Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Efficacy and Safety of Budesonide/Glycopyrrolate/Formoterol Fumarate Metered Dose Inhaler Formulated Using Co-Suspension Delivery Technology in Japanese Patients with COPD: A Subgroup Analysis of the KRONOS Study
Authors Ichinose M, Fukushima Y , Inoue Y , Hataji O , Ferguson GT , Rabe KF , Hayashi N , Okada H , Takikawa M, Bourne E, Ballal S, DeAngelis K , Aurivillius M, Dorinsky P , Reisner C
Received 26 June 2019
Accepted for publication 21 November 2019
Published 23 December 2019 Volume 2019:14 Pages 2979—2991
DOI https://doi.org/10.2147/COPD.S220850
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Masakazu Ichinose,1 Yasushi Fukushima,2 Yoshikazu Inoue,3 Osamu Hataji,4 Gary T Ferguson,5 Klaus F Rabe,6 Nobuya Hayashi,7 Hiroshi Okada,7 Mami Takikawa,7 Eric Bourne,8 Shaila Ballal,9 Kiernan DeAngelis,10 Magnus Aurivillius,11 Paul Dorinsky,8 Colin Reisner9
1Department of Respiratory Medicine, Tohoku University Graduate School of Medicine, Sendai, Japan; 2Department of Internal Medicine, Fukuwa Clinic, Tokyo, Japan; 3Clinical Research Center, National Hospital Organization, Kinki-Chuo Chest Medical Center, Osaka, Japan; 4Respiratory Center, Matsusaka Municipal Hospital, Matsusaka, Japan; 5Pulmonary Research Institute of Southeast Michigan, Farmington Hills, MI, USA; 6LungenClinic Grosshansdorf and Christian-Albrechts University Kiel, Airway Research Center North, Member of the German Center for Lung Research (DZL), Grosshansdorf, Germany; 7AstraZeneca K.K., Osaka, Japan; 8AstraZeneca, Durham, NC, USA; 9AstraZeneca, Morristown, NJ, USA; 10Formerly of AstraZeneca, Durham, NC, USA; 11AstraZeneca, Gothenburg, Sweden
Correspondence: Masakazu Ichinose
Department of Respiratory Medicine, Tohoku University Graduate School of Medicine, Sendai, Japan
Tel +81-22-717-8534
Email [email protected]
Background: KRONOS, a Phase III, multicenter, randomized, double-blind study (NCT02497001) conducted in Canada, China, Japan, and the USA, assessed the efficacy and safety of budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler (BGF MDI), a triple fixed-dose combination therapy, relative to dual therapies in patients with moderate-to-very severe COPD. Here we present findings from the Japanese subgroup of KRONOS.
Methods: Patients received BGF MDI 320/18/9.6μg, glycopyrrolate/formoterol fumarate (GFF) MDI 18/9.6μg, budesonide/formoterol fumarate (BFF) MDI 320/9.6μg, or budesonide/formoterol fumarate dry powder inhaler (BUD/FORM DPI) 400/12μg twice-daily for 24 weeks. The primary endpoint was the change from baseline in morning pre-dose trough forced expiratory volume in 1 s (FEV1) over Weeks 12–24. Symptoms, quality of life, exacerbations, and safety were also assessed.
Results: In total, 416 Japanese patients (21.9% of the global KRONOS population) were randomized and treated with BGF MDI (n=139), GFF MDI (n=138), BFF MDI (n=70), or BUD/FORM DPI (n=69). Nominally significant improvements in the change from baseline in morning pre-dose trough FEV1 over Weeks 12–24 were observed for BGF MDI vs GFF MDI (least squares mean [LSM] difference 37 mL, 95% confidence interval [CI] 3, 72; P=0.0337) and BFF MDI (67 mL; 95% CI 25, 109; P=0.0020). Treatment with BGF MDI led to a nominally significant reduction in the rate of moderate/severe exacerbations vs GFF MDI (rate ratio 0.40, 95% CI 0.19, 0.83; P=0.0142). Compared with dual therapies, numerical improvements were observed with BGF MDI for Transition Dyspnea Index focal score and the change from baseline in Evaluating Respiratory Symptoms in COPD total score (P≤0.3899). All treatments were generally well tolerated.
Conclusion: BGF MDI nominally significantly improved lung function and numerically improved symptoms vs GFF MDI and BFF MDI. BGF MDI nominally significantly reduced exacerbations vs GFF MDI in Japanese patients with COPD. Efficacy and safety findings were generally comparable to those in the global KRONOS population.
