Back to Journals » Cancer Management and Research » Volume 13
Efficacy and Safety of Anlotinib in Patients with Advanced Non-Small Cell Lung Cancer: A Real-World Study
Received 15 February 2021
Accepted for publication 25 April 2021
Published 20 May 2021 Volume 2021:13 Pages 4115—4128
DOI https://doi.org/10.2147/CMAR.S304838
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Qiuxia Zhong, Zhihua Liu
Department of Radiation Oncology, The Affiliated Cancer Hospital of Nanchang University, Nanchang, Jiangxi, People’s Republic of China
Correspondence: Zhihua Liu
Department of Radiation Oncology, The Affiliated Cancer Hospital of Nanchang University, No. 519 East Beijing Road, Nanchang, 330029, People’s Republic of China
Tel +86 791-88313632
Email [email protected]
Background: Anlotinib is a multi-target tyrosine kinase inhibitor (TKI) independently developed by China, which can inhibit tumor angiogenesis and tumor cell proliferation. The ALTER 0303 study has suggested that anlotinib improved overall survival (OS) and progression-free survival (PFS) in the treatment of advanced non-small cell lung cancer (NSCLC). However, in the real world, the efficacy and safety of anlotinib is not clear. Although relevant retrospective studies have confirmed the efficacy and safety of anlotinib, the sample size is small. And the OS was not observed because of the follow-up time was short. Further studies are still essential to evaluate the efficacy and safety of anlotinib in patients with advanced NSCLC in real-world settings. Related studies have preliminarily shown that anlotinib combined with whole-brain radiotherapy (WBRT) can significantly prolong the survival of patients with brain metastases of NSCLC. This study also discusses the best treatment strategies of patients with brain metastases.
Methods: A retrospective study was conducted on 206 patients with advanced NSCLC who had treated with anlotinib. The primary endpoints were PFS and OS. The secondary endpoints were objective response rate (ORR), disease control rate (DCR) and safety. Kaplan–Meier survival curves were applied to evaluate the efficacy. Univariate analysis was performed by Log rank testing. Cox regression analysis was utilized to evaluate the significance of potential risk factors obtained from the univariate analysis.
Results: The median PFS (mPFS) was 4.0 (95% CI: 3.607– 4.393) months, univariate analysis revealed that patients with longer PFS included epidermal growth factor receptor (EGFR) mutation-negative, Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 1, no brain metastases, no liver metastases, no adrenal metastases, or ≤ 2 distant metastases. Cox regression analysis indicated that patients with EGFR-negative and ECOG PS ≤ 1 had longer PFS. The median OS (mOS) was 8(95% CI: 6.495– 9.505) months. EGFR mutation-negative, previous thoracic radiation therapy, no brain metastases, or ≤ 2 distant metastases were independent positive predictors of OS. The results of Cox regression indicated that the patients without previous thoracic radiation therapy (hazard ratio: 1.855; 95% CI: 1.162-2.960; p=0.010) had shortened OS. The objective response rate was 10.2%, and the disease control rate was 78.2%. The main treatment-related adverse events (AEs) were generally tolerated. All AEs observed during the trial were controlled after dose reduction or symptomatic treatments, and no death was found to be associated with anlotinib.
Conclusion: Anlotinib was well tolerated and effective in patients with advanced NSCLC. Patients with EGFR mutation-negative and ECOG PS ≤ 1 had longer PFS, and patients without previous thoracic radiation therapy (HR: 1.855, 95% CI 1.162– 2.960; P = 0.010) had shorter OS. Further investigations are needed because of small sample.
