Back to Journals » Cancer Management and Research » Volume 10
Efficacy and safety of de-escalation bone-modifying agents for cancer patients with bone metastases: a systematic review and meta-analysis
Authors Liu C , Wang L, Liu L, Zhuang J, Tang S, Zhang TS, Zhou C, Feng F , Liu R, Zhang J, Zhang T, Gao C, Li H, Li J, Sun C
Received 10 June 2018
Accepted for publication 24 July 2018
Published 21 September 2018 Volume 2018:10 Pages 3809—3823
DOI https://doi.org/10.2147/CMAR.S176811
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Cun Liu,1 Lu Wang,1 Lijuan Liu,2 Jing Zhuang,2 Shifeng Tang,2 Tiansong Zhang,3 Chao Zhou,2 Fubin Feng,2 Ruijuan Liu,2 Jinmei Zhang,4 Tingting Zhang,1 Chundi Gao,5 Huayao Li,5 Jia Li,6 Changgang Sun2,7
1College of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, Shandong Province, People’s Republic of China; 2Department of Oncology, Weifang Traditional Chinese Hospital, Weifang, Shandong Province, People’s Republic of China; 3Department of Traditional Chinese Medicine, Jing’an District Central Hospital, Shanghai, People’s Republic of China; 4Department of Endocrinology, Weifang Traditional Chinese Hospital, Weifang, Shandong, Province People’s Republic of China; 5First School of Clinical Medicine, Shandong University of Traditional Chinese Medicine, Jinan, Shandong Province, People’s Republic of China; 6College of Basic Medicine, Weifang Medical University, Weifang, Shandong Province, People’s Republic of China; 7Department of Oncology, Affiliated Hospital of Weifang Medical University, Weifang, Shandong Province, People’s Republic of China
Background: Compared with application of bone-modifying agents (BMAs) every 4 weeks, it is unclear whether 12-weekly de-escalated therapy can be used as a substitute strategy.
Methods: A systematic search of PubMed, EMBASE, and the Cochrane Register of Controlled Trials until November 22, 2017, was performed. Randomized controlled trials (RCTs) were included to assess skeletal-related event (SRE) rates, adverse events, and bone turnover biomarkers, comparing 12-weekly de-escalated treatments with standard 4-weekly dosage regimens. Risk ratios (RRs) with 95% CIs were pooled in fixed-effect meta-analyses.
Results: A total of eight citations were eligible comprising 2,878 patients: zoledronate (three studies, 2,650 patients), pamidronate (two studies, 68 patients), and denosumab (three studies, 160 patients). Summary RR (0.98; 95% CI 0.87–1.12; P=0.82) for SRE rates between de-escalated and standard arms was produced when seven low risk of bias trials (695 patients) were pooled, and results without statistical significance also appeared in the analysis of adverse events and bone turnover biomarkers. Due to the limited sample size and methodological differences, the data for skeletal morbidity rates (SMRs), time to first SRE, serum C-telopeptide (sCTx) levels, and hypocalcemia were not combined, but systematic review still obtained similar indistinguishableness.
Conclusion: In this meta-analysis of randomized clinical trials, the results “appeared” to show non-inferiority of the 12-weekly treatment. Due to the difference in available data, the results for bisphosphonates are more solid than for the receptor activator of nuclear factor-κB ligand (RANKL) antibodies.
Keywords: bone-modifying agents, bone metastasis, cancer, de-escalated treatment, meta-analysis
Introduction
Approximately 70% of the patients with multiple myeloma or advanced malignant tumors (especially with highest prevalence in breast and prostate cancers) are associated with a common clinical problem of bone metastasis.1 Malignant bone diseases caused by bone metastases can severely damage the stability of normal bones and result in life-limiting skeletal-related events (SREs), including pathological fractures and nerve compression, which may require palliative radiotherapy or bone surgery and can also cause hypercalcemia and a decrease in quality of life2–5 or even lead to a higher risk of death.6 Bone-modifying agents (BMAs), including bisphosphonates and receptor activator of nuclear factor-κB ligand (RANKL) inhibitor, can inhibit osteoclast-mediated bone resorption.7 This treatment has been tested to reduce the incidence of skeletal morbidity in patients with bone metastases8–10 and is widely used clinically. However, the best interval of drug delivery is still controversial.