Keywords: co-suspension delivery technology, ICS/LAMA/LABA, inhaled corticosteroid, long-acting muscarinic antagonist, long-acting β2-agonist, Japan
Introduction
The Nippon COPD Epidemiology study, conducted in 2000, estimated that at least 8.6% of Japanese adults (aged ≥40 years) have airflow limitation indicative of COPD.1 In 2015, COPD was the tenth leading cause of death in Japan;2 however, public awareness of the disease is low, with fewer than 20% of adults reporting that they were aware of COPD in a 2012 survey.3 Japan has the highest percentage of adults over the age of 65 in the world,4 and thus the burden of COPD is expected to grow with the aging of the population.
Treatment of COPD is typically initiated with a single bronchodilator (long-acting muscarinic antagonist [LAMA] or long-acting β2-agonist [LABA]), with the addition of a second bronchodilator and/or an inhaled corticosteroid (ICS), if necessary, to control symptoms and decrease the risk of exacerbations.5 Triple ICS/LAMA/LABA combinations are currently recommended by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) as a step-up treatment for patients who experience further exacerbations on dual therapy (with step-up from LAMA/LABA recommended particularly for those with blood eosinophil levels ≥100 cells/mm3), or have persistent breathlessness on ICS/LABA therapy.5 Notably, the Japanese Respiratory Society (JRS) only recommends ICS-containing therapies for patients with COPD who have features of asthma-COPD overlap (ACO),6 which include bronchodilator responsiveness >400 mL and elevated blood eosinophil levels (≥300 cells/mm3).7,8 In line with these recommendations, ICS use tends to be lower in Japanese patients with COPD compared to global patient populations, based on observations from international clinical trials.9,10
A greater benefit of triple therapies vs. dual therapies on lung function, symptoms, and exacerbations has been supported by several recent studies;11–15 however, triple therapy has not been extensively studied in Japanese patients with COPD, who may differ from Western patients in their demographics, clinical characteristics, and pharmacological treatment history.16–18 Budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler (BGF MDI) is a triple fixed-dose combination of an ICS, a LAMA, and a LABA formulated using co-suspension delivery technology that is currently in global development for COPD. In a pivotal 24-week, Phase III, multicenter trial (KRONOS; NCT02497001), which included patients from Japan, BGF MDI showed benefits on lung function, symptoms, and exacerbations vs. corresponding LAMA/LABA and ICS/LABA therapies, with a similar safety profile.15 Here we report findings from the Japanese subgroup of KRONOS to assess the effects of BGF MDI in Japanese patients with COPD and discuss these in the context of results from the global population.15
Materials and Methods
Study Design
Details of the study design have been previously published.15 Briefly, KRONOS was a double-blind, parallel-group, multicenter, Phase III randomized trial, conducted in Canada, China, Japan, and the USA. Patients were randomized 2:2:1:1 to receive BGF MDI 320/18/9.6 μg, glycopyrrolate/formoterol fumarate (GFF) MDI 18/9.6 μg, budesonide/formoterol fumarate (BFF) MDI 320/9.6 μg, or open-label budesonide/formoterol dry powder inhaler (BUD/FORM DPI; Symbicort Turbuhaler®) 400/12 μg twice-daily for 24 weeks. For all treatments, each dose was administered as two inhalations. BUD/FORM DPI 400/12 μg was administered as two inhalations of 200/6 μg (metered dose, corresponding to a total daily delivered dose of 320/9 μg). The 18 μg dose of glycopyrrolate and 9.6 μg dose of formoterol fumarate are equivalent to 14.4 µg and 10 µg, respectively, of glycopyrronium and formoterol fumarate dihydrate (delivered doses).
The study was conducted in accordance with Good Clinical Practice, including the Declaration of Helsinki. The protocol and informed consent form were approved by appropriate institutional review boards (full details have been published).15 All patients provided written informed consent before screening.
Patients
Inclusion and exclusion criteria for KRONOS have been published.15 Briefly, patients were current or former smokers, 40–80 years of age, with an established clinical history of COPD, which for Japanese patients was defined according to the JRS guidelines19 and confirmed by the investigator. Patients had moderate-to-very severe COPD, defined as a post-bronchodilator forced expiratory volume in 1 s (FEV1) ≥25% and <80%, according to predicted normal values using reference norms for Japan.19,20 All patients were symptomatic (COPD Assessment Test [CAT] score ≥10) despite receiving two or more inhaled maintenance therapies for at least 6 weeks before screening. There was no requirement for a history of COPD exacerbations in the previous year. Exclusion criteria included, but were not limited to, a current diagnosis of asthma or any other respiratory disorder (other than COPD) which, in the opinion of the investigator, could influence the study results.
