Back to Journals » Drug Design, Development and Therapy » Volume 17
Efficacy and Safety of a Subanesthetic Dose of Esketamine Combined with Propofol in Patients with Obesity Undergoing Painless Gastroscopy: A Prospective, Double-Blind, Randomized Controlled Trial
Authors Zheng L , Wang Y, Ma Q, Liang W, Zhang X, Ren Z, Qin W, Meng F, Li Y , Fan G , Yin N
Received 14 February 2023
Accepted for publication 24 April 2023
Published 4 May 2023 Volume 2023:17 Pages 1347—1356
DOI https://doi.org/10.2147/DDDT.S408076
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Longbin Zheng,1,* Yiteng Wang,2,* Qing Ma,1,* Wenbo Liang,1 Xiaojing Zhang,1 Zhiqiang Ren,1 Weimin Qin,1 Fan Meng,1 Yuhong Li,1 Guoxiang Fan,1 Ning Yin1
1Department of Anesthesiology, Sir Run Run Hospital, Nanjing Medical University, Nanjing, Jiangsu Province, People’s Republic of China; 2Department of Anesthesiology, Xinchang County People’s Hospital, Xinchang, Zhejiang Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ning Yin, Department of Anesthesiology, Sir Run Run Hospital, Nanjing Medical University, No. 109 Longmian Avenue, Nanjing, Jiangsu Province, 211112, People’s Republic of China, Email [email protected]
Purpose: Patients with obesity are more susceptible to hypoxemia. Anesthetic management for patients with obesity undergoing painless gastroscopy presents a severe challenge for anesthesiologists. Esketamine is a NMDA antagonist that has been proven to be beneficial for ameliorating respiratory depression owing to its sympathomimetic effect; however, there are no relevant reports on its use in patients with obesity. We designed a randomized controlled trial to evaluate whether esketamine can be the ideal adjuvant to propofol sedation in patients with obesity undergoing painless gastroscopy.
Patients and Methods: A total of 104 patients with obesity undergoing painless gastroscopy were randomly divided into group C (propofol+saline) and group S (propofol+esketamine 0.25 mg/kg). Anesthesia was induced by 2 mg/kg propofol with saline or esketamine. The consumption of propofol, hemodynamic parameters, duration of procedure, induction time, postoperative awakening time, and orientation recovery time were recorded. Adverse events and satisfaction scores were also recorded.
Results: Propofol consumption was 274.4± 22.6 mg and 201.3± 16.6 mg in groups C and S, respectively. The induction time of groups C and S were 25.4± 2.3 s and 17.8± 1.9 s, respectively. The postoperative awakening times of groups C and S were 6.2± 1.1 min and 4.8± 1.3 min, respectively. Hemodynamic parameters were more stable in group S than in group C. The incidence of adverse events such as injection pain, hypoxemia, hypotension, bradycardia, choking, and body movement were significantly lower in group S. The satisfaction scores of the endoscopist and anesthesiologist were (4.58± 0.49 vs 3.71± 0.83) and (4.75± 0.44 vs 3.33± 0.92), respectively.
Conclusion: The combination of propofol and esketamine (0.25 mg/kg) improves the safety and reduces the incidence of adverse events in patients with obesity during painless gastroscopy. Thus, this method is worthy of clinical application.
Clinical Trials Registration: ChiCTR 2200062547.
Keywords: esketamine, propofol, patients with obesity, painless gastroscopy
Corrigendum for this paper has been published.
Introduction
Painless gastroscopy has become a widely used approach for the diagnosis and treatment of digestive tract diseases.1 Propofol is the most commonly used anesthetic in painless gastroscopy due to the advantage of rapid onset and recovery.2 However, an increased dose is needed to fulfill the anesthetic requirement as propofol has no analgesic effect, which increases the risk of dosage-dependent complications such as hypoxemia and hypotension.3 Even though opioids are widely applied in perioperative analgesia clinically, the inhibitory effect of respiration leads to a high risk in clinical application.4
With the improvement in health awareness, more patients with obesity opt to have painless gastroscopy as a part of physical health examination. Patients with obesity are more susceptible to hypoxemia due to the reduced functional residual volume and the fat deposition caused by airway narrowing.5 The focus of anesthetic management for patients with obesity in painless gastroscopy is to ensure safety of the airway, which presents a severe challenge for anesthesiologists. Therefore, searching for a safe and effective anesthetic protocol for patients with obesity remains an urgent task.