In general, the dosing interval for BMAs is every 3–4 weeks.11 This dose regimen was developed from studies on hypercalcemia patients and co-administration with standard anti-cancer agents, rather than on convincing pharmacodynamics and contrastive studies.12,13 The pharmacokinetic studies found that terminal half-lives of bisphosphonate and denosumab were both longer.14,15 With the prolongation of the overall survival expectancy of the patients with advanced malignant tumors, the toxic effects may increase gradually with the long accumulation periods of BMAs, primarily manifested in jaw osteonecrosis, renal adverse events, hypocalcemia, and bone pain.16 Therefore, increasing attention has been paid to whether de-escalation dosing could provide the same efficacy as the standard dosage regimen while improving adherence and safety.17
We conducted this research to summarize all available evidence from randomized controlled trial (RCT) studies18–25 regarding the comparison between 12-weekly de-escalation treatments and standard 4-weekly dosage regimens and to provide a quantitative assessment. If de-escalation shows non-inferiority, its clinical application will undoubtedly reduce the cost of medical treatment and the waste of medical resources.
Methods
The present systematic review was in compliance with PRISMA statement26 and has been registered in the PROSPERO database (CRD42017083426). A complete PRISMA checklist is provided in Table S1.27,28
Research question
The research issues are expressed in the framework of population–intervention–comparator–outcomes-study design (PICOS) as “Comparison of the benefit (skeletal morbidity rate [SMR], SRE, time to first SRE) and harm (osteonecrosis of jaw, renal toxicity, bone pain, hypocalcemia) of BMA administration to cancer patients with bone metastases every 12 weeks or every 4 weeks”.
Literature-search strategy
Under the guidance of the comprehensive and systematic search strategy formulated by evidence-based experts, PubMed, EMBASE, and Cochrane Library were independently searched by two investigators (CL and LW) and updated until November 22, 2017. No filters, limits, and publication date or language restrictions were enforced. Complete search strategies are shown in Table S2. To test the sensitivity of the search strategy and find any other relevant publications, reference lists of multiple articles and pertinent reviews were checked manually.
Inclusion and exclusion criteria
The article inclusion criteria applied to the stage 1 review (title and abstract reading) were as follows: 1) cancer patients with bone metastasis; 2) randomized clinical trial; and 3) de-escalated treatment (12-weekly) compared with standard treatment (4-weekly) using the same BMAs. Stage 2 review (full-text reading) inclusion criteria for application were as follows: 1) administration contains 4-weekly dose and 12-weekly dose; 2) included at least one end point of the following: SREs, SMR (which was defined as the number of occurrences of any SRE, allowing for only one event in any 3-week interval, divided by the time at risk in years), time to first on-study SRE, adverse events, serious adverse events (SAEs), renal adverse events, osteonecrosis of the jaw, cardiac events, bone pain, radiation to bone, gastrointestinal events, hypocalcemia, or bone turnover marker (urine N-telopeptide [uNTx] or urine N-telopeptide corrected for creatinine [uNTx/Cr] or serum C-telopeptide [sCTx]).
The exclusion criteria were as follows: 1) conference abstracts or 2) not treated with same BMAs or contained different doses in two arms. If data from the same study cohort resulted in more than one publication, data for different outcomes were required to be included, whereas if the results were the same, the most recent or complete report was used to prevent the duplication of data from patients from one cohort.
Data extraction and study quality assessment
Two authors independently performed data extraction and quality assessment, disagreements were resolved by consensus, and a third senior author was consulted when necessary. For all standard research, data collection was performed using a predefined standardized grid (Table 1), including the following entries: first author, year of publication, country, study design information, sample size, mean age, patient inclusion criteria, outcomes assessed, duration, industry funded, and study status. Specific outcomes were separately collected and are shown in Table 2, including SRE, adverse events, SAEs, renal adverse events, osteonecrosis of the jaw, bone pain, radiation to bone, and bone turnover marker (reduction of uNTx).