Study Endpoints
This manuscript reports findings according to the Japan/China regulatory approach, in which most endpoints were assessed over Weeks 12–24. Data for the global KRONOS study population are presented for comparison. Lung function endpoints were change from baseline in morning pre-dose trough FEV1 over Weeks 12–24 (primary) and over 24 weeks (secondary). Post-dose spirometry measurements were not conducted in Japan, and therefore the additional secondary lung function endpoints from the Japan/China approach (FEV1 area under the curve from 0 to 4 hrs [AUC0–4] over Weeks 12–24 and peak change from baseline in FEV1 within 4 hrs post-dosing over Weeks 12–24) are not available in the Japanese population.
Secondary symptom and quality of life endpoints included Transition Dyspnea Index (TDI) focal score over Weeks 12–24, change from baseline in St George’s Respiratory Questionnaire (SGRQ) total score over Weeks 12–24, change from baseline in mean daily number of puffs of rescue medication over 24 weeks, and time to clinically important deterioration (CID; defined as one of the following: a ≥100 mL decrease from baseline in trough FEV1 OR a ≥4 point increase from baseline in SGRQ total score OR a TDI focal score of –1 point or less OR a treatment-emergent moderate or severe COPD exacerbation occurring up to Week 24). Other efficacy endpoints included the rate of moderate or severe COPD exacerbations over 24 weeks and change from baseline in mean daily Evaluating Respiratory Symptoms (E-RS) total score over Weeks 12–24. CID and moderate/severe exacerbations were defined in KRONOS, as reported by Ferguson et al.15
Safety was assessed via adverse event monitoring, 12-lead electrocardiograms, clinical laboratory testing, and vital sign measurements. Cases of pneumonia and major adverse cardiovascular events (MACE) were adjudicated by an external clinical endpoint committee (CEC) to ensure that appropriate pre-defined and clinically consistent criteria were met for these outcomes. Safety data were reviewed by an external data monitoring committee.
Statistical Analysis
Although the Japanese subgroup analysis was pre-specified, no adjustments for multiplicity were made. Therefore, all P-values and statistical significance reported in this manuscript are nominal. The Japanese modified intent-to-treat (mITT) and safety populations were subsets of the KRONOS mITT and safety populations that included the patients enrolled at sites in Japan. The comparisons of interest for the Japan/China statistical approach were BGF MDI vs. BFF MDI and vs. GFF MDI (both for superiority) and BFF MDI vs. BUD/FORM DPI (for non-inferiority; assessed in the global population only).
The primary endpoint was analyzed using a linear repeated measures model which included the following covariates: baseline trough FEV1, % reversibility to albuterol, baseline eosinophil count, visit, treatment, treatment-by-visit interaction, and ICS use at screening. Analyses of secondary endpoints used similar models with their respective baseline measures as covariates. Time to CID was analyzed using a Cox proportional hazards model, and the rate of moderate or severe exacerbations was analyzed using a negative binomial model. A post hoc analysis of the rate of moderate or severe exacerbations according to baseline reversibility to albuterol was also performed in the Japanese population. Further details of the statistical analysis have been previously published.15
Results
Study Population
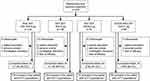
Of the 1902 patients who were randomized in the KRONOS study globally, a total of 416 (21.9%) were randomized and treated in Japan (Figure 1). All were included in the Japanese safety and mITT populations (mean age 69.5 years, 94.2% male, mean post-albuterol FEV1 51.9% of the predicted normal value; Table 1).
 |
Table 1 Demographic and Baseline Disease Characteristics (Japanese Safety Population) |
The most common COPD medication in the Japanese population at study entry was a LAMA plus a LABA (66.6%) followed by triple therapy consisting of an ICS, a LAMA, and a LABA (19.7%); in total, 31.5% of patients were receiving an ICS-containing therapy at screening. Approximately half of Japanese patients had a blood eosinophil count ≥150 cells/mm3 (52.4%) and a minority had counts ≥300 cells/mm3 (13.2%). Reversibility (defined as improvement in FEV1 of ≥12% and ≥200 mL following administration of albuterol) was observed in 37.5% of Japanese patients, and 3.6% of patients had reversibility ≥400 mL. Overall, most Japanese patients had no history of COPD exacerbations in the year prior to study entry (80.0%). The proportion of patients in the Japanese population who had experienced ≥1 exacerbation in the past 12 months was highest in the BGF MDI group (25.9%) and comparable across the dual therapy groups (range: 15.9–17.4%; Table 1).