Esketamine, a right-lateral dismantled fission of ketamine, is the N-methyl-D-aspartic acid (NMDA) antagonist that can provide unique dissociative anesthesia. The anesthetic effect of esketamine is twice as potent as ketamine and its potency is approximately three times higher than ketamine, which is related to its stronger effect on the NMDA-receptor.6 Due to the better analgesic effect and higher clearance rate, esketamine shows a lower incidence of side reaction than ketamine at the equivalent analgesic dose, resulting in a faster recovery.7 Recent studies have suggested that the application of esketamine is beneficial for ameliorating hemodynamic and respiratory depression due to the sympathomimetic effect,8 while there is no relevant report for patients with obesity. Owing to advantages such as short elimination half-life, rapid awakening, and less respiratory depression,7 esketamine may be the ideal adjuvant to propofol sedation in painless gastroscopy procedures.
In this study, we performed a prospective, double-blind, randomized controlled trial to determine the efficacy of esketamine versus placebo combined with propofol in patients with obesity undergoing painless gastroscopy. No previous study has evaluated the effect of esketamine on patients with obesity. Therefore, this is the first research focused on the efficacy and safety of esketamine combined with propofol in patients with obesity undergoing painless gastroscopy. We speculate that the combination of esketamine and propofol may be an optimized protocol with fewer adverse events.
Methods
Ethics and Trial Registration
The study protocol was approved by the Medical Ethics Committee of the Sir Run Run Hospital, Nanjing Medical University (Ethics Number: 2021-SR-031), and the study was registered in the Chinese Clinical Trial Registry (ChiCTR 2200062547; 08/11/2022). All participants signed an informed consent form. This study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines for Good Clinical Practice. Written informed consent was obtained from all participants.
Inclusion and Exclusion Criteria
A prospective, double-blind, randomized controlled trial was performed in Sir Run Run Hospital between August 12, 2022, and February 10, 2023. Patients scheduled for painless gastroscopy were eligible for participation in this trial if they were 1) 18–64 years old; 2) had an American Society of Anesthesiologists (ASA) physical status I or II; 3) had a body mass index (BMI) ≥28 kg/m2.
The exclusion criteria were as follows: 1) severe cardiovascular or cerebrovascular disease; 2) digestive tract obstruction; 3) respiratory infection; 4) history of diabetes, hypertension, or hypotension; 5) severe snoring and sleep apnea syndrome; 6) history of opioid and esketamine addiction; 7) allergy to drugs used in the present study; 8) psychosocial disease or cognitive dysfunction.
Randomization and Blinding
All patients provided written informed consent, and were then divided into two groups using a computer-generated random number table via restricted randomization. With this list kept in a sealed envelope so that only nursing staff members without any relation to the research could access them. According to the randomization results, the patients were assigned to either group C or group S. In group C, patients were injected with normal saline placebo and propofol (2 mg/kg); in group S, patients were injected with esketamine (0.25 mg/kg) and propofol (2 mg/kg). To maintain blinding to the research, an independent research nurse prepared and distributed the medications in identical syringes labelled with study numbers only.
Study Interventions
All patients routinely fasted before the operation, and no preoperative medication was given. After entering the operation room, venous access was established immediately, and the left lateral position was taken. Noninvasive blood pressure (NIBP), electrocardiogram (ECG), heart rate (HR), and pulse oxygen saturation (SPO2) were routinely monitored. Oxygen was administered at 2–4 L/min via a nasal cannula.
For induction of anesthesia, a bolus intravenous injection of esketamine (0.25 mg/kg) or the same volume of normal saline placebo was administered, followed by immediate intravenous injection of propofol (2 mg/kg). When the eyelash reflex disappeared and there was no significant body movement, the endoscopist began the endoscopy. The level of sedation was assessed using the Ramsay Sedation Scale. Additional doses of propofol (0.5 mg/kg) were administered to achieve a Ramsay sedation score of 5 or 6 and to treat stress responses such as body movement and choking. Once the SpO2 fell below 95%, procedures for opening the airway, such as head-tilt, chin lift, and jaw-thrust, were performed instantly by the anesthesiologist. If the SpO2 fell below 90% and hypoxemia had not ameliorated following the treatments, the operation was stopped immediately, and the gastroscope was removed. Then, pure oxygen was administered via a mask, with positive pressure ventilation if necessary. Ephedrine 6 mg was injected when SBP was below 30% of the baseline level, and atropine 0.5 mg was injected when HR was below 45/min. Other perioperative adverse events were recorded and managed in accordance with clinical operation standards.