Cochrane Collaboration’s assessment tool29 was used to assess the risk of bias, and special attention was paid to the following items that usually represent the quality of the RCT:30 random sequence generation, allocation concealment, blinded (participants, personnel and outcome assessment), incomplete outcome data, free of selective reporting, and free of other bias.
Data analyses
Meta-analysis was performed where enough data were available. Binary outcomes were synthesized using risk ratio (RR). All summary estimates were reported with point estimates and corresponding 95% CIs. If data were considered unsuitable for meta-analysis based on study characteristics, a narrative approach to summary of study-specific results was employed. The statistical heterogeneity across studies was assessed using the Cochran Q and I2 statistics with significance defined as Q test ≤0.10 or I2 >50%.31 The random-effects model was selected as a result of the existence of significant heterogeneity; if not, the fixed-effect model was performed to combine results. Due to the limitations of available data, sensitivity or subgroup analyses were not executed. The analyses described earlier were implemented through Review Manager 5.3 (Cochrane Collaboration, Oxford, UK).
Results
Search results
The flow diagram illustrates the identification process of electronic search and study selection based on eligible and excluded trials (Figure 1). Our systematic literature search identified 8,989 potentially relevant publications; after duplicate removal and the first screening of titles and abstracts, 8,974 were excluded. We had a full review for the remaining 15 records, of which seven articles were excluded: one32 focused upon zoledronate dose rather than dose frequency, one33 did not include necessary outcomes, one34 focused on the comparison with denosumab and bisphosphonate, while not providing separate data from each subgroup, and four35-38 were published as meeting abstracts without end point data. Eventually, eight publications were identified for the meta-analysis.
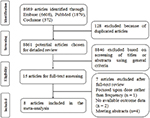  | Figure 1 Flow chart of article screening and selection process. |
Study and patients’ characteristics
The characteristics and data extraction of qualified studies included in the meta-analysis are summarized in Tables 1 and 2. The age of participants across studies ranged from 55 to 65 years. A total of 2,878 participants from eight RCTs were included. Among the studies, three18–20 studies evaluated reduced-frequency dosing treatment with zoledronate, two21,22 with pamidronate, and three23–25 with denosumab.
Two of the studies19,24 involved a series of malignancies with bone metastasis including breast cancer, prostate cancer, and multiple myeloma, and the rest were breast cancer as the main research object.18,20–23,25 Two articles,23,25 respectively, reported the results of a study by Lipton et al at different time points (13 and 25 weeks), so the data extracted from the two were considered attributable to the same study. In addition, of all the articles included, two studies were published in 2017,19,20 two in 2013,18,22 one in 2014,21 and three between 2007 and 2009.23–25 In terms of experimental design, the dose and frequency were consistent across studies using zoledronate (4 mg), pamidronate (90 mg), and denosumab (180 mg) every 4 weeks vs every 12 weeks.
Quality assessment
All included articles18–25 were evaluated for risk bias using the Cochrane Collaboration tools. Four studies18–20,24 were considered high risk of bias in blinding of the outcome assessment field. Although other articles also showed an uncertain risk of bias in several fields, overall, the majority of RCTs exhibited lower risk of bias (Figure 2).
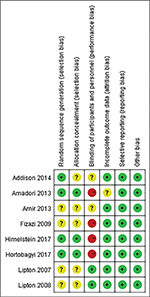  | Figure 2 Risk of bias assessment. Note: Green represents low risk of bias; red represents high risk of bias; and yellow represents unclear risk of bias. |
Findings – SREs
The included studies reported multiple outcome estimates related to the risk of SREs comparing de-escalated with standard dose, including the SRE rate (the proportion of patients with at least one SRE on study),18–25 the time to first on-study SRE,19,20 and the SMR.18,20
As shown in Figure 3, data for the SRE rates were available in all the included studies. The combined RRs showed that de-escalated was not superior to the standard arm in SRE rates (RR 0.98; 95% CI 0.87–1.12; P=0.82) with no significant heterogeneity (I2=0%; P=0.96).
  | Figure 3 Meta-analysis results for skeletal-related events. |
Among the ZOOM study,18 the SMR ratio (4-weekly arm vs 12-weekly arm) was 0.97 (95% CI 0.60–1.57; P=0.896). In addition, the mean (SD) SMR was 0.50 (1.50) and 0.46 (1.06) events annually for de-escalated vs the standard arm in the OPTIMIZE-2 study.20 Both findings suggested that the 12-weekly de-escalated was not inferior to the 4-weekly treatment. Regarding the time to first SRE, there was no statistically significant difference between treatment arms (HR 1.06; 95% CI 0.70–1.60; P=0.79) for OPTIMIZE-2.20 Median times to first SRE were also reported by Himelstein et al,19 which were 15.7 vs 16.8 (4-weekly arm vs 12-weekly arm). As a result of the differences in data type, no consolidation analysis for SMR or time to first SRE was conducted.