Keywords: anlotinib, advanced non-small cell lung cancer, efficacy, safety
Introduction
According to the Global Cancer statistics 2020, lung cancer is the most common one and the leading cause of cancer deaths in the world.1 Due to the biological characteristics and tumor heterogeneity of lung cancer, it is prone to recur and lead to distant metastases, ending up with poor prognosis. Majority of patients present with locally advanced or metastatic disease at time of diagnosis, and only a minority of these patients can be treated with surgery, and the 5-year OS rate of patients with advanced lung cancer is less than 17%.2 There are a variety of methods to control the lung cancer, such as systemic chemotherapy, local radiotherapy, targeted therapy, immunotherapy and so on. Systemic chemotherapy is recommended as standard treatment in advanced NSCLC.3 However, how to choose therapeutic regimen in patients who failed second-line or third-line treatment is the current research focus. Tumor angiogenesis plays a central role in tumor growth and metastases, and is regulated by vascular endothelial growth factor receptor, platelet-derived growth factor, fibroblast growth factor and other vascular growth factors.4 Anlotinib (AL3818) is a newly developed oral small-molecule receptor tyrosine kinase (RTK) inhibitor that targets vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR) and stem cell growth factor receptor (c-Kit).5 Therefore, anlotinib can effectively inhibit both tumour angiogenesis and tumour cell proliferation.6 The ALTER 0303 trial confirmed that the overall survival of the patients treated with anlotinib is improved compared with placebo (9.6 months vs 6.3 months; P=0.002). And the mPFS of patients treated with anlotinib could reach 5.4 months.7 Previous clinical studies have also demonstrated that anti-angiogenesis plus chemotherapy significantly prolongs PFS and OS compared to chemotherapy alone in patients with advanced non-squamous NSCLC.8,9 And many studies have already demonstrated that immunotherapy could increase antiangiogenic effect and antiangiogenic agents can also activate the immune system, thus concurrent application might exert a synergistic effect between immunotherapy and anti-angiogenesis.10 However, in the real world, the efficacy and safety of anlotinib combined with chemotherapy or combined with immunotherapy need to be further explored. In the present study, we conducted a retrospective study to assess the efficacy and toxicity of anlotinib in advanced NSCLC in real-world practice.
Methods
Patient Characteristics
A total of 206 patients diagnosed with advanced NSCLC in The Affiliated Cancer Hospital of Nanchang University were analyzed retrospectively from June 2018 to December 2020. The characteristics of the patients were collected, including gender, age, pathological type, smoking status, EGFR status, ECOG PS, number of treatment lines, clinical stage, brain metastases, liver metastases, bone metastases, adrenal metastases, pleural metastases, previous antivascular drug therapy, previous thoracic radiation therapy and number of distant metastases. This study was approved by the Ethics Committee of The Affiliated Cancer Hospital of Nanchang University.
Inclusion Criteria
The inclusion criteria for patients were the following: (I) Histological or cytological diagnosis of lung cancer according to histopathological criteria (World Health Organization, 2015). (II) All patients were confirmed to have advanced or recurrent stage IIIB/IV NSCLC according to the TNM classification (version 7). (III) Disease progression after at least 1 line of chemotherapy and TKI therapy for all patients with driver alterations (EGFR mutation or ALK rearrangement) as well as disease progression after at least 2 lines of chemotherapy for all patients without driver alterations. (IV) All patients were confirmed recurrence or metastases identified in chest, abdominal or brain computed tomography (CT) scans and/or bone scans.
Exclusion Criteria
The exclusion criteria for patients were the following: (I) Malignant tumor patients with other serious diseases. (II) The patients received local therapy such as thoracic radiation therapy or interventional therapy during the treatment of anlotinib. (III) Abnormal coagulation function or bleeding tendency. (IV) Uncontrolled hypertension.
Therapeutic Methods
Anlotinib was given orally, once daily (12mg, 10mg, 8mg) on days 1–14 of a 21-day cycle. The initial drug dose of anlotinib was judged by the clinicians based on the patients’conditions. Patients with severe adverse events can gradually reduce the dose from 12mg to 8 mg. If the diseased progressed or intolerance due to adverse events, treatment was discontinued.
Efficacy Evaluation
The efficacy of anlotinib was evaluated once every two cycles. Efficacy was evaluated by computed tomography (CT) and magnetic resonance imaging (MRI) according to Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1). Objective tumor responses included complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). The DCR was defined as the addition of objective response and stabilization rates (CR+PR+SD). The primary endpoint was PFS and OS. PFS was defined as the time from the beginning of anlotinib treatment to tumor progression or patient death. OS was defined as the time from the beginning of anlotinib treatment to patient death. The secondary endpoint was ORR and DCR. The data cutoff was December 31, 2020.