Lung Function
For the primary endpoint of change from baseline in morning pre-dose trough FEV1 over Weeks 12–24, nominally significant improvements for BGF MDI vs. GFF MDI (least squares mean [LSM] difference 37 mL, 95% confidence interval [CI] 3, 72 mL; P=0.0337) and vs. BFF MDI (LSM difference 67 mL, 95% CI 25, 109 mL; P=0.0020; Table 2, Figure 2) were observed in Japanese patients. Similarly, in the global population, BGF MDI significantly improved morning pre-dose trough FEV1 over Weeks 12–24 compared to GFF MDI (LSM difference 20 mL, 95% CI 1, 39 mL; P=0.0424) and BFF MDI (LSM difference 77 mL, 95% CI 53, 100 mL; P<0.0001; Table 2). BFF MDI was non-inferior to BUD/FORM DPI for the change from baseline in morning pre-dose trough FEV1 over Weeks 12–24 in the global per-protocol (LSM difference –11 mL, 95% CI –39, 17 mL) and mITT populations (LSM difference –15 mL, 95% CI –42, 12 mL), using a non-inferiority margin of –50 mL for the lower bound of the 95% CI. Similar treatment differences for BGF MDI vs. GFF MDI and BFF MDI were observed in both the Japanese and global populations for the secondary endpoint of change from baseline in morning pre-dose trough FEV1 over 24 weeks (Table 2, Figure 2).
 |
Table 2 Primary and Secondary Lung Function Endpoints (Efficacy Estimand; mITT Population) |
While post-dose lung function assessments were not performed in the Japanese population, the secondary endpoints of FEV1 AUC0–4, and peak change from baseline in FEV1 within 4 hrs post-dosing over Weeks 12–24 were significantly larger for BGF MDI vs. BFF MDI (both P<0.0001) but not vs. GFF MDI in the global population Supplementary Table 1). For FEV1 AUC0–4, BFF MDI was non-inferior to BUD/FORM DPI using a margin of –75 mL in the global per-protocol (LSM difference –13 mL, 95% CI –50, 24 mL), and mITT (LSM difference –16 mL, 95% CI –52, 20 mL) populations.
Symptoms and Quality of Life Endpoints
In the PDF version of the article, the units are broken over the line from the figures. Please ensure that these are kept together.Overall, changes from baseline in symptoms and quality of life endpoints were smaller in the Japanese vs. the global population (Table 3). This reflected less severe symptom burden at baseline in Japanese patients, as evidenced by higher Baseline Dyspnea Index (BDI) scores, lower SGRQ and CAT total scores, and lower use of rescue medication compared to the overall population.15 In the Japanese population, treatment with BGF MDI numerically improved TDI focal scores over Weeks 12–24 vs. GFF MDI and BFF MDI (LSM differences 0.17 [95% CI –0.17, 0.50] and 0.22 [95% CI –0.18, 0.63], respectively; P≤0.3279; Table 3). Slightly smaller treatment differences in TDI focal score favoring BGF MDI vs. GFF MDI and BFF MDI were also seen in the global population (Table 3).
 |
Table 3 Symptom and Quality of Life Endpoints (Efficacy Estimand; mITT Population) |
BGF MDI numerically improved SGRQ total score over Weeks 12–24 vs. GFF MDI in both the Japanese (LSM difference –1.52, 95% CI –3.60, 0.55; P=0.1495) and global (LSM difference –1.10, 95% CI –2.29, 0.10; P=0.0717) populations (Table 3). A small numerical improvement for BGF MDI vs. BFF MDI in SGRQ total score was observed in the global population (LSM difference –0.52, 95% CI –1.99, 0.95; P=0.4893) but not in the Japanese population (LSM difference 0.20, 95% CI –2.33, 2.73; P=0.8766; Table 3).
BGF MDI numerically decreased E-RS total scores in Japanese patients (indicating an improvement in symptoms) vs. GFF MDI (LSM difference –0.89, 95% CI –1.86, 0.07; P=0.0701) and vs. BFF MDI (LSM difference –0.52, 95% CI –1.70, 0.66; P=0.3899). There were also small numerical differences in E-RS scores in favor of BGF MDI vs. both dual comparators in the global population (Table 3).
Changes from baseline in daily rescue medication use over 24 weeks were small and similar across treatment groups in both the Japanese (range: 0.0 to –0.3 puffs/day) and global (range: –0.4 to –0.7 puffs/day) populations (P≤0.4439; Table 3).