Bone radiotherapy, as one of the important definitions of SRE, was also, respectively, analyzed in two studies (12-weekly arm: 25 events; 4–weekly arm: 31 events).20,22 Differences with no statistical significance between the two arms are shown in the pooled analysis (RR 0.80; 95% CI 0.49–1.30; P=0.36) (Figure 4).
  | Figure 4 Meta-analysis results for bone radiotherapy. |
Finding – adverse events
A series of data of side effects and toxicities were analyzed. Overall, AEs occurred in 802 patients (12-weekly arm: 389 events; 4-weekly arm : 413 events) and SAEs occurred in 185 patients (12-weekly arm: 88 events; 4-weekly arm: 97 events). Summary RRs were produced, respectively, RR 0.96 (95% CI 0.89–1.04; P=0.38) for AEs and RR 0.91 (95% CI 0.70–1.17; P=0.44) for SAEs, both were not statistically significant, and high statistical heterogeneity (I2=66%; P=0.05) was observed for AEs (Table 2 and Figure 5), whereas there was no significant heterogeneity (I2=0%; P=0.78) in SAEs (Table 2 and Figure 6).
  | Figure 5 Meta-analysis results for adverse events. |
  | Figure 6 Meta-analysis results for serious adverse events. |
In addition, we conducted a meta-analysis of several common toxic outcomes. The results all showed no statistically significant reductions. Only two studies18,25 have reported bone pain data, the comparison showed a summary RR of 0.81 (95% CI 0.42–1.55; P=0.52) between de-escalated (15 events) and standard (19 events) arms, and low statistical heterogeneity was found (I2=0%; P=0.71) (Figure 7). Data for renal adverse events were available from four studies.18–20,22 Similar indifference was found (RR 0.67; 95% CI 0.39–1.16; P=0.15) between de-escalated (21 events) and standard (31 events) arms with low statistical heterogeneity (I2=0%; P=0.55) (Figure 8). Five studies18–20,22,23 provided available data for osteonecrosis of the jaw, but only three18–20 were included in the meta-analysis for the presence of 0 events in both groups.22,23 Comparison showed a summary RR of 0.58 (95% CI 0.30–1.12; P=0.11) between de-escalated (1,385 events) and standard (1,389 events) arms, and low statistical heterogeneity was observed (I2=0%; P=0.38) (Figure 9). Finally, we did not carry out meta-analysis for hypocalcemia because there was only one set of available data. The research19 showed that regardless of any grade of hypocalcemia or grade 4 hypocalcemia, no significant differences existed between the 4-weekly group and the 12-weekly group.
  | Figure 7 Meta-analysis results for bone pain. |
  | Figure 8 Meta-analysis results for renal adverse events. |
  | Figure 9 Meta-analysis results for osteonecrosis of the jaw. |
Finding – bone turnover biomarkers
The study of Amadori et al18 provided a significant increase in the N-terminal telopeptide concentration in the 12-weekly group vs 4-weekly group from 6 months (12.2% vs –2.3%; P=0.0111) to 12 months (12.2% vs 0.0%; P=0.0465). However, this open-label result was not reproduced by a double-blind design of Hortobagyi et al.20 When we gathered the other available data,24,25 no statistically significant results (RR 0.90; 95% CI 0.75–1.08; P=0.37) were obtained (Figure 10).
  | Figure 10 Meta-analysis results for reduction of urine N-telopeptide. |
The research on sCTx also showed different results. Addison’s research21 based on the REFORM cohort provided a statistically significant greater increase in sCTx (median of 131 vs 17, P=0.034) when comparing treatment group 2 (12-weekly arm) with group 1 (4-weekly arm). However, the observation point was only at baseline and week 12, when sCTx levels were measured for 48 weeks, the outcome changed (73.7% in control arm; 68.4% in de-escalated arm; P=0.64). No statistical analysis was performed for sCTx due to heterogeneity of the data.