Upon completion of endoscopic examinations, patients were transferred to the recovery room for at least 30 min. The patients could leave the operating room when they were fully awake, had a PADSS score ≥9, and had stable vital signs.
This study was designed as a prospective, double-blind, randomized controlled trial. All patients received intravenous sedation by the same anesthesiologist, and all data were collected by another anesthesiologist, both of whom were unaware of the patient allocation group. All procedures were performed by the same endoscopist.
Outcomes
The consumption of propofol was recorded. Systolic blood pressure (SBP), diastolic blood pressure (DBP), HR, and SPO2 were recorded before (T1) and immediately after anesthesia induction (T2), immediately before (T3) and after (T4) gastroscope insertion, and at the end of the surgery (T5). The duration of the procedure (insertion of gastroscope to withdrawal of gastroscope), induction time (anesthesia induction to disappearance of eyelash reflex), postoperative awakening time (the end of operation to consciousness return), and orientation recovery time (consciousness return to normal walking) were recorded. Adverse events included injection pain, hypoxemia (SpO2 <90% for ≥10 s), body movement, choking, bradycardia, hypotension (blood pressure <30% of the basal blood pressure), nausea, vomiting, dizziness, and delirium. The satisfaction of the endoscopist and anesthesiologist was recorded. Satisfaction was evaluated using a 5-point Likert scale (1=very dissatisfied, 5=highly satisfied).
Statistical Analysis
SPSS 26.0 (SPSS, Inc., IL, USA) was used for all statistical analyses. The normality of the data distribution was examined by the Shapiro–Wilk test. Normally distributed data are expressed as mean ± standard deviation (SD), while non-normally distributed data are expressed as median and interquartile range. Data were compared between groups using unpaired t-tests, chi-squared tests, Fisher’s exact test, or Kruskal–Wallis one-way ANOVAs with Dunn’s multiple comparison test, as appropriate. P values<0.05 were considered statistically significant.
Results
Between August 2022 and February 2023, 891 patients were assessed for eligibility, and 778 were excluded before randomization. Overall, 113 patients were randomly allocated: 58 in group C and 55 in group S. Among these, nine patients were dropped from the analysis: two were excluded for having a cold and seven for withdrawing consent. Thus, 104 patients were finally analyzed in our study, and detailed participant information is shown in Figure 1.
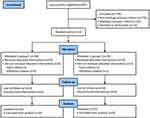 |
Figure 1 Study population flow diagram. |
Patient Inclusion and Characteristics
Figure 1 shows the flow diagram of this study. A total of 104 patients met the inclusion criteria and were randomized into two groups. The demographic characteristics and baseline values of the patients are shown in Table 1. There were no significant differences in the characteristics, comorbidities, and baseline values of patients between the two groups.
 |
Table 1 The Demographic Characteristic of Patients |
The Duration of Procedure and Propofol Consumption
In group C, the average duration was 7.1±0.9 min, with longest duration of 10.8 min and shortest duration of 5.2 min. In group S, the average duration was 6.8±1.1 min, with longest duration of 11.1 min and shortest duration of 4.8 min. There was no statistical difference in the duration of the procedure between the two groups (P>0.05) (Table 2). Propofol consumption was 274.4±22.6 and 201.3±16.6 mg in groups C and S, respectively. Propofol consumption in group S was significantly lower than that in group C (P<0.05) (Table 2).
 |
Table 2 The Duration of Procedure and Propofol Consumption |
Evaluation of Anesthesia-Related Indices
The induction time of groups C and S were 25.4±2.3 s and 17.8±1.9 s, respectively. The induction time was significantly shorter in group S than in group C. The postoperative awakening times of groups C and S were 6.2±1.1 min and 4.8±1.3 min, respectively. The postoperative awakening time in group S was significantly shorter than that in group C. There was no significant difference in orientation recovery time between the two groups (Table 3).
 |
Table 3 The Evaluation of Anesthesia-Related Indices |
Hemodynamic Results
There was no significant difference in SBP, DBP, HR, and SpO2 between the two groups at T1 (P>0.05). HR, SBP, DBP, and SpO2 were significantly lower at T2, T3, and T4 in group C than in group S (P<0.05). SpO2 was significantly lower at T5 in group C than in group S (P<0.05). There was no significant difference in SBP, DBP, and HR between the two groups at T5 (P>0.05) (Figure 2).
