comparison of serum markers for muscle damage, surgical blood loss, postoperative recovery, and surgical site pain after extreme lateral interbody fusion with percutaneous pedicle screws or traditional open posterior lumbar interbody fusion. BMC Musculoskelet Disord. 2017;18(1):415. doi:10.1186/s12891-017-1775-y
39. Yoo JS, Ahn J, Buvanendran A, et al. Multimodal analgesia in pain management after spine surgery. J Spine Surg. 2019;5(Suppl S2):S154–S159. doi:10.21037/jss.2019.05.04
40. Ivanusic J, Konishi Y, Barrington MJ. A cadaveric study investigating the mechanism of action of erector spinae blockade. Reg Anesth Pain Med. 2018;43(6):567–571. doi:10.1097/AAP.0000000000000789
41. Hamilton DL, Manickam B. The erector spinae plane block. Reg Anesth Pain Med. 2017;42(2):276. doi:10.1097/AAP.0000000000000565
42. El-Boghdadly K, Pawa A. The erector spinae plane block: plane and simple. Anaesthesia. 2017;72(4):434–438. doi:10.1111/anae.13830
43. Liang X, Zhou W, Fan Y. Erector spinae plane block for spinal surgery: a systematic review and meta-analysis. Korean J Pain. 2021;34(4):487–500. doi:10.3344/kjp.2021.34.4.487
44. Xiao X, Zhu T, Wang L, et al. Efficacy of postoperative analgesia by erector spinal plane block after lumbar surgery: a systematic review and meta-analysis of randomized controlled trials. Comput Math Methods Med. 2022;2022:3264142. doi:10.1155/2022/3264142
45. Duan M, Xu Y, Fu Q. Efficacy of erector spinae nerve block for pain control after spinal surgeries: an updated systematic review and meta-analysis. Front Surg. 2022;9:845125. doi:10.3389/fsurg.2022.845125
46. Casati A, Putzu M. Bupivacaine, levobupivacaine and ropivacaine: are they clinically different? Best Pract Res Clin Anaesthesiol. 2005;19(2):247–268. doi:10.1016/j.bpa.2004.12.003
47. McClellan KJ, Faulds D. Ropivacaine: an update of its use in regional anaesthesia. Drugs. 2000;60(5):1065–1093. doi:10.2165/00003495-200060050-00007
48. De Cassai A, Bonanno C, Padrini R, et al. Pharmacokinetics of lidocaine after bilateral ESP block. Reg Anesth Pain Med. 2021;46(1):86–89. doi:10.1136/rapm-2020-101718
49. Tsaousi GG, Pourzitaki C, Aloisio S, et al. Dexmedetomidine as a sedative and analgesic adjuvant in spine surgery: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. 2018;74(11):1377–1389. doi:10.1007/s00228-018-2520-7
50. Talke P, Stapelfeldt C, Lobo E, et al. Effect of α2B-adrenoceptor polymorphism on peripheral vasoconstriction in healthy volunteers. Anesthesiology. 2005;102(3):536–542. doi:10.1097/00000542-200503000-00010
51. Sukegawa S, Higuchi H, Inoue M, et al. Locally injected dexmedetomidine inhibits carrageenin-induced inflammatory responses in the injected region. Anesth Analg. 2014;118(2):473–480. doi:10.1213/ANE.0000000000000060
52. Yi-Han W, Rong T, Jun L, et al. Dexmedetomidine combined with ropivacaine for erector spinae plane block after posterior lumbar spine surgery: a randomized controlled trial. BMC Musculoskelet Disord. 2022;23(1):235. doi:10.1186/s12891-022-05198-9
53. Chin KJ, Adhikary S, Sarwani N, et al. The analgesic efficacy of pre-operative bilateral erector spinae plane (ESP) blocks in patients having ventral hernia repair. Anaesthesia. 2017;72(4):452–460. doi:10.1111/anae.13814
54. Alici HA, Ahiskalioglu A, Aydin ME, et al. High volume single injection lumbar erector spinae plane block provides effective analgesia for lower extremity herpes zoster. J Clin Anesth. 2019;54:136–137. doi:10.1016/j.jclinane.2018.11.009
55. Tulgar S, Balaban O. Spread of local anesthetic in erector spine plane block at thoracic and lumbar levels. Reg Anesth Pain Med. 2019;44(1):134–135. doi:10.1136/rapm-2018-000027
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