Adverse Events
The adverse events were evaluated according to the Common Terminology Criteria for Adverse Events version 4.0.
Statistical Methods
The data were analyzed by SPSS 23.0, the enumeration data are expressed as rate or constituent ratio, X2 test was used for comparison between groups. The PFS and OS between the groups was analyzed by Kaplan–Meier survival curve. A P value <0.05 was considered statistically significant.
Results
Clinical Characteristics
A total of 206 patients with non-small cell lung cancer were treated, there were 142 males (68.9%) and 64 females (31.1%), ages ranged from 29 to 86 years old, with an average age of 60. The histologic subtypes were squamous cell carcinoma in 81 patients (39.3%) and adenocarcinoma in 121 patients (58.7%). 135 patients had a history of smoking (65.5%) and 71 patients (34.5%) were never-smokers, 23 patients (11.2%) had EGFR mutations, none had anaplastic lymphoma kinase (ALK) rearrangement. 89 patients (43.2%) had performance status (PS) of 0–1, 117 patients (56.8%) had PS of 2. All patients were treated with anlotinib, ninety-one patients (44.2%) received anlotinib as third-line therapy and 100 patients (48.5%) as further-line treatment. 28 patients (13.6%) were pathologically staged as stage III, 178 patients (86.4%) were clinically staged as stage IV, 122 patients (59.2%) were treated with anlotinib alone, 84 patients (40.8%) received combined therapy with anlotinib. 36 patients (17.5%) had previously received thoracic radiotherapy, 170 patients (82.5%) had not received thoracic radiotherapy. There were 104 patients (50.5%) who received antivascular drug therapy including bevacizumab, endostar, and apatinib. The baseline characteristics are shown in Table 1.
 |
Table 1 Baseline Characteristics of Patients |
Clinical Efficacy
Kaplan–Meier survival analysis indicated that the mPFS of all patients was 4 (95% CI: 3.607–4.393) months. (Figure 1A). Univariate analysis revealed that the group of patients with longer PFS included EGFR-negative, ECOG PS≤1, no brain metastases, no liver metastases, no adrenal metastases, ≤2 distant metastases (Figure 1B–G). There were no significant correlations among gender (p=0.548), age (p=0.554), smoking history (p=0.572), pathological type (p=0.312), pathological stage (p=0.180), previous EGFR-TKI treatment (p=0.184), previous thoracic radiation therapy (p=0.453), previous antiangiogenic treatments (p=0.296), bone metastases (p=0.076), pleural metastases (p=0.457)(Table 2). Cox regression analysis demonstrated that EGFR mutation and ECOG PS could significantly affect the PFS of patients, patients with EGFR mutation-negative and ECOG PS ≤1 had longer PFS (Table 3). The mOS was 8 (95% CI: 6.495–9.505) months. (Figure 2A), and univariate analysis revealed that the following subgroups of patients had longer OS (P<0.05): EGFR mutation-negative, previous thoracic radiation therapy, without brain metastases, ≤2 distant metastases (Figure 2B–E). Sex, age, smoking history, pathology, ECOG PS, clinical stage, previous EGFR-TKI treatment, previous antiangiogenic treatments, liver metastases, bone metastases, adrenal metastases, pleural metastases had no influence on OS (Table 4). The four significant factors were subsequently analyzed by Cox proportional hazards regression analysis. The results of Cox regression indicated that only the patients without previous thoracic radiation therapy (hazard ratio: 1.855; 95% CI: 1.162–2.960; P = 0.010) had shortened OS (Table 5). The ORR was 10.2%, and the DCR was 78.2%.