Incidence of Adverse Events
There were no significant clinical complications during the study. The incidences of injection pain, hypoxemia, hypotension, bradycardia, choking, and body movement were significantly lower in group S compared with group C (P<0.05). No nausea, vomiting, dizziness, or delirium was observed in either group (Table 4).
 |
Table 4 Incidence of Adverse Events |
The Satisfactions of Endoscopist and Anesthesiologist
The satisfaction score of endoscopist (4.58±0.49 vs 3.71±0.83) and anesthesiologist (4.75±0.44 vs 3.33±0.92) were significantly higher in group S compared to group C (Figure 3).
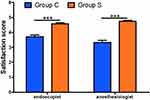 |
Figure 3 The satisfactions of endoscopist and anesthesiologist. ***P<0.001. |
Discussion
In the present study, our results suggest that the combination of esketamine and propofol maintains hemodynamic stability and decreases the consumption of propofol and incidence of respiratory depression, as well as shortens the induction time and awakening time in patients with obesity undergoing painless gastroscopy. Esketamine combined with propofol could also improve the satisfaction scores of endoscopist and anesthesiologist. Taken together, our data demonstrate that the combination of esketamine and propofol might be a more effective and safer anesthesia protocol for patients with obesity in painless gastroscopy. No previous study evaluated the effect of esketamine on patients with obesity. Therefore, this is the first research focused on the efficacy and safety of esketamine in patients with obesity undergoing painless gastroscopy, which has a great clinical significance and is worthy of further clinical application.
It is well known that there is a high risk of airway management in patients with obesity due to the altered airway anatomy, including limited neck extension, shortened neck, and fat accumulation in the pharyngeal wall.5 Due to its lipophilic properties, the distribution volume of propofol is higher in patients with obesity and a higher dose of propofol is required to reach the sedative level.9 Therefore, anesthetic management for patients with obesity in painless gastroscopy presents a challenge to anesthesiologists. It is necessary to seek an effective and safe anesthetic agent combined with propofol for patients with obesity undergoing painless gastroscopy. Esketamine, as the right-lateral dismantled fission of ketamine, is a NMDA receptor antagonist that can produce unique dissociative anesthesia and display an analgesic effect twice that of ketamine.6 Previous research has suggested that esketamine can reduce adverse events such as hypotension and hypoxemia in painless gastrointestinal endoscopy;8 however, there is no relevant report for patients with obesity. According to previous data,10 a subanesthetic dose of esketamine was selected to evaluate the anesthetic effect in patients with obesity undergoing painless gastroscopy in the present study.
It has been suggested that esketamine can attenuate nociceptive stimuli and inhibit nociceptive transmission via dissociative anesthesia, therefore reducing the dosage of anesthetics.11 The results of this study suggest that the additional use of esketamine could effectively reduce the requirement for propofol, which is consistent with the results of previous studies. Eberl et al found that the combination of low-dose esketamine (0.15 mg/kg) and propofol reduced propofol consumption significantly in patients undergoing endoscopic retrograde cholangiopancreatography (ERCP).12 Our results showed that the incidence of stress response, induction time, and awakening time were lower in group S than in group C. This may be attributed to the synergistic effect of the two agents on different receptors.13 Propofol exerts the anesthetic role mainly by activating GABA receptors, while esketamine exhibits both sedative and analgesic effects primarily by inhibiting NMDA receptors.8 The synergistic effect of drug combination leads to the reduction of propofol consumption, and provides a more efficient and safe anesthetic management.