Discussion
Dosing intervals have increasingly been questioned, although the standard application of BMAs is once every 4 weeks, which was obtained from studies of hypercalcemia patients who received anticancer agents39 and has long been guiding clinical practice.10,40 Terminal treatment of cancer patients, especially palliative care, requires a shift from “problem-based, disease-oriented” care to “goal-oriented, integrated” care. The balance between the long-term use of BMA-related side effects41 and the therapeutic benefits of advanced cancer patients needs deliberation. Thus, increasing interest is focused on the de-escalated treatment strategies. If curative effects of de-escalation treatment to less frequent dosing is concordant with administration of 4-weekly, it can effectively reduce health care costs and relieve medical pressure.42
The primary outcome is health-related quality of life in this research. The results showed that the 12-weekly de-escalated treatment regimen is not inferior to the 4-weekly dosage regimen for patients with bone-metastatic cancer, regardless of whether they had completed the standard 1 year of BMA treatment before, which challenged the current guidelines.43–45 The incidence of SRE is a composite frequently used end point of skeletal complications in patients with bone metastases.46,47 We had observed that the average probability of SREs in different experiments was of great disparity. This finding is not only due to the different frequency requirements for imaging but also because of different decision-making models and treatment thresholds among different clinicians. Although the nature of the SRE is uncertain but still plausible, no statistical significance was shown in the final results between study arms. For frequencies of adverse events and toxicity, although limited to different measuring tools, part of the study of small sample data and not sufficient follow-up time, overall, the results showed non-inferiority of the de-escalated treatment. The role of bone turnover biomarkers as a substitute for subsequent SRE risks is increasingly questioned,48 and it is still a common clinical method used because it is simpler and easier.4,49 The results were observed to be different in these studies, but we observed that with the extension of follow-up time and the increase in sample size, the bone turnover biomarker levels tended to not be different between the two groups. In fact, we do not have much hope for a positive outcome for the de-escalated arms; the end is really the same. However, de-escalated scheduling is sufficient to satisfy our predefined definition of non-inferiority. Meanwhile, the limitations of data should be taken into consideration, and its clinical feasibility remains to be verified by large sample experiments. At that time, indiscrimination between the two groups may have a substantial impact on medical decisions.
Since 2000, the American Society of Clinical Oncology (ASCO) guidelines have suggested the use of BMAs indefinitely.16 For this long-term treatment model, a larger interval of medication will undoubtedly reduce the cost for patients. Actually, the current guidelines recommend a “one size fits all” approach.50 It is suggested that all patients receive the same dose and frequency of BMAs, regardless of their potential risks or needs, which is obviously unreasonable in the current era of personalized medicine. For example, with different pharmacokinetic and efficacy properties, denosumab, as a new bisphosphonate alternative drug,51 was invariably observed to be marginally more effective than zoledronate in preventing SREs and improving quality of life.52,53 Meanwhile, excellent effectiveness brings a considerable extra cost. The study of Shapiro et al54 has shown that the mean cost of the treatment strategy is nine-fold higher for denosumab than generic zoledronate every 3 months. Therefore, making appropriate treatment strategies is a test to clinicians. What is important is to limit medical waste and economic loss caused by the overuse of the treated individuals while guaranteeing their health and rights.