 |
Table 2 Univariate Analysis of PFS |
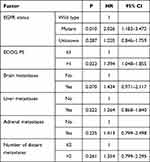 |
Table 3 Cox Regression of PFS |
 |
Table 4 Univariate Analysis of OS |
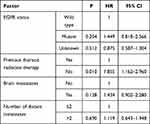 |
Table 5 Cox Regression of OS |
Safety
All patients completed the toxicity assessment. The incidence of grade 3 toxicity was 33.5% (69/206). The common grade 3 adverse events were hand-foot syndrome 30 (14.6%), hypertension 20 (9.7%) and hemoptysis 9 (4.4%). No grade 4 adverse events were observed in all patients. None of the patients stopped using anlotinib because of treatment-related adverse events. 35 patients needed dose reductions because of hypertension and hand-foot syndrome. The adverse events is listed in Table 6.
 |
Table 6 Treatment-Related Adverse Events |
Discussion
Lung cancer is the most frequent cause of cancer-related deaths worldwide, non–small cell lung cancer (NSCLC) account for 80% to 85% of lung cancers, and majority of patients present with locally advanced or metastatic disease at time of diagnosis.11 Platinum-based systemic chemotherapy is the standard treatment to control lung cancer. However, it is heterogeneity that results in the development of drug resistance. Many patients have progressed after chemotherapy because of multiple drug resistance (MDR) of tumor cells.12 Furthermore, many patients can not tolerate the adverse reactions of chemotherapeutic drugs, resulting in poor treatment efficacy and poor prognosis. With the rapid development of cancer treatment strategies, therapies for lung cancer are entering a new era of therapeutic strategies with targeted therapy and immunotherapy. Patients initially achieve some clinical benefits, followed by disease progression. Although the survival of patients with positive driving genes and positive PD-L1 has been greatly improved, after their applying targeted therapy for 10 to 18 months, most of them suffer from secondary gene mutations or abnormal signal pathways, which contribute to drug resistance.13,14 The patients with negative driving gene and low expression of PD-L1 have poor efficacy of targeted therapy and immunotherapy. In addition, targeted therapy and immunotherapy may bring serious side effects. Therefore there is an urgent need for a kind of drugs with efficacy and safety. At present, the commonly used treatment is anti-angiogenic drugs alone or in combination with other drugs. Anlotinib is a novel small molecule multi-target tyrosine kinase inhibitor, which can effectively inhibit tumor angiogenesis and promote tumor vascular normalization. In the real world, the clinical efficacy and safety of anlotinib remains uncertain. The results of this study show that anlotinib is effective in patients with advanced NSCLC and the adverse effects can be tolerated.
The ALTER 0302 trail is a Phase II clinical study, have demonstrated the efficacy and safety of anlotinib for the first time.15 In the ALTER 0303 Phase III trail, anlotinib as third-line and further therapy is well tolerated and offers significantly improved PFS (5.4 months vs 1.4 months, P<0.001) and OS (9.6 months vs 6.3 months, P<0.002) compared with placebo among Chinese patients.7 This study included 206 patients with advanced non-small cell lung cancer. The primary endpoints were PFS and OS, secondary endpoints were ORR, DCR and adverse events. The results showed that the mPFS of advanced NSCLC patients was 4 (95% CI: 3.607–4.393) months, and the mOS was 8 (95% CI: 6.495–9.505) months, the ORR was 10.2% and the DCR was 78.2%. The results of this study show that the mOS and mPFS of patients are worse than those of previous studies.7,15 The explanations considered here were as follows. The first is the difference in ECOG PS. Previous studies have shown that ECOG PS is an important factor for predicting the prognosis of patients with NSCLC.16 There were more patients with PS score > 1 included in our study. The second is the clinical stage of patients. Clinical stage is an important factor affecting the survival of patients, and the proportion of patients with IV stage included in this study is up to 86%.