Hemodynamic fluctuations is the most frequent intraoperative adverse event in painless gastroscopy.14 Zhan et al reported that there was no significant difference with or without esketamine administration (0.05, 0.1 and 0.2 mg/kg) in terms of hemodynamic characteristics in painless gastroscopy.15 However, the hemodynamics were more stable as the doses of esketamine were 0.25 and 0.5 mg/kg.16 This might be because the dosages of esketamine were low, and the sympathetic excitatory effect of esketamine may be offset by cardiovascular depression of propofol.15 We recorded hemodynamic characteristics and found no significant difference between the groups before operation. However, HR, SBP, and DBP decreased immediately after anesthesia induction in group C. With the addition of esketamine, the decreasing trends mentioned above disappeared, and the patients exhibited more stable vital signs, which is similar to the results of previous studies,17 and the sympathetic excitatory effect of esketamine may be the reason for the stable hemodynamic.
Our data showed that the combination of esketamine and propofol shortened the induction time in this study. However, previous studies have shown that there was no difference in induction time between propofol alone and ketamine combined with propofol in painless gastroenteroscopy.18 The higher potency of esketamine compared with ketamine might be crucial for the shorter induction time. Some studies have suggested that esketamine has no significant effect on the awakening time of patients sedated with propofol,15 which is inconsistent with the findings of this study. Our data showed that the awakening time of patients sedated with propofol was significantly shortened by esketamine administration. This may be due to the fact that the propofol requirement of patients with obesity was greater in group C, and administration of esketamine significantly reduced the propofol dosage.
Patients with obesity are more vulnerable to hypoxemia due to a greater dose requirement of propofol, and fat deposition around the neck narrows the upper airway.19,20 In severe cases, intraoperative oxygen desaturation can threaten the safety of patients.21,22 Therefore, it is of great clinical value to prevent intraoperative hypoxemia during painless gastroscopy in patients with obesity. Our data showed no significant difference in BMI between the two groups, which indicates that the risk of respiratory depression was similar in both groups. To avoid injury caused by prolonged hypoxemia, the length of observation was set at 10 s.23,24
Due to the similar pharmacologic actions as ketamine, esketamine has sympathomimetic effects on bronchial smooth muscle and results in bronchodilation, thus improving respiratory depression,8 as demonstrated in previous research.12 Our data suggests a certain degree of decline in peripheral blood oxygen saturation in both groups after anesthesia induction. Compared with group C, the incidence of hypoxemia was significantly lower in group S, suggesting that the additional application of esketamine improved respiratory depression in patients with obesity undergoing painless gastroscopy. A possible underlying mechanism may be related to the sympathomimetic effect. Due to the increased sympathetic tone, voluntary breathing and airway reflexes are maintained, and esketamine therefore stabilizes breathing.25 Furthermore, propofol is related to a higher risk of respiratory inhibition in a dose-dependent manner.26 Hence, the reduced dosage of propofol is another key factor for the lower incidence of hypoxemia.
In addition, postoperative adverse reactions such as nausea, vomiting, dizziness, and delirium were analyzed, but none of them appeared in our results. A possible reason is that the patients included in this study were less than 65 years of age, and the intraoperative anesthetic dosages were appropriate. In conclusion, esketamine is safe and effective for patients with obesity undergoing painless gastroscopy.
To comprehensively verify the anesthetic effect, the satisfaction of the endoscopist and anesthesiologist during the treatment process was evaluated in this study. The data demonstrated that the satisfaction scores of the endoscopist and anesthesiologist in group S were significantly higher than those in group C, which may be due to the following reasons. First, hypoxemia occurred more frequently in group C, which resulted in a greater number of interruptions, thus reducing the coherence in the operation. Second, the postoperative awakening time was shorter, intraoperative vital signs were more stable, and the incidence of adverse effects was lower in group S, which contributed to more controlled anesthetic management. Third, the incidence of injection pain due to propofol administration was significantly lower in group S, which alleviates the anxiety and discomfort of patients.
This study had several limitations. First, the sample size was relatively small, with certain limitations and the possibility of statistical deviation. Second, even though a higher dose of esketamine may result in a more potent sedative effect and further reduce propofol consumption, the incidence of adverse effects may increase accordingly. Therefore, the optimum dosage of esketamine has yet to be determined. Finally, patients aged > 65 years were excluded from this study. We considered that the optimal sedation regimen for elderly patients should be determined according to the results of this study. In the future, prospective randomized controlled trial studies with a larger sample capacity should be performed to verify the results of this study.
Conclusions
In conclusion, the combination of propofol and esketamine was associated with a shorter induction time and awakening time, less propofol consumption, more stable hemodynamics, and lower incidence of adverse events compared with propofol alone in patients with obesity undergoing painless gastroscopy. Significant advantages were shown in terms of the satisfaction of endoscopist and anesthesiologist. Therefore, this method is worthy of further clinical application.
