Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Efficacy and safety of a novel, nebulized glycopyrrolate for the treatment of COPD: effect of baseline disease severity and age; pooled analysis of GOLDEN 3 and GOLDEN 4
Authors Ohar J , Tosiello R , Goodin T, Sanjar S
Received 21 August 2018
Accepted for publication 26 November 2018
Published 18 December 2018 Volume 2019:14 Pages 27—37
DOI https://doi.org/10.2147/COPD.S184808
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Jill Ohar,1 Robert Tosiello,2 Thomas Goodin,2 Shahin Sanjar2
1Department of Internal Medicine, Wake Forest University School of Medicine, Winston-Salem, NC, USA; 2Sunovion Pharmaceuticals Inc., Marlborough, MA, USA
Background: The efficacy and safety of nebulized glycopyrrolate inhalation solution (GLY), administered twice daily (BID) via the innovative eFlow® Closed System nebulizer (PARI Pharma GmbH, Starnberg, Germany), were demonstrated in two replicate, placebo-controlled, 12-week Phase III studies (GOLDEN 3 and GOLDEN 4). This report evaluates the efficacy and safety of GLY by baseline disease severity and age in the pooled GOLDEN 3 and GOLDEN 4 patient population (N=1,294).
Methods: Patients were grouped by baseline predicted post-bronchodilator FEV1 (<50%, ≥50%) and age (<65, ≥65, ≥75 years).
Results: GLY (25 and 50 µg BID) produced significant improvements in trough FEV1 in FEV1% predicted <50% (0.070 L, 0.079 L) and ≥50% (0.112 L, 0.126 L) subgroups (P<0.01 vs placebo), and in patients aged <65 (0.056 L, 0.086 L), ≥65 (0.140 L, 0.124 L), and ≥75 (0.144 L, 0.120 L) years (P<0.05 vs placebo). St George’s Respiratory Questionnaire (SGRQ) total score was significantly improved with GLY 25 and 50 µg BID (P<0.05 vs placebo) in FEV1% predicted <50% (-3.237, -3.061) and ≥50% (-3.392, -2.322) and in <65 years (-3.447, -2.318) and ≥65 years (-3.053, -3.098) subgroups. In patients aged ≥75 years, GLY 25 µg reduced SGRQ total score by -6.278 units (P<0.01 vs placebo). The incidence of treatment-emergent adverse events was similar between GLY and placebo across all subgroups, and the overall incidence of cardiovascular events was low.
Conclusions: Nebulized GLY improved lung function and health status and was well tolerated over 12 weeks in patients with moderate-to-very-severe COPD, irrespective of baseline disease severity and age.
Clinical trial registration: NCT02347761, NCT02347774.
Keywords: age, COPD, disease severity, long-acting muscarinic antagonist, LAMA, nebulizer, nebulized glycopyrrolate
Plain language summary
COPD is a debilitating illness that results from limited airflow within the lungs. Patients with COPD have symptoms such as cough and breathlessness. About four of every ten people in the US aged 65 years or older have COPD. Aging is associated with a progressive decline in lung function. This means that older patients can have more severe disease at the time of diagnosis compared with younger patients. Bronchodilators are drugs that open up the airways, help relieve symptoms, and thereby increase the ability to carry out activities of daily living. However, older age and more severe disease can make bronchodilator therapy less effective. This may be due to these patients finding it harder to inhale medication (for example, due to muscle weakness or problems with coordination), or having other conditions (such as arthritis or dementia) that may affect the person’s ability to use the inhalation device effectively. Nebulized glycopyrrolate is a long-acting bronchodilator belonging to the LAMA (long-acting muscarinic antagonist) class of drugs, and is approved in the US for the long-term treatment of COPD. The drug is delivered through a nebulizer using normal breathing, which may be useful for patients who find it difficult to use other handheld inhalation devices. This analysis found that nebulized glycopyrrolate improved lung function and symptoms across age groups and disease severities, with a good overall safety profile.