A study suggested that patients with EGFR-positive were more sensitive to antiangiogenic drugs.17 The results of ALTER 0303 trail showed that EGFR mutations did not affect mPFS and mOS. In our study, the PFS of EGFR-positive patients appeared worse than that for EGFR-positive patients. There are probably many reasons for this result. First of all, the proportion of EGFR-positive patients in this study is small, and there are only 23 EGFR-positive patients in our study. It was inadequate to draw conclusions in terms of the efficacy of anlotinib. And most of EGFR-positive patients are complicated with brain metastases (69%) or liver metastases (43%), resulting in poor OS. Secondly, further analysis of the baseline characteristics of patients showed that the EGFR positive patients whose KPS score was low and they are all older than that with EGFR positive patients. And 15 (7.3%) patients were adjusted to 8 mg because they could not tolerate the toxicity of anlotinib, which may cause the OS and PFS of patients to be worse than those of EGFR negative patients. Thirdly, Zhao et al found that EGFR wild-type is resistant to radiation, while EGFR mutant is more sensitive to radiation.18 Related studies have proved that the use of upfront radiotherapy can improve OS and intracranial PFS (iPFS) in EGFR positive patients with brain metastases.19 Because of the side effects of whole brain radiotherapy on neurocognitive function, most patients with EGFR mutations in this study were treated with EGFR-TKIs previously. After first-line or second-line treatment, salvage craniocerebral radiotherapy is considered only when the intracranial lesions progressed or accompanied with severe brain metastases symptoms, which may lead to the shortening of OS in EGFR positive patients in this study. Finally, the methods used for detection of EGFR mutations may affect the results. As we all know, liquid biopsy technique is not sensitive to biopsy tissue. In our study, most of patients performed liquid biopsy rather than using biopsy tissue, which may affect the accuracy of the final results. In addition, the proportion of EGFR status unknown patients in this study was up to 70.4%, and many of their EGFR status may be EGFR positive. All of the above reasons may affect the OS and PFS of patients.
Previous studies have shown that liver and brain metastases may be adverse prognostic factors for patients with lung cancer.20,21 Univariate analysis also showed that brain metastases and liver metastases were latent risk factors affecting the survival of patients with lung cancer. A retrospective study showed that ECOG PS score, smoking history and age were predictors of treatment efficacy of anlotinib.16 This retrospective study also identified a poor PS as independent negative predictors of OS, smoking history and age did not significantly influence mOS. IMpower150 trail have shown that immunotherapy combined with chemotherapy and antivascular therapy is more effective than chemotherapy combined with bevacizumab in patients with advanced non-squamous cell NSCLC.22 Beyond and Revel have also shown that the combination of antiangiogenic drugs and chemotherapy can significantly prolong PFS and OS in patients with advanced NSCLC compared with chemotherapy alone.8,9 At present, some studies have shown that anti-angiogenic drugs can activate the immune system and overcome resistance to immunotherapy, as well as play a synergistic anti-tumor effect with immunotherapy.23 Impower 150 study significantly prolonged OS in patients with liver metastases and EGFR/ALK gene mutation, which provided a new choice for first-line treatment of advanced non-squamous NSCLC.22 The JVDF study is a Phase I clinical study that evaluated the efficacy and safety of ramucirumab combined with pembrolizumab with previously treated advanced NSCLC.24 The results showed a manageable safety profile, the ORR was 42.3%, DCR was 84.6%, and the mPFS was 9.3 months. The study also showed that immune treatment combined with anti-angiogenesis therapy was more effective in patients with high expression of PD-L1. In our study, no significant difference was seen in PFS between patients treated with anlotinib alone and those treated with combination therapy, but the mOS of patients in combined treatment group was 11 months, while patients treated with anlotinib alone was only 7 months. The mOS of patients treated with combination therapy was significantly prolonged, although there is no difference in statistics, we believe that anlotinib combined with immunotherapy or chemotherapy may also be an option for the patients with advanced NSCLC. The small sample size could account for the failure of statistical