Data Sharing Statement
All data generated or analyzed during this study were included in the published article. Further inquiries about the datasets can be directed to the corresponding author: Professor Ning Yin on reasonable request.
Acknowledgments
Appreciate for the support from the endoscopists and nursing teams of the Sir Run Run Hospital, Nanjing Medical University.
Funding
This work was supported by the Nanjing Health Science and Technology Development Special Fund (No. YKK21259).
Disclosure
The authors have no competing interests in this work.
References
1. Xu C, He L, Ren J, et al. Efficacy and Safety of Remimazolam Besylate Combined with Alfentanil in Painless Gastroscopy: a Randomized, Single-Blind, Parallel Controlled Study. Contrast Media Mol Imaging. 2022;2022:7102293. doi:10.1155/2022/7102293
2. Qiu Y, Hou H, Zhang J, et al. The effect of preoperative sleep quality on the target plasma concentration of propofol and postoperative sleep in patients undergoing painless gastroscopy. BMC Anesthesiology. 2023;23(1):9. doi:10.1186/s12871-022-01957-2
3. Zhang J, Cairen Z, Shi L, et al. Remimazolam versus propofol for procedural sedation and anesthesia: a systemic review and meta-analysis. Minerva Anestesiol. 2022;88(12):1035–1042. doi:10.23736/S0375-9393.22.16817-3
4. Dahan A, van Lemmen M, Jansen S, et al. Buprenorphine: a treatment and cause of opioid-induced respiratory depression. Br J Anaesth. 2022;128(3):402–404. doi:10.1016/j.bja.2021.12.001
5. Shobatake R, Itaya-Hironaka A, Yamauchi A, et al. Intermittent Hypoxia Up-Regulates Gene Expressions of Peptide YY (PYY), Glucagon-like Peptide-1 (GLP-1), and Neurotensin (NTS) in Enteroendocrine Cells. Int J Mol Sci. 2019;20(8):1849. doi:10.3390/ijms20081849
6. Liu P, Zhang SS, Liang Y, Gao ZJ, Gao W, Dong BH. Efficacy and Safety of Esketamine Combined with Antidepressants for Treatment-Resistant Depression: a Meta-Analysis. Neuropsychiatr Dis Treat. 2022;18:2855–2865. doi:10.2147/NDT.S388764
7. Wang J, Huang J, Yang S, et al. Pharmacokinetics and Safety of Esketamine in Chinese Patients Undergoing Painless Gastroscopy in Comparison with Ketamine: a Randomized, Open-Label Clinical Study. Drug Des Devel Ther. 2019;13:4135–4144. doi:10.2147/DDDT.S224553
8. Jonkman K, van Rijnsoever E, Olofsen E, et al. Esketamine counters opioid-induced respiratory depression. Br J Anaesth. 2018;120(5):1117–1127. doi:10.1016/j.bja.2018.02.021
9. Dargin J, Medzon R. Emergency department management of the airway in obese adults. Ann Emerg Med. 2010;56(2):95–104. doi:10.1016/j.annemergmed.2010.03.011
10. Long YQ, Feng CD, Ding YY, et al. Esketamine as an Adjuvant to Ciprofol or Propofol Sedation for Same-Day Bidirectional Endoscopy: protocol for a Randomized, Double-Blind, Controlled Trial With Factorial Design. Front Pharmacol. 2022;13:821691. doi:10.3389/fphar.2022.821691
11. Trimmel H, Helbok R, Staudinger T, et al. S(+)-ketamine: current trends in emergency and intensive care medicine. Wien Klin Wochenschr. 2018;130(9–10):356–366. doi:10.1007/s00508-017-1299-3
12. Eberl S, Koers L, van Hooft J, et al. The effectiveness of a low-dose esketamine versus an alfentanil adjunct to propofol sedation during endoscopic retrograde cholangiopancreatography: a randomised controlled multicentre trial. Eur J Anaesthesiol. 2020;37(5):394–401. doi:10.1097/EJA.0000000000001134
13. Ebru TK, Resul K. Comparison of ketamine-propofol mixture (ketofol) and midazolam-meperidine in endoscopic retrograde cholangiopancretography (ERCP) for oldest old patients. Ther Clin Risk Manag. 2019;15:755–763. doi:10.2147/TCRM.S201441