Introduction
COPD affects a substantial proportion of older patients, with 35% of those diagnosed in the United States aged ≥65 years.1,2 Approximately 30% of patients with COPD have severe-to-very-severe airflow limitation at diagnosis.3,4 Lung function declines with age due to physiologic (eg, reduction in the strength of respiratory muscles) and anatomic (eg, loss of supporting structures in the lung parenchyma) changes in the peripheral airways, with a greater deterioration observed in patients aged >70 years.5,6 This causes a decrease in FEV1, which decreases with age to a greater extent than other commonly assessed lung volumes.7
Bronchodilators are central to pharmacologic therapy for COPD and are used clinically to relieve symptoms, reduce the frequency and severity of exacerbations, and improve health status.1 For maintenance bronchodilator monotherapy, long-acting muscarinic antagonists (LAMAs) are preferred over long-acting beta2-agonists (LABAs),1 as some LAMAs have demonstrated a reduction in exacerbation rate compared with LABAs.8,9
Importantly, advanced age and more severe airflow limitation at treatment initiation reduce the effect of bronchodilator therapy,7,10 and are associated with higher rates of device handling errors.11 The presence of fewer comorbidities was significantly associated with high physician-reported confidence in inhaler use, which in turn was associated with a more favorable COPD-related health status.12 The correct use of handheld devices, such as dry powder inhalers and metered dose inhalers, depends on several factors that may be impaired in older patients, including the ability to generate sufficient inspiratory flow, cognitive function, adequate breath/actuation coordination, dexterity, and hand strength.6,13–17
Nebulized therapy may be appropriate in patients who have difficulty using handheld devices, as drug administration occurs with normal tidal breathing. Glycopyrrolate inhalation solution (GLY; Lonhala®, Sunovion Pharmaceuticals Inc., Marlborough, MA, USA) 25 μg twice daily (BID) delivered by the innovative eFlow® Closed System nebulizer (Magnair®, PARI Pharma GmbH, Starnberg, Germany) was approved by the US Food and Drug Administration for the long-term maintenance treatment of airflow obstruction in patients with COPD in December 2017.18 The efficacy and safety was demonstrated by data from the 12-week Phase III Glycopyrrolate for Obstructive Lung Disease Via Electronic Nebulizer (GOLDEN) 3 and GOLDEN 4 studies (NCT02347761 and NCT02347774, respectively).19 This pooled subgroup analysis assessed the effect of age and disease severity (post-bronchodilator FEV1% predicted) at baseline on physiologic and symptomatic responses, as measured by lung function, health status, and safety.
Methods
Study design
GOLDEN 3 and GOLDEN 4 (Figure 1) were prospectively designed to enroll patients representative of the general COPD population by including those with very severe disease, a history of cardiovascular (CV) risk factors, and continuing background LABA therapy (limited by protocol to approximately 30% of patients), with or without additional inhaled corticosteroid therapy.
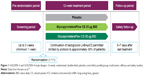  | Figure 1 GOLDEN 3 and GOLDEN 4 study designs: 12-week, randomized, double-blind, placebo-controlled, parallel group, multicenter, efficacy and safety studies. |
Albuterol (salbutamol), as rescue medication, and ipratropium bromide, as supplemental medication, were permitted.
Patients
Briefly, key eligibility criteria, reported previously,19 included males or females ≥40 years of age, current or ex-smokers with ≥10 pack-year smoking history, a clinical diagnosis of moderate-to-very-severe COPD (as defined by the GOLD 2014 Report),1 and qualifying post-bronchodilator (ipratropium 68 μg) spirometry (FEV1 ≤80% of predicted normal, FEV1 >0.7 L, and FEV1/forced vital capacity ratio <0.70).
The GOLDEN 3 (SUN101–301: project approval number 28481) and GOLDEN 4 (SUN101–302: project approval number 28482) study protocols were approved by Quorum Review IRB North American (US and Canadian) Board (Panel II) prior to patient enrollment, and were conducted in accordance with the protocols, International Council for Harmonization Good Clinical Practice guidelines, and the Declaration of Helsinki. All patients provided written informed consent.