CID and COPD Exacerbations
The percentage of Japanese patients experiencing a CID was comparable across the BGF MDI, GFF MDI, and BFF MDI groups (range: 66–69%), and slightly higher in the BUD/FORM DPI group (74%; Table 4). Hazard ratios for time to CID were 0.93, 0.89, and 0.81 for BGF MDI vs. GFF MDI, BFF MDI, and BUD/FORM DPI, respectively (Table 4).
 |
Table 4 CIDa and COPD Exacerbations (Efficacy Estimand; Japanese mITT Population) |
Annualized rates of moderate or severe COPD exacerbations in Japanese patients over 24 weeks were 0.32, 0.82, 0.23, and 0.28 for BGF MDI, GFF MDI, BFF MDI, and BUD/FORM DPI, respectively (Table 4). BGF MDI nominally significantly reduced the rate of moderate or severe exacerbations vs. GFF MDI (rate ratio 0.40, 95% CI 0.19, 0.83; P=0.0142) but not vs. BFF MDI (1.39, 95% CI 0.47, 4.13; P=0.5556). However, the number of subjects with exacerbations in the BFF MDI and BUD/FORM DPI groups was small (Table 4), and therefore no definitive conclusions can be derived from these comparisons. In a post hoc analysis of COPD exacerbations by reversibility at baseline, the lower rate of moderate or severe exacerbations for BGF MDI compared to GFF MDI was observed in both reversible (model-estimated rate 0.25 vs. 0.96 per year, respectively) and non-reversible (0.32 vs. 0.71, respectively) patients in the Japanese population.
Safety
The percentage of Japanese patients who experienced at least one treatment-emergent adverse event (TEAE) ranged from 59.4% in the BUD/FORM DPI group to 72.9% in the BFF MDI group (Table 5). Overall, the most commonly observed TEAEs in Japanese patients were nasopharyngitis (19.7%) and bronchitis (5.3%). The incidence of serious TEAEs was comparable across treatment groups (range: 7.9–10.1%; Table 5); the most frequent serious TEAEs in the Japanese population overall were COPD (2.9%) and pneumonia (1.0%). Eight patients in the Japan subgroup (1.9%) had confirmed pneumonia events, seven of which occurred in the BGF MDI group (5.0%); the incidence of confirmed pneumonia ranged from 0% to 0.7% across the other three treatment groups. Of the seven confirmed cases of pneumonia in the BGF MDI, only one was considered by the investigator to be treatment-related. A total of three Japanese patients (0.7%) had an event confirmed as MACE: two cases of non-fatal myocardial infarction (both in the BUD/FORM DPI group), and one cardiovascular death in the GFF MDI group. Neither of the two deaths in the Japan subgroup that resulted from TEAEs reported during the study were considered by the investigator to be related to study treatment (one each in the GFF MDI and BFF MDI groups resulting from cardio-respiratory arrest and squamous cell carcinoma, respectively). No clinically meaningful trends in clinical laboratory values, vital signs, or electrocardiogram parameters were observed in any of the treatment groups.
 |
Table 5 Summary of Adverse Events (Japanese Safety Population) |
Discussion
The KRONOS study provided support for the use of triple therapy in a global population of symptomatic patients with COPD (CAT score ≥10 despite treatment with two or more inhaled maintenance therapies), the majority of whom had not experienced an exacerbation in the past year.15 Here, we present findings from a pre-specified analysis of the Japan subgroup of KRONOS, demonstrating that triple therapy improved lung function compared to both dual therapies in Japanese patients with COPD and also reduced exacerbations compared to LAMA/LABA therapy.