There are several limitations to this study that should be mentioned, as well. First, the study contained different BMA types and the duration of BMAs used before enrollment and did not always report common clinical end points. Therefore, certain studies tend to play a dominant role in inherently few research samples when carrying out a specific end point summary analysis. Second, the duration of BMAs in these studies is noteworthy, because the life expectancy of partial cancer patients with bone metastases may be close to or shorter than the median length of follow-up of these studies, especially for the special group of elderly patients.55 Additionally, with the aggravation of the disease, the loss of follow-up becomes common. Both may lead to inability to fully evaluate the relationship between exposure and outcome. In addition, our research only included the patients with breast cancer, prostate cancer, and multiple myeloma, although bone metastases are common in many types of cancers.56 Several studies (NCT02051218; NCT00320710; NCT00320710; and NCT02721433) have not included other types of cancer patients as well. Therefore, whether the results can be generalized to other types of cancers is still uncertain and needs further investigation. What has to be further mentioned is that the sample size is very limited, especially for RANKL inhibitor. This may result in the instability, even false positive rate, of the outcomes to a certain extent. Despite these limitations, it is worth noting that there is consistency in all trial results. There were no signs of significant differences between the de-escalation and control arms for different BMAs used and outcome events.
Conclusion
After summarizing and analyzing all available data obtained to date, there appears to be no difference in outcomes between 12-weekly de-escalated therapy and 4-weekly dosage regimen. The longer-interval dose is a better choice, both from a health care resource perspective and a financial perspective. It is important to determine whether each type of cancer can benefit from this and whether the high heterogeneity of SRE risk among different individuals is suitable for unified Clinical Governance. Further precision research is needed in the future to adequately advise clinicians and patients on the optimal dosage regimen of BMAs in various clinical settings.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (grant number: 81473513).
Author contributions
The research project was designed by Cun Liu, Lu Wang, and Changgang Sun; organized by Cun Liu, Lu Wang, Lijuan Liu, Jing Zhuang, Shifeng Tang, Fubin Feng, Jinmei Zhang, and Tingting Zhang; and executed by Cun Liu and Lu Wang. Statistical analysis was designed by Cun Liu, Lu Wang, Tiansong Zhang, Chundi Gao, and Huayao Li; executed by Cun Liu, Lu Wang, Tiansong Zhang, Fubin Feng, Tingting Zhang, and Jia Li; and reviewed and critiqued by Changgang Sun, Lijuan Liu, Chao Zhou, Ruijuan Liu, and Jinmei Zhang. The first draft of the manuscript was written by Cun Liu and Lu Wang, and the manuscript was reviewed and critiqued by Lijuan Liu, Tiansong Zhang, and Changgang Sun. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Hofbauer LC, Rachner TD, Coleman RE, Jakob F. Endocrine aspects of bone metastases. Lancet Diabetes Endocrinol. 2014;2(6):500–512. | ||
Clément-Demange L, Clézardin P. Emerging therapies in bone metastasis. Curr Opin Pharmacol. 2015;22:79–86. | ||
Brown JE, Cook RJ, Major P, et al. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst. 2005;97(1):59–69. | ||
Brown JE, Thomson CS, Ellis SP, Gutcher SA, Purohit OP, Coleman RE. Bone resorption predicts for skeletal complications in metastatic bone disease. Br J Cancer. 2003;89(11):2031–2037. | ||
Gómez García S, Clemons M, Amir E. Rethinking end-points for bone-targeted therapy in advanced cancer. Eur J Cancer. 2016;63:105–109. | ||
Saad F, Lipton A, Cook R, Chen YM, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110(8):1860–1867. | ||
Green JR, Müller K, Jaeggi KA. Preclinical pharmacology of CGP 42’446, a new, potent, heterocyclic bisphosphonate compound. J Bone Miner Res. 1994;9(5):745–751. | ||
Coleman RE. Bisphosphonates: clinical experience. Oncologist. 2004;9(Suppl 4):14–27. | ||