14. Tang L, Ye C, Wang N, et al. The median effective doses of propofol combined with two different doses of nalbuphine for adult patients during painless gastroscopy. Front Pharmacol. 2022;13:1014486. doi:10.3389/fphar.2022.1014486
15. Zhan Y, Liang S, Yang Z, et al. Efficacy and safety of subanesthetic doses of esketamine combined with propofol in painless gastrointestinal endoscopy: a prospective, double-blind, randomized controlled trial. BMC Gastroenterol. 2022;22(1):391. doi:10.1186/s12876-022-02467-8
16. Yang H, Zhao Q, Chen HY, et al. The median effective concentration of propofol with different doses of esketamine during gastrointestinal endoscopy in elderly patients: a randomized controlled trial. Br J Clin Pharmacol. 2022;88(3):1279–1287. doi:10.1111/bcp.15072
17. Zhu T, Zhao X, Sun M, et al. Opioid-reduced anesthesia based on esketamine in gynecological day surgery: a randomized double-blind controlled study. BMC Anesthesiol. 2022;22(1):354. doi:10.1186/s12871-022-01889-x
18. Yin S, Hong J, Sha T, et al. Efficacy and Tolerability of Sufentanil, Dexmedetomidine, or Ketamine Added to Propofol-based Sedation for Gastrointestinal Endoscopy in Elderly Patients: a Prospective, Randomized, Controlled Trial. Clin Ther. 2019;41(9):1864–1877.e0. doi:10.1016/j.clinthera.2019.06.011
19. Zheng P, Jiang D, Liu C, et al. Nitric Oxide Inhalation Therapy Attenuates Postoperative Hypoxemia in Obese Patients with Acute Type A Aortic Dissection. Comput Math Methods Med. 2022;2022:9612548. doi:10.1155/2022/9612548
20. Zhou R, Wang HT, Gu W. Efficacy of High-Flow Nasal Cannula versus Conventional Oxygen Therapy in Obese Patients during the Perioperative Period: a Systematic Review and Meta-Analysis. Can Respir J. 2022;2022:4415313. doi:10.1155/2022/4415313
21. Meidert AS, Choukèr A, Praun S, et al. Exhaled Breath and Oxygenator Sweep Gas Propionaldehyde in Acute Respiratory Distress Syndrome. Molecules. 2020;26(1):145. doi:10.3390/molecules26010145
22. Shao LJ, Hong FX, Liu FK, Wan L, Xue FS. Prospective, randomized comparison of two supplemental oxygen methods during gastroscopy with propofol mono-sedation in obese patients. World J Clin Cases. 2021;9(20):5479–5489. doi:10.12998/wjcc.v9.i20.5479
23. Bluth T, Serpa Neto A, Schultz MJ, et al.; Writing Committee for the PROBESE Collaborative Group of the PROtective VEntilation Network (PROVEnet) for the Clinical Trial Network of the European Society of Anaesthesiology. Effect of Intraoperative High Positive End-Expiratory Pressure (PEEP) With Recruitment Maneuvers vs Low PEEP on Postoperative Pulmonary Complications in Obese Patients: a Randomized Clinical Trial. JAMA. 2019;321(23):2292–2305. doi:10.1001/jama.2019.7505
24. Kulkas A, Duce B, Leppänen T, et al. Severity of desaturation events differs between hypopnea and obstructive apnea events and is modulated by their duration in obstructive sleep apnea. Sleep Breath. 2017;21(4):829–835. doi:10.1007/s11325-017-1513-6
25. Huang X, Ai P, Wei C, et al. Comparison of the Effects of Esketamine/Propofol and Sufentanil/Propofol on the Incidence of Intraoperative Hypoxemia during Bronchoscopy: protocol for a Randomized, Prospective, Parallel-Group Trial. J Clin Med. 2022;11(15):4587. doi:10.3390/jcm11154587
26. Li X, Lv X, Jiang Z, et al. Application of Intravenous Lidocaine in Obese Patients Undergoing Painless Colonoscopy: a Prospective, Randomized, Double-Blind, Controlled Study. Drug Des Devel Ther. 2020;14:3509–3518. doi:10.2147/DDDT.S266062
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