Overall, efficacy and safety findings were comparable between the Japanese and global populations, with the degree of variation expected given the smaller sample size of the Japan subgroup, especially in the BFF MDI and BUD/FORM DPI groups due to the 2:2:1:1 randomization ratio. While both the global and Japanese populations were similar in terms of airflow limitation, exacerbation history, and eosinophil count, the Japanese population was slightly older on average and had slightly higher mean BDI focal scores and lower SGRQ and CAT total scores at baseline, indicating a slightly lower symptom burden overall.15 In addition, use of ICS at baseline was lower in Japanese patients relative to the global population. These demographic differences are generally consistent with the results of other subgroup analyses of Japanese patients with COPD who participated in similar global Phase III clinical studies of LAMA/LABA therapies.10,21
For morning pre-dose trough FEV1, treatment comparisons showed larger improvements with BGF MDI compared to GFF MDI and BFF MDI, which were generally consistent between the Japan and global populations. BGF MDI also showed numerical benefits on symptoms endpoints vs. GFF MDI and BFF MDI in Japanese patients, with treatment differences similar to the global population. The variability in the magnitude of improvement from baseline in the Japanese population compared to the global population was consistent with the smaller sample size and the fact that Japanese patients reported less severe symptoms and higher quality of life at baseline compared to the overall patient population.15
All three ICS-containing treatments showed a marked effect on exacerbation rates vs. LAMA/LABA therapy in Japanese patients. This is notable as the current JRS guidelines only recommend ICS treatment for patients with evidence of ACO.6 However, patients with a current diagnosis of asthma were not permitted to enroll in KRONOS. Additionally, only a small proportion of the Japanese subpopulation showed characteristic features of ACO,7,8 with only 3.6% having reversibility to albuterol ≥400 mL, and 13.2% having a blood eosinophil count ≥300 cells/mm3 at baseline. Moreover, the exacerbation findings were consistent across both reversible and non-reversible patients, suggesting that the response to ICS treatment in Japanese patients was not due to an asthma component. While no meaningful differences in exacerbation rates were observed between the triple and dual ICS-containing therapies in the Japan subgroup (in contrast to the numerical improvement seen with BGF MDI vs. BFF MDI in the global population), this may be a result of baseline differences in prior exacerbation history between the BGF MDI group and both ICS/LABA groups as well as the smaller sample size of the Japan population. Indeed, the small sample size and the relatively low number of exacerbation events in Japanese patients resulted in an estimate with high variability, and thus these findings should be interpreted with caution. The slightly lower overall rate of exacerbations in Japanese patients compared to the global population is consistent with other Japanese and Asian subgroup analyses in COPD,9,10,22 as well as the fact that 80% of the Japanese population had no history of exacerbation in the previous year. The reduction in exacerbations observed with ICS-containing therapies compared to GFF MDI suggests that many symptomatic patients in Japan could benefit from the addition of an ICS to their treatment regimen (regardless of exacerbation history), as only 31.5% were receiving ICS therapy at study entry (compared to 71.8% of the global population).
Overall, all treatments were generally well tolerated in Japanese patients, and the safety profile of BGF MDI was comparable to the results from the global population. These findings are in agreement with a previous Phase I study of BGF MDI, which found that exposure and tolerability were comparable between Western and Japanese healthy subjects.23 Although some numerical differences were observed in the adverse event profile between the Japanese and global KRONOS populations,15 the differences were generally small in magnitude and to be expected, based on the smaller sample size of the Japanese subgroup. With regard to pneumonia, an important safety outcome with ICS-containing therapies in COPD, the highest incidence of confirmed pneumonia was observed in the BGF MDI group (n=7; 5.0%), and the incidence was similar between the ICS/LABA and LAMA/LABA groups (n=0–1; 0–0.7%). This was most likely a chance finding based on several factors. Firstly, there was no clear trend for a higher incidence of pneumonia in the budesonide-containing treatment groups (BGF MDI, 5.0%; BFF MDI 0%; BUD/FORM DPI, 0%) compared to GFF MDI (0.7%). Secondly, there was an imbalance in the baseline exacerbation history across treatment groups that may have contributed to the imbalance in the incidence of pneumonia. Specifically, the percentage of patients who had experienced an exacerbation in the previous year was highest in the BGF MDI group (25.9%) vs. all three dual therapy groups (range: 15.9–17.4%), and a previous exacerbation is a risk factor for pneumonia in patients with COPD.24 Accordingly, five of the seven patients with confirmed pneumonia in the BGF MDI group had a history of exacerbation in the past 12 months, while the patient in the GFF MDI group who developed pneumonia did not have a prior history of exacerbation. Furthermore, the incidence of pneumonia in the global population was comparable for all treatments (range: 1.3–1.9%),15 and overall rates of pneumonia across groups were comparable between the Japanese (1.9%) and global (1.7%) populations. Finally, in an extension of the current study, which evaluated Japanese patients enrolled in KRONOS for an additional 28 weeks, the incidence of pneumonia during Weeks 24–52 of treatment was similar across the BGF MDI (4.3%) and dual therapy treatment groups (range: 2.9–5.7%).25 Across the 52-week study, five of the 13 patients in the BGF MDI group with confirmed pneumonia (and none of the 11 patients in the other three treatment groups) had experienced an exacerbation in the previous year.
A limitation of the study is the small sample size in the Japanese subgroup, requiring the conclusions to be interpreted with caution; however, this factor is somewhat mitigated by the generally similar results between the global and Japanese populations.
Conclusions
BGF MDI nominally significantly improved lung function and numerically improved symptoms compared with LAMA/LABA (GFF MDI) and ICS/LABA (BFF MDI) combination therapies. BGF MDI also nominally significantly reduced the rate of moderate or severe COPD exacerbations vs. GFF MDI in Japanese patients with COPD despite the fact that these patients did not meet criteria for asthma-COPD overlap. Efficacy and safety findings were generally comparable to the global KRONOS study population. These results suggest that triple therapy with BGF MDI is efficacious and well tolerated in Japanese patients with moderate-to-very severe COPD, and may be a useful treatment option for those who experience continued symptoms on dual therapy, regardless of exacerbation history and bronchodilator reversibility.