Clemons M, Gelmon KA, Pritchard KI, Paterson AH. Bone-targeted agents and skeletal-related events in breast cancer patients with bone metastases: the state of the art. Curr Oncol. 2012;19(5):259–268. | ||
Holen I, Coleman RE. Bisphosphonates as treatment of bone metastases. Curr Pharm Des. 2010;16(11):1262–1271. | ||
Hutton B, Addison C, Mazzarello S, et al. De-escalated administration of bone-targeted agents in patients with breast and prostate cancer-A survey of Canadian oncologists. J Bone Oncol. 2013;2(2):77–83. | ||
Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med. 1996;335(24):1785–1791. | ||
Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29(9):1125–1132. | ||
Cremers SC, Papapoulos SE, Gelderblom H, et al. Skeletal retention of bisphosphonate (pamidronate) and its relation to the rate of bone resorption in patients with breast cancer and bone metastases. J Bone Miner Res. 2005;20(9):1543–1547. | ||
Doshi S, Sutjandra L, Zheng J, et al. Denosumab dose selection for patients with bone metastases from solid tumors. Clin Cancer Res. 2012;18(9):2648–2657. | ||
van Poznak C, Somerfield MR, Barlow WE, et al. Role of Bone-Modifying Agents in Metastatic Breast Cancer: An American Society of Clinical Oncology-Cancer Care Ontario Focused Guideline Update. J Clin Oncol. 2017;35(35):3978–3986. | ||
Bouganim N, Dranitsaris G, Amir E, Clemons M. Optimising the use of bone-targeted agents in patients with metastatic cancers: a practical guide for medical oncologists. Support Care Cancer. 2011;19(11):1687–1696. | ||
Amadori D, Aglietta M, Alessi B, et al. Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): a phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol. 2013;14(7):663–670. | ||
Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of Longer-Interval vs Standard Dosing of Zoledronic Acid on Skeletal Events in Patients With Bone Metastases: A Randomized Clinical Trial. JAMA. 2017;317(1):48–58. | ||
Hortobagyi GN, van Poznak C, Harker WG, et al. Continued Treatment Effect of Zoledronic Acid Dosing Every 12 vs 4 Weeks in Women With Breast Cancer Metastatic to Bone: The OPTIMIZE-2 Randomized Clinical Trial. JAMA Oncol. 2017;3(7):906–912. | ||
Addison CL, Pond GR, Zhao H, et al. Effects of de-escalated bisphosphonate therapy on bone turnover biomarkers in breast cancer patients with bone metastases. Springerplus. 2014;3:577. | ||
Amir E, Freedman O, Carlsson L, et al. Randomized feasibility study of de-escalated (every 12 wk) versus standard (every 3 to 4 wk) intravenous pamidronate in women with low-risk bone metastases from breast cancer. Am J Clin Oncol. 2013;36(5):436–442. | ||
Lipton A, Steger GG, Figueroa J, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007;25(28):4431–4437. | ||
Fizazi K, Lipton A, Mariette X, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27(10):1564–1571. | ||
Lipton A, Steger GG, Figueroa J, et al. Extended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapy. Clin Cancer Res. 2008;14(20):6690–6696. | ||
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. | ||
Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. 2011;39(2):91–92. | ||
Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. | ||
Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. | ||
Chan KK, Glenny AM, Weldon JC, Furness S, Worthington HV, Wakeford H. Interventions for the treatment of oral and oropharyngeal cancers: targeted therapy and immunotherapy. Cochrane Database Syst Rev. 2015;12(12):CD010341. | ||
Song F, Sheldon TA, Sutton AJ, Abrams KR, Jones DR. Methods for exploring heterogeneity in meta-analysis. Eval Health Prof. 2001;24(2):126–151. | ||
Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer. 2001;91(7):1191–1200. | ||
Kuchuk I, Beaumont JL, Clemons M, Amir E, Addison CL, Cella D. Effects of de-escalated bisphosphonate therapy on the Functional Assessment of Cancer Therapy-Bone Pain, Brief Pain Inventory and bone biomarkers. J Bone Oncol. 2013;2(4):154–157. | ||
Body JJ, Lipton A, Gralow J, et al. Effects of denosumab in patients with bone metastases with and without previous bisphosphonate exposure. J Bone Miner Res. 2010;25(3):440–446. | ||
Coleman RE, Wright J, Houston S, et al. Randomized trial of marker-directed versus standard schedule zoledronic acid for bone metastases from breast cancer. J Clin Oncol. 2012;30(15 Suppl):511. | ||