significance, only 27 patients were included in the combination immunotherapy group and 57 patients in the combination chemotherapy group. The efficacy of anlotinib combined with chemotherapy and immunotherapy in the future is worthy of further discussion. In addition, previous thoracic radiation therapy was also an important independent prognostic factor in this study. Univariate and multivariate analysis show that when the patients had previous thoracic radiation therapy, the mOS of patients was 16 months, which was much longer than that of patients without previous thoracic radiation therapy (7 months), the difference is statistically significant (P<0.009). However, previous studies did not draw a significant conclusion.25 Previous studies have shown that brain metastases is an important factor affecting the poor prognosis of patients with lung cancer.21 In a retrospective study, 2 patients who received underwent local radiotherapy during the treatment of anlotinib had a positive intracranial response.26 In our study, 37 patients with brain metastases were further analyzed, and the conclusions were as follows: the mOS of all patients with brain metastases was 7 months, of which the mOS of patients treated with anlotinib alone was 7 months, and that of patients with combined chemotherapy or immunotherapy was 10 months, the difference is statistically significant (P=0.002). It is suggested that the efficacy of combined treatment of patients with brain metastases is better. The results of the II phase clinical study of anlotinib combined with WBRT in the treatment of advanced non-small cell lung cancer patients with brain metastases were reported at the annual meeting of the European Society for Medical Oncology 2020.27 The intracranial ORR and DCR were 60% and 90% respectively, the iPFS was 87.5% at 6 months, and the median extracranial PFS was 8.7 months, and the adverse reactions were controllable, suggesting that anlotinib combined with WBRT provides a new treatment option for patients with multiple brain metastases in NSCLC. However, the sample size of patients in this study was small, and a total of 20 patients were included in this study. In our study, there were 47 patients with brain metastases, of which 11 patients received simultaneous craniocerebral radiotherapy. The results showed that local craniocerebral radiotherapy or whole brain radiotherapy could prolong the mOS of patients with NSCLC (5 months VS 7 months, P=0.001). Although the sample size was small, the results showed that patients with NSCLC complicated with brain metastases might obtain additional survival benefits during local craniocerebral radiotherapy or whole brain radiotherapy with anlotinib.
Anlotinib is a novel small molecule multi-target tyrosine kinase inhibitor, and the most common adverse reaction during treatment is hypertension and hand-foot syndrome.6 The common adverse events in our study were fatigue, followed by hand-foot syndrome and hypertension. The common grade 3 AEs were hand and foot syndrome and hypertension. The incidence of hemoptysis was 46 (22.3%) and grade 3 was 9 (4.4%). The incidence of hemoptysis is higher than that of previous studies, which may be due to the fact that the proportion of patients with squamous cell carcinoma is 39.3%. No patient suffered from grade 4 side effects. No death related to the treatment of anlotinib. The serious grade 3 AEs were manageable with supportive treatment or with dose reduction.
The limitations of this study are as follows: first, this study is a single-center retrospective study. The sample size is small, so the results might be baised and need the support of prospective clinical studies with large sample size. Secondly, EGFR status was unknown in 70% of the total population, so it is impossible to determine the efficacy of mutation state on the curative effect. Then, quality of life was not evaluated in detail. Finally, we did not pay attention to the treatment after anlotinib resistance, which may affect the OS of patients.
Conclusion
In conclusion, we elucidated the efficacy and safety of anlotinib in a real-world setting. Anlotinib is effective in the treatment of advanced NSCLC, which can bring longer survival benefits for the patients whose ECOG PS is 0–1 and patients who have previously received thoracic radiotherapy. Patients with brain metastases may get additional survival benefits during treatment with anlotinib combined with craniocerebral radiotherapy. Anlotinib combined with chemotherapy or immunotherapy is effective and the toxicity is tolerable. Further studies are needed to demonstrate the efficacy and safety of anlotinib combined with WBRT.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of The Affiliated Cancer Hospital of Nanchang University and conformed to the provisions of the Declaration of Helsinki. Since the study is a retrospective study, it did not damage the health and interests of patients, and protected their privacy and personal information, the study is exempted from informed consent.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A, et al. Cancer Statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Nagasaka M, Gadgeel SM. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev Anticancer Ther. 2018;18(1):63–70. doi:10.1080/14737140.2018.1409624