Abbreviations
ACO, asthma-COPD overlap; AUC, area under the curve; BDI, Baseline Dyspnea Index; BFF, budesonide/formoterol fumarate; BGF, budesonide/glycopyrrolate/formoterol fumarate; BUD/FORM DPI, budesonide/formoterol dry powder inhaler; CEC, clinical endpoint committee; CI, confidence interval; CID, clinically important deterioration; E-RS, Evaluating Respiratory Symptoms; FEV1, forced expiratory volume in 1 s; GFF, glycopyrrolate/formoterol fumarate; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroid; JRS, Japanese Respiratory Society; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LSM, least squares mean; MACE, major adverse cardiovascular events; MDI, metered dose inhaler; mITT, modified intent-to-treat; SGRQ, St George’s Respiratory Questionnaire; TDI, Transition Dyspnea Index; TEAE, treatment-emergent adverse event.
Ethics Approval and Informed Consent
Patients provided written informed consent prior to screening, and the study was conducted in accordance with Good Clinical Practice, including the Declaration of Helsinki and the International Council for Harmonisation. The protocol was approved by local institutional review boards (names have been previously published).15 The names of all institutional review boards can be found in Supplementary Table 2.
Data Sharing Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Acknowledgments
The authors would like to thank all the patients and their families and the team of investigators, research nurses, and operations staff involved in these studies. Medical writing support, under the direction of the authors, was provided by Julia King, PhD, of CMC Connect, a division of McCann Health Medical Communications Ltd, Glasgow, UK and was funded by AstraZeneca, Gaithersburg, USA, in accordance with Good Publication Practice (GPP3) guidelines.26
Author Contributions
HO, MT, EB, SB, KdA, PD, and CR contributed to the conception or design of the study; MI, YF, YI, OH, GTR, KFR, HO, MT, EB, KdA, and MA participated in the acquisition of reported data; NH and SB contributed to the statistical analysis of the data. All authors participated in the analysis and interpretation of data reported. All authors reviewed or critically revised the manuscript, provided final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Funding
The KRONOS study was supported by AstraZeneca.
Disclosure
MI reports personal fees from AstraZeneca, during the conduct of the study; and personal fees from Kyorin, Nippon Boehringer Ingelheim, and Novartis Pharma, outside of the submitted work. YI reports personal fees from GlaxoSmithKline, Nippon Boehringer Ingelheim, Nobelpharma, and Shionogi & Co., outside of the submitted work. OH reports personal fees from AstraZeneca, Nippon Boehringer Ingelheim, and Novartis Pharma; and research funding from AstraZeneca, Daiichi Sankyo, GlaxoSmithKline, Kyorin Pharm, Nippon Boehringer Ingelheim, Novartis Pharma, and Ono Pharm. GTF reports grants, personal fees, and non-financial support from AstraZeneca during the conduct of the study; grants, personal fees, and non-financial support from AstraZeneca, Boehringer Ingelheim, Novartis, Pearl – a member of the AstraZeneca Group, and Sunovion; grants and personal fees from Theravance; and personal fees from Circassia, GlaxoSmithKline, Innoviva, Mylan, Sanofi, and Verona, outside of the submitted work. KFR reports personal fees from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi Pharmaceuticals, InterMune, Novartis, Sanofi, Roche, and Teva; and grants from the Ministry of Education and Science, Germany, outside of the submitted work. NH, HO, and MT are employees of AstraZeneca K.K., Japan. EB, SB, MA, PD, and CR are employees of AstraZeneca. KdA is a former employee of AstraZeneca. The authors report no other conflicts of interest in this work.
References
1. Fukuchi Y, Nishimura M, Ichinose M, et al. COPD in Japan: the Nippon COPD epidemiology study. Respirology. 2004;9(4):458–465. doi:10.1111/res.2004.9.issue-4
2. Nomura S, Sakamoto H, Glenn S, et al. Population health and regional variations of disease burden in Japan, 1990–2015: a systematic subnational analysis for the Global Burden of Disease Study 2015. Lancet. 2017;390(10101):1521–1538. doi:10.1016/S0140-6736(17)31544-1
3. Omori H, Yoshimoto D, Kumar M, Goren A. Prevalence, awareness, characteristics, and health outcomes associated with COPD at-risk status among adults in Japan. Expert Rev Pharmacoecon Outcomes Res. 2016;16(4):501–512. doi:10.1586/14737167.2016.1104250
4. The World Bank. Population ages 65 and above (% of total); 2017. Available from: https://data.worldbank.org/indicator/SP.POP.65UP.TO.ZS.