Amir E, Freedman O, Carlsson L, et al. P4-16-08: Pilot Randomized Trial of De-Escalated (q12 Weekly) Versus Standard (q3-4 Weekly) Intravenous Bisphosphonates in Women with Low-Risk Bone Metastases from Breast Cancer. Cancer Res. 2011;71(24 Suppl): P4-16-08P4-16-08-P4-16-08. | ||
Templeton AJ, Stalder L, Albiges Sauvin L, et al. Prevention of symptomatic skeletal events with denosumab administered every 4 weeks versus every 12 weeks: A noninferiority phase III trial (SAKK 96/12, REDUSE). Ann Oncol. 2014;25(Suppl 4):iv540–iv541. | ||
Body J, Fizazi K, Steger GG, et al. Effects of denosumab on bone turnover: Results from two randomized phase 2 trials in patients with solid tumors. Ann Oncol. 2008;19(S8)viii275–viii276. | ||
Kimmel DB. Mechanism of action, pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. J Dent Res. 2007;86(11):1022–1033. | ||
Kuchuk I, Mazzarello S, Butterfield K, Appleton A, Addison CL, Clemons M. Oral care and the use of bone-targeted agents in patients with metastatic cancers: A practical guide for dental surgeons and oncologists. J Bone Oncol. 2013;2(1):38–46. | ||
Mariotti A. Bisphosphonates and osteonecrosis of the jaws. J Dent Educ. 2008;72(8):919–929. | ||
Xie J, Diener M, Sorg R, Wu EQ, Namjoshi M. Cost-effectiveness of denosumab compared with zoledronic acid in patients with breast cancer and bone metastases. Clin Breast Cancer. 2012;12(4):247–258. | ||
National Comprehensive Cancer Network (NCCN) [homepage on the Internet]. Clinical Practice Guidelines in Oncology. Breast Cancer. Version 2; 2017. Accessed September 6, 2018. https://www.nccn.org/professionals/physician_gls/default.aspx. | ||
National Comprehensive Cancer Network (NCCN) [homepage on the Internet]. Clinical Practice Guidelines in Oncology. Multiple Myeloma. Version 3; 2017. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed September 6, 2018. | ||
National Comprehensive Cancer Network (NCCN) [homepage on the Internet]. Clinical Practice Guidelines in Oncology. Prostatic cancer. Version 2; 2017. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed September 6, 2018. | ||
Major PP, Cook R. Efficacy of bisphosphonates in the management of skeletal complications of bone metastases and selection of clinical endpoints. Am J Clin Oncol. 2002;25(6 Suppl 1):S10–S18. | ||
Hussain A, Aly A, Daniel Mullins C, Qian Y, Arellano J, Onukwugha E. Risk of skeletal related events among elderly prostate cancer patients by site of metastasis at diagnosis. Cancer Med. 2016;5(11):3300–3309. | ||
Clamp A, Danson S, Nguyen H, Cole D, Clemons M. Assessment of therapeutic response in patients with metastatic bone disease. Lancet Oncol. 2004;5(10):607–616. | ||
Coleman R, Costa L, Saad F, et al. Consensus on the utility of bone markers in the malignant bone disease setting. Crit Rev Oncol Hematol. 2011;80(3):411–432. | ||
Kuchuk I, Clemons M, Addison C. Time to put an end to the “one size fits all” approach to bisphosphonate use in patients with metastatic breast cancer? Curr Oncol. 2012;19(5):e303–e304. | ||
Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–176. | ||
Zhang Z, Pu F, Shao Z. The skeletal-related events of denosumab versus zoledronic acid in patients with bone metastases: A meta-analysis of randomized controlled trials. J Bone Oncol. 2017;9:21–24. | ||
Zhang X, Hamadeh IS, Song S, et al. Osteonecrosis of the Jaw in the United States Food and Drug Administration’s Adverse Event Reporting System (FAERS). J Bone Miner Res. 2016;31(2):336–340. | ||
Shapiro CL, Moriarty JP, Dusetzina S, et al. Cost-Effectiveness Analysis of Monthly Zoledronic Acid, Zoledronic Acid Every 3 Months, and Monthly Denosumab in Women With Breast Cancer and Skeletal Metastases: CALGB 70604 (Alliance). J Clin Oncol. 2017;35(35):3949–3955. | ||
Body JJ, Coleman R, Clezardin P, et al. International Society of Geriatric Oncology (SIOG) clinical practice recommendations for the use of bisphosphonates in elderly patients. Eur J Cancer. 2007;43(5):852–858. | ||
Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165–176. |
Supplementary materials
  | Table S1 PRISMA checklist |
  | Table S2 Search strategy |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