4. Priti SH, Jeffrey JW, Christoph M, et al. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2017;52(2):117–124. doi:10.1016/j.semcancer.2017.12.002
5. Lin B, Song X, Yang D, et al. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFR-βand FGFR1. Gene. 2018;654(undefined):77–86. doi:10.1016/j.gene.2018.02.026
6. Syed YY. Anlotinib: first Global Approval. Drugs. 2018;78(10):1057–1062. doi:10.1007/s40265-018-0939-x
7. Han B, Li K, Wang Q, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA ONCOL. 2018;4(11):1569–1575. doi:10.1001/jamaoncol.2018.3039
8. Zhou C, W YL, Chen G, et al. BEYOND: A Randomized, double-blind, placebo-controlled, multicenter, phase iii study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33(19):2197–2204. doi:10.1200/JCO.2014.59.4424
9. Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665–673. doi:10.1016/S0140-6736(14)60845-X
10. Georganaki M, Hooren L, Dimberg A. Vascular targeting to increase the efficiency of immune checkpoint blockade in cancer. Front Immunol. 2018;9(undefined):3081. doi:10.3389/fimmu.2018.03081
11. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. doi:10.3322/caac.21565
12. Wang D, Wang W, Zhu B, Wang X. Lung cancer heterogeneity and new strategies for drug therapy. Annu Rev Pharmacol Toxicol. 2018;58(1):531–546. doi:10.1146/annurev-pharmtox-010716-104523
13. Lin JJ, Shaw AT. Resisting resistance: targeted therapies in lung cancer. Trends Cancer. 2016;2(7):350–364. doi:10.1016/j.trecan.2016.05.010
14. Morgillo F, Della Corte CM, Fasano M, et al. Mechanisms of resistance to EGFR- targeted drugs: lung cancer. ESMO Open. 2016;1(3). doi:10.1136/esmoopen-2016-000060.
15. Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302. Br J Cancer. 2018;118(5):654–661. doi:10.1038/bjc.2017.478
16. Yang SJ, Zhang W, Chen Q, et al. Clinical investigation of the efficacy and safety of anlotinib with immunotherapy in advanced non-small cell lung cancer as third-line therapy: aretrospective study. Cancer Manag Res. 2020;12:10333–10340. doi:10.2147/CMAR.S280096
17. Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):625–635. doi:10.1016/S1470-2045(19)30035-X
18. Zhao L, Cai X, Chen D, et al. Therapeutic effect of whole brain radiotherapy on advanced NSCLC between EGFR TKI-naïve and TKI-resistant. Radiation Oncol. 2020;15:1. doi:10.1186/s13014-019-1454-2
19. Yu X, Fan Y. Real-world data on prognostic factors for overall survival in egfr-mutant non-small-cell lung cancer patients with brain metastases. J Cancer. 2019;10(15):3486–3493. doi:10.7150/jca.30292
20. Castañón E, Rolfo C, Viñal D, et al. Impact of epidermal growth factor receptor (EGFR) activating mutations and their targeted treatment in the prognosis of stage IV non-small cell lung cancer (NSCLC) patients harboring liver metastases. J Transl Med. 2015;257(13).
21. Sørensen JB, Hansen HH, Hansen M, et al. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol. 1988;6(9):1474–1480. doi:10.1200/JCO.1988.6.9.1474
22. Reck M, Mok T, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7(5):387–401. doi:10.1016/S2213-2600(19)30084-0
23. Ciciola P, Cascetta P, Bianco C, et al. Combining immune checkpoint inhibitors with anti-angiogenic agents. J Clin Med. 2020;9(3):675. doi:10.3390/jcm9030675
24. Herbst RS, Arkenau HT, Santana-Davila R, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol. 2019;20(8):1109–1123. doi:10.1016/S1470-2045(19)30458-9
25. Shao L, Wang W, Song Z, et al. The efficacy and safety of anlotinib treatment for advanced lung cancer. Onco Targets Ther. 2019;12:6549–6554. doi:10.2147/OTT.S205674
26. Yao J, Wang Z, Sheng J, et al. Efficacy and safety of combined immunotherapy and antiangiogenic therapy for advanced non-small cell lung cancer: atwo-center retrospective study. Int Immunopharmacol. 2020;89. doi:10.1016/j.intimp.2020.107033
27. Zhu X. Anlotinib combined with whole brain radiation therapy (WBRT) for advanced non-small lung cancer with multiple brain metastases: An open-label, single-arm phase II trial. ESMO. 2020;1386p. doi:10.1016/j.ijrobp.2020.03.038
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