5. Global Initiative for Chronic Obstructive Lung Disease. 2019 Report: global strategy for prevention, diagnosis and management of COPD; 2019. Available from: http://www.goldcopd.org.
6. The Japanese Respiratory Society. Guidelines for the diagnosis and treatment of COPD, 5th Edition (in Japanese); 2018. Available from: http://www.jrs.or.jp/modules/guidelines/index.php?content_id=112.
7. Yanagisawa S, Ichinose M. Definition and diagnosis of asthma-COPD overlap (ACO). Allergol Int. 2018;67(2):172–178. doi:10.1016/j.alit.2018.01.002
8. Sin DD, Miravitlles M, Mannino DM, et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J. 2016;48(3):664–673. doi:10.1183/13993003.00436-2016
9. Fukuchi Y, Fernandez L, Kuo HP, et al. Efficacy of tiotropium in COPD patients from Asia: a subgroup analysis from the UPLIFT trial. Respirology. 2011;16(5):825–835. doi:10.1111/j.1440-1843.2011.01982.x
10. Ichinose M, Taniguchi H, Takizawa A, et al. The efficacy and safety of combined tiotropium and olodaterol via the Respimat® inhaler in patients with COPD: results from the Japanese sub-population of the Tonado® studies. Int J Chron Obstruct Pulmon Dis. 2016;11:2017–2027. doi:10.2147/COPD.S110389
11. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
12. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391(10125):1076–1084. doi:10.1016/S0140-6736(18)30206-X
13. Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016;388(10048):963–973. doi:10.1016/S0140-6736(16)31354-X
14. Lipson DA, Barnacle H, Birk R, et al. FULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(4):438–446. doi:10.1164/rccm.201703-0449OC
15. Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med. 2018;6(10):747–758. doi:10.1016/S2213-2600(18)30327-8
16. Takahashi S, Betsuyaku T. The chronic obstructive pulmonary disease comorbidity spectrum in Japan differs from that in western countries. Respir Investig. 2015;53(6):259–270. doi:10.1016/j.resinv.2015.05.005
17. Nishimura M. Similarities and differences between East and West in COPD. Respirology. 2016;21(8):1340–1341. doi:10.1111/resp.2016.21.issue-8
18. Landis SH, Muellerova H, Mannino DM, et al. Continuing to confront COPD international patient survey: methods, COPD prevalence, and disease burden in 2012–2013. Int J Chron Obstruct Pulmon Dis. 2014;9:597–611. doi:10.2147/COPD.S61854
19. The Japanese Respiratory Society. Guidelines for the diagnosis and treatment of COPD, 4th Edition (in Japanese); 2013. Available from: http://www.jrs.or.jp/.
20. Sasaki H, Nakamura M, Kida K, et al. Reference values for spirogram and blood gas analysis in Japanese adults. J Jpn Respir Soc. 2001;39(5):S1–S17.
21. Ichinose M, Nishimura M, Akimoto M, et al. Tiotropium/olodaterol versus tiotropium in Japanese patients with COPD: results from the DYNAGITO study. Int J Chron Obstruct Pulmon Dis. 2018;13:2147–2156. doi:10.2147/COPD
22. Hashimoto S, Ikeuchi H, Murata S, Kitawaki T, Ikeda K, Banerji D. Efficacy and safety of indacaterol/glycopyrronium in Japanese patients with COPD: a subgroup analysis from the SHINE study. Int J Chron Obstruct Pulmon Dis. 2016;11:2543–2551. doi:10.2147/COPD
23. Dorinsky P, DePetrillo P, Siddiqui S, Maes A, Reisner C. Safety and pharmacokinetics of budesonide/glycopyrronium/formoterol fumarate dihydrate metered dose inhaler (BGF MDI) in healthy adult subjects of Japanese descent. Pulm Pharmacol Ther. 2018;51:18–25. doi:10.1016/j.pupt.2018.05.001
24. Müllerova H, Chigbo C, Hagan GW, et al. The natural history of community-acquired pneumonia in COPD patients: a population database analysis. Respir Med. 2012;106(8):1124–1133. doi:10.1016/j.rmed.2012.04.008
25. Ichinose M, Fukushima Y, Inoue Y, et al. Long-term safety and efficacy of budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler formulated using co-suspension delivery technology in Japanese patients with COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:2993–3002
26. Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med. 2015;163(6):461–464. doi:10.7326/M15-0288
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.