Efficacy and safety assessments
Subgroup analyses by baseline disease severity and age were prespecified. Intent-to-treat (ITT) and safety populations pooled from GOLDEN 3 and GOLDEN 4 comprised all patients receiving at least one dose of study drug and, for efficacy, one post-dose pulmonary function assessment. Efficacy was assessed in all patients who received double-blind study drug, regardless of their completion status. Treatment effect during the time patients remained on randomized therapy was analyzed (on-treatment data). Patients who discontinued the randomized treatment before week 12 continued to be followed and were analyzed using retrieved dropout data (all collected data). Efficacy and safety analyses were performed on on-treatment and all collected data, and as the primary study results were similar for both datasets,19 only on-treatment data are presented.
The primary efficacy endpoint was change from baseline trough FEV1 at week 12. Additional efficacy endpoints included change from baseline health status measured by St George’s Respiratory Questionnaire (SGRQ) total score and SGRQ responder rate (defined as a ≥4-unit reduction in SGRQ total score) at week 12.
Treatment-emergent adverse events (TEAEs) were coded according to MedDRA v15.1. CV events of special interest were examined using standardized MedDRA query analysis and included cardiac arrhythmia, arrhythmia-related events, cardiac failure, ischemic heart disease, QT prolongation, and myocardial infarction. Other assessments in the overall population included heart rate, blood pressure, electrocardiogram (ECG), and clinical laboratory measures. Holter monitoring was conducted at visit 0 and at week 12 in a subpopulation (N=153) of GOLDEN 3.
Statistical analysis
Least square (LS) means for the difference between GLY and placebo for FEV1 were calculated using a mixed model for repeated measures, with change from baseline trough FEV1 as the response variable, and treatment group, CV risk, background LABA use, visit week, visit-week-by-treatment-group interaction, and baseline FEV1 as covariates. Change from baseline SGRQ total score at week 12, as the response variable, was assessed by analysis of covariance. ORs and CIs were computed for SGRQ responder rates. No adjustments were made for multiple treatment comparisons in this post hoc analysis. Safety data were analyzed using descriptive statistics.
All statistical procedures were performed using SAS v9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Patient demographics and baseline characteristics
Overall, 1,111/1,294 (86%) patients randomized (GOLDEN 3, N=653; GOLDEN 4, N=641) completed treatment. The mean age was 63.2 years (range: 40–87 years), and the majority of patients were male (56.0%) and Caucasian (89.6%).
Patient subgroups were defined by baseline post-bronchodilator FEV1% predicted (<50%: N=555; ≥50%: N=737) and age (<65 years: N=705; ≥65 years: N=588). The ≥75 years age group (N=126), a subgroup of the ≥65 years age group, was analyzed separately.
Background LABA use was higher in patients with FEV1% predicted <50% compared with those with FEV1% predicted ≥50%. The proportion of patients with CV risk factors was similar in all FEV1% predicted subgroups (Table 1). Background LABA use was slightly higher and a greater proportion had existing CV risk factors among patients aged ≥65 years compared with those aged <65 years (Table 2).
The overall use of inhaled respiratory medication prior to the studies was lower in the FEV1% predicted ≥50% subgroup than the FEV1% predicted <50% subgroup, with the exception of steroids, which were used by a similar proportion of patients in each subgroup. Proton pump inhibitors were used by a numerically higher proportion of patients with FEV1% predicted ≥50% compared with FEV1% predicted <50%.
Prior to entering the studies, the overall use of inhaled long-acting respiratory medications (including steroids) showed numerical increase with increasing age. Generally, short-acting beta2-agonist use was higher in patients aged <65 years compared with older patients, and short-acting muscarinic antagonist use was similar across age groups. Previous use of statins and antiplatelet agents showed an overall increase with age.
Efficacy
GLY 25 μg and 50 μg BID produced significant, clinically important improvements in the primary endpoint of LS mean placebo-adjusted change from baseline in trough FEV1 at week 12 (0.094 L and 0.104 L, respectively; P<0.001). Placebo-adjusted change from baseline in trough FEV1 with GLY was numerically higher in the FEV1% predicted ≥50% subgroup (25 μg: 0.112 L; 50 μg: 0.126 L) than in the FEV1% predicted <50% subgroup (25 μg: 0.070 L; 50 μg: 0.079 L) (Figure 2A). Placebo-adjusted change from baseline in trough FEV1 with GLY 25 μg and 50 μg was numerically higher in the older age groups (≥65 years: 0.140 L and 0.124 L; ≥75 years: 0.144 L and 0.120 L, respectively) compared with the <65 years subgroup (0.056 L and 0.086 L, respectively) (Figure 2B).
Significant improvements (P<0.05) in LS mean SGRQ total score were observed at week 12 in the overall population with GLY 25 μg (−3.710) and 50 μg (−3.078) vs placebo (−0.364).
Improvement in SGRQ total score was observed with GLY in both the <50% and ≥50% FEV1% predicted subgroups (25 μg: −3.237 and −3.392, P<0.01 vs placebo; 50 μg: −3.061 and −2.322, P<0.05 vs placebo; Figure 3A). GLY produced significant improvement in placebo-adjusted SGRQ total score in both <65 years and ≥65 years subgroups (25 μg: −3.447 and −3.053, P<0.01 and P<0.05; 50 μg: −2.318 and −3.098, both P<0.01). The minimum clinically important difference, defined as a reduction of ≥4 units in SGRQ total score, was exceeded in the GLY 25 μg ≥75 years subgroup (−6.278; P<0.01 vs placebo), but not in the corresponding GLY 50 μg subgroup (−2.441; Figure 3B).
In the FEV1% predicted <50% subgroup, GLY 50 μg was associated with a significant increase in SGRQ responder rate vs placebo (OR: 1.658; 95% CI: 1.029–2.672), while GLY 25 μg resulted in a significant increase vs placebo in the FEV1% predicted ≥50% subgroup (OR: 1.730; 95% CI: 1.166–2.567; Figure 4A). Significant improvement in SGRQ responder rate was observed with GLY 25 μg and 50 μg doses in patients aged ≥65 years (1.985; 95% CI: 1.253–3.143 and OR: 2.008; 95% CI: 1.250–3.224 respectively; Figure 4B).
Safety
Overall, treatment with GLY 25 μg and 50 μg BID led to a lower incidence of TEAEs vs placebo (43.4%, 50.7%, and 52.3%, respectively), serious TEAEs (3.0%, 4.2%, and 5.6%, respectively), and TEAEs leading to discontinuation of study drug (5.1%, 3.9%, and 9.3%, respectively).
In the FEV1% predicted subgroups, the incidence of TEAEs was similar for GLY and placebo (Table 3). Overall, cough and COPD worsening were the most frequent TEAEs, occurring in 7.9% and 9.5% of the FEV1% predicted <50% subgroup and 8.3% and 6.6% of the FEV1% predicted ≥50% subgroup, respectively. Incidence of COPD worsening and dyspnea was higher in all treatment groups in the FEV1% predicted <50% subgroup compared with the ≥50% subgroup. The incidence of serious TEAEs was lower with GLY than placebo in both FEV1% predicted subgroups, with a higher overall incidence in the <50% subgroup compared with the ≥50% subgroup.
The incidence of TEAEs was numerically higher for all treatment groups in patients aged ≥65 years compared with <65 years (Table 4). Cough and COPD worsening were the most frequent TEAEs overall (<65 years: 7.4% and 8.2%; ≥65 years: 9.0% and 8.0%; ≥75 years: 7.9% and 4.0%, respectively). The incidence of serious TEAEs was lower with GLY than placebo across all age subgroups, with fewer events in the <65 years subgroup versus ≥65 years subgroup.
Incidence of TEAEs leading to discontinuation was similar in both FEV1% predicted <50% and ≥50% subgroups, and lower with GLY (25 μg: 4.9% and 5.3%; 50 μg: 3.6% and 4.2%, respectively) vs placebo (10.7% and 8.3%, respectively). In GLY patients in the FEV1% predicted <50% and ≥50% subgroups, the most common TEAEs leading to discontinuation were dyspnea and cough, respectively.
Across age subgroups, GLY 25 μg and 50 μg resulted in a lower incidence of TEAEs leading to discontinuation compared with placebo (<65 years: 3.9%, 3.9%, and 5.9%; ≥65 years: 6.5%, 3.9%, and 12.9%; ≥75 years: 6.4%, 2.6%, and 9.8%, respectively). In patients aged <65 years and ≥65 years treated with GLY, the most common TEAEs leading to discontinuation were cough and dyspnea, respectively.
Few CV events of special interest were seen; the incidence with placebo was similar to GLY in patients with baseline FEV1% predicted <50% (placebo: 2.3%; GLY 25 μg: 1.1%; GLY 50 μg: 3.1%), and higher than GLY in the baseline FEV1% predicted ≥50% subgroup (placebo: 2.8%; GLY 25 μg: 2.0%; GLY 50 μg: 1.3%). Incidence rates for CV events of special interest were generally similar between the <65 (placebo: 1.8%; GLY 25 μg: 2.2%; GLY 50 μg: 1.6%) and ≥65 years age groups (placebo: 3.3%; GLY 25 μg: 1.0%; GLY 50 μg: 2.8%), but numerically higher in the ≥75 years subgroup (placebo: 4.9%; GLY 25 μg: 4.3%; GLY 50 μg: 5.3%).
The overall number of major adverse CV events was small (Tables 5 and 6); the overall incidence rate (IR) for GLY 50 μg was similar to placebo (IR: 23.3 and 16.4 per 1,000 patient-years, respectively). There were no clinically relevant changes in laboratory measures, blood pressure, heart rate, Holter monitoring (GOLDEN 3 only), or ECG parameters in the overall population.
Discussion
Analysis of pooled data from the GOLDEN 3 and GOLDEN 4 studies showed efficacy of nebulized GLY in subgroups of older patients and those with moderate-to-very-severe airflow limitation, resulting in significant improvements in change from baseline trough FEV1 at 12 weeks and in patient-reported outcomes. GLY produced a decrease in SGRQ total score in all subgroups, as well as improved responder rates compared with placebo, including older age group and lower FEV1% predicted subgroups.
As with GLY, a non-inferiority study found that treatment with the LAMAs tiotropium and umeclidinium significantly improved change from baseline in trough FEV1 at 12 weeks regardless of disease severity, and patients with moderate COPD showed a substantially larger increase in trough FEV1 compared with patients with severe COPD.20 A comparable LAMA monotherapy study investigating age subgroups is not evident in the literature. The improvement in FEV1 and SGRQ irrespective of age21 or disease severity at baseline,22–24 observed with GLY are similar to those found with several LAMA/LABA combinations.
GLY was well tolerated and generated no additional safety signals. The most common TEAEs across all treatments and subgroups were cough, dyspnea, and COPD worsening. TEAE incidences were similar across FEV1 subgroups and were higher in patients aged ≥65 years compared with those aged <65 years.
Treatment with GLY resulted in fewer TEAEs leading to discontinuation compared with placebo in all subgroups. There were few CV events of special interest; the incidence was numerically higher in the ≥75 years subgroup for all treatments, which may be due to a numerically higher percentage of patients ≥75 years having high CV risk at baseline (72.3%–87.8%) compared with patients <65 years (52.4%–55.1%).
The main limitation of this paper is the post-hoc nature of the analyses which precludes controlling for multiplicity. However, this limitation is mitigated by the size of the observed differences in the subgroups, the consistency of the results across variables and both doses, and the clinical meaningfulness of the results. Although the individual studies were not powered to provide differences between subgroups, combining the data did allow for meaningful statistical analyses. Results for the ≥75 years subgroup should be interpreted with caution due to the small number of patients, even though significant improvements in trough FEV1 in both GLY dose groups, and a significant reduction in SGRQ total score for the GLY 25 μg group were reported for this subgroup. It must be noted that although the subgroup definitions used herein (disease severity: FEV1% predicted ≥50% and <50%; age: <65 years and ≥65 years, with an additional subgroup of ≥75 years) differed from those in the study protocols (disease severity: FEV1% predicted ≥50%, ≥30% to <50%, and <30%; age: <65 years, 65–74 years, and ≥75 years), we believe that the results support our conclusion that efficacy and safety were not affected by disease severity and age of the patient at baseline.
Due to the increase in the global incidence of COPD25,26 and the aging population (it is estimated that approximately 20% of the US population will be aged ≥65 years by 2030),27 optimizing disease management in those patients who have age-related physical, functional, and cognitive impairments is an important goal. These impairments can increase errors in handling inhalers11 and, consequently, have a substantial impact on patients’ compliance with therapeutic regimen to administer treatment correctly. In addition, chronic comorbidities increase the risk for treatment nonadherence.6,13 Treatment options that do not rely on the requirement for specific coordination or attainment of an inspiratory flow threshold during administration may be preferable in this patient population.28,29
Conclusion
In this secondary analysis of two Phase III clinical trials, nebulized GLY demonstrated statistically significant and clinically important improvements in lung function and health status over 12 weeks in patients with moderate-to-very-severe COPD, irrespective of baseline disease severity and age, including those aged ≥75 years. Both doses of GLY were well tolerated and generated no additional overall or CV safety signals across all baseline FEV1% predicted and age subgroups. Nebulized GLY provides an additional treatment option for the management of COPD, including in patients who may have difficulty using handheld inhalers. Appropriate selection of the drug delivery device is important in COPD management and may depend on disease severity, age, cognitive function, and physical ability.
Data sharing statement
Sunovion Pharmaceuticals Inc. is part of a clinical trial data sharing consortium that facilitates access for qualified researchers to selected anonymized clinical trial data. For up-to-date information on data availability please visit https://www.clinicalstudydatarequest.com/Study-Sponsors.aspx and click on ‘Sunovion’.
Acknowledgments
The studies were funded by Sunovion Pharmaceuticals Inc. Medical writing support was provided by Linda Townsend PhD of FireKite, an Ashfield company, part of UDG Healthcare plc, and was funded by Sunovion Pharmaceuticals Inc. Aspects of these data were presented in an oral presentation: Ohar J, et al, “The efficacy and safety of a novel, nebulized glycopyrrolate for the treatment of COPD: Effect of baseline lung function and age” at the CHEST meeting, Toronto, Canada, October 28–November 1, 2017.
Author contributions
All authors had full access to the study data, conducted the analysis, and take responsibility for the integrity of the data and the accuracy of the analysis. All authors contributed to drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. Jill Ohar contributed to data collection; Robert Tosiello contributed to study design.
Disclosure
Jill Ohar has served on advisory boards for Sunovion Pharmaceuticals Inc., AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Mylan, and Theravance, and has provided expert witness testimony for: Wallace & Graham, Levy Konigsberg, Goldenberg Heller & Antognoli, Simon Greensone Panatier Bartlett, Williams Kherkher Hart, Gori Julian & Associates, Simmons Hanley Conroy, and Elrod Pope. Robert Tosiello, Thomas Goodin, and Shahin Sanjar are employees of Sunovion Pharmaceuticals Inc. The authors report no other conflicts of interest in this work.
References
Global Initiative for Chronic Obstructive Lung Disease Inc. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD, 2018. [Guidelines]; 2018. Available from: http://goldcopd.org/. Accessed July 26, 2018. | ||
Wheaton AG, Cunningham TJ, Ford ES, Croft JB. Centers for Disease Control and Prevention (CDC). Employment and activity limitations among adults with chronic obstructive pulmonary disease – United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(11):289–295. | ||
Mapel DW, Dalal AA, Blanchette CM, Petersen H, Ferguson GT. Severity of COPD at initial spirometry-confirmed diagnosis: data from medical charts and administrative claims. Int J Chron Obstruct Pulmon Dis. 2011;6:573–581. | ||
Cabrera López C, Casanova Macario C, Marín Trigo JM, et al. Comparison of the 2017 and 2015 Global Initiative for Chronic Obstructive Lung Disease Reports. Impact on grouping and outcomes. Am J Respir Crit Care Med. 2018;197(4):463–469. | ||
Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1(3):253–260. | ||
Taffet GE, Donohue JF, Altman PR. Considerations for managing chronic obstructive pulmonary disease in the elderly. Clin Interv Aging. 2014;9:23–30. | ||
Schermer T, Heijdra Y, Zadel S, et al. Flow and volume responses after routine salbutamol reversibility testing in mild to very severe COPD. Respir Med. 2007;101(6):1355–1362. | ||
Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364(12):1093–1103. | ||
Decramer ML, Chapman KR, Dahl R, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med. 2013;1(7):524–533. | ||
Anthonisen NR, Lindgren PG, Tashkin DP, et al. Bronchodilator response in the lung health study over 11 yrs. Eur Respir J. 2005;26(1):45–51. | ||
Wieshammer S, Dreyhaupt J. Dry powder inhalers: which factors determine the frequency of handling errors? Respiration. 2008;75(1):18–25. | ||
Amin AN, Ganapathy V, Roughley A, Small M. Confidence in correct inhaler technique and its association with treatment adherence and health status among US patients with chronic obstructive pulmonary disease. Patient Prefer Adherence. 2017;11:1205–1212. | ||
Barbour KE, Helmick CG, Boring M, Brady TJ. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation – United States, 2013–2015. MMWR Morb Mortal Wkly Rep. 2017;66(9):246–253. | ||
Mahler DA, Waterman LA, Ward J, Gifford AH. Comparison of dry powder versus nebulized beta-agonist in patients with COPD who have suboptimal peak inspiratory flow rate. J Aerosol Med Pulm Drug Deliv. 2014;27(2):103–109. | ||
Filuk R. Delivery system selection: clinical considerations. Am Health Drug Benefit. 2008;1(Suppl 8):13–17. | ||
Barrons R, Pegram A, Borries A. Inhaler device selection: special considerations in elderly patients with chronic obstructive pulmonary disease. Am J Health Syst Pharm. 2011;68(13):1221–1232. | ||
Gray SL, Williams DM, Pulliam CC, Sirgo MA, Bishop AL, Donohue JF. Characteristics predicting incorrect metered-dose inhaler technique in older subjects. Arch Intern Med. 1996;156(9):984–988. | ||
LONHALA MAGNAIR (glycopyrrolate) inhalation solution [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2018. | ||
Kerwin E, Donohue JF, Goodin T, Tosiello R, Wheeler A, Ferguson GT. Efficacy and safety of glycopyrrolate/eFlow® CS (nebulized glycopyrrolate) in moderate-to-very-severe COPD: Results from the glycopyrrolate for obstructive lung disease via electronic nebulizer (GOLDEN) 3 and 4 randomized controlled trials. Respir Med. 2017;132:238–250. | ||
Feldman G, Maltais F, Khindri S, et al. A randomized, blinded study to evaluate the efficacy and safety of umeclidinium 62.5 μg compared with tiotropium 18 μg in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:719–730. | ||
Ferguson GT, Karpel JP, Clerisme-Beaty E, Grönke L, Voß F, Buhl R. Efficacy and safety of tiotropium + olodaterol maintenance treatment in patients with COPD in the TONADO® and OTEMTO® studies: a subgroup analysis by age. Int J Chron Obstruct Pulmon Dis. 2016;11:2701–2710. | ||
Singh D, Gaga M, Schmidt O, et al. Effects of tiotropium + olodaterol versus tiotropium or placebo by COPD disease severity and previous treatment history in the OTEMTO® studies. Respir Res. 2016;17(1):73. | ||
Ferguson GT, Fležar M, Korn S, et al. Efficacy of tiotropium + olodaterol in patients with chronic obstructive pulmonary disease by initial disease severity and treatment intensity: a post hoc analysis. Adv Ther. 2015;32(6):523–536. | ||
Chapman KR, Bateman ED, Chen H, Hu H, Fogel R, Banerji D. QVA149 improves lung function, dyspnea, and health status independent of previously prescribed medications and COPD severity: A subgroup analysis from the SHINE and ILLUMINATE studies. Chronic Obstr Pulm Dis. 2015;2(1):48–60. | ||
Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J Glob Health. 2015;5(2):020415. | ||
Burney P, Suissa S, Soriano JB, et al. The pharmacoepidemiology of COPD: recent advances and methodological discussion. Eur Respir J Suppl. 2003;43:1s–44s. | ||
Ferrucci L, Giallauria F, Guralnik JM. Epidemiology of aging. Radiol Clin North Am. 2008;46(4):643–652. | ||
Rau JL. Practical problems with aerosol therapy in COPD. Respir Care. 2006;51(2):158–172. | ||
Dhand R, Dolovich M, Chipps B, Myers TR, Restrepo R, Farrar JR. The role of nebulized therapy in the management of COPD: evidence and recommendations. COPD. 2012;9(1):58–72. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.









