Back to Journals » International Journal of Women's Health » Volume 9
Efficacy and safety of a flexible extended regimen of ethinylestradiol/drospirenone for the treatment of dysmenorrhea: a multicenter, randomized, open-label, active-controlled study
Authors Momoeda M , Kondo M, Elliesen J, Yasuda M, Yamamoto S, Harada T
Received 13 February 2017
Accepted for publication 31 March 2017
Published 2 May 2017 Volume 2017:9 Pages 295—305
DOI https://doi.org/10.2147/IJWH.S134576
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Mikio Momoeda,1 Masami Kondo,2 Joerg Elliesen,3 Masanobu Yasuda,2 Shigetomo Yamamoto,4 Tasuku Harada5
1Department of Integrated Women’s Health, St Luke’s International Hospital, Tokyo, 2Product Development, Bayer Yakuhin Ltd, Osaka, Japan; 3Global Clinical Development, Bayer AG, Berlin, Germany; 4Medical Affairs, Bayer Yakuhin Ltd, Osaka, 5Department of Obstetrics and Gynecology, Tottori University Faculty of Medicine, Tottori, Japan
Background: Dysmenorrhea is a common condition in women, which is characterized by menstrual pain. Low-dose estrogen/progestin combined oral contraceptives have been shown to reduce the severity of dysmenorrhea symptoms, and a 28-day cyclic regimen of ethinylestradiol/drospirenone (28d regimen) is approved for this indication in Japan.
Aim: The aim of this study was to assess the safety and efficacy of a flexible extended regimen of ethinylestradiol/drospirenone (flexible regimen) in Japanese women with dysmenorrhea.
Methods: This multicenter, open-label study was performed in Japanese women with dysmenorrhea who, after a baseline observational phase, were randomized to receive ethinylestradiol 20 µg/drospirenone 3 mg in a flexible regimen (one tablet each day for 24–120 days followed by a 4-day tablet-free interval) or in the standard 28d regimen (one tablet each day for 24 days, followed by 4 days of placebo tablets for six cycles). The primary endpoint was the number of days with dysmenorrhea of at least mild intensity over a 140-day evaluation period. Dysmenorrhea scores, bleeding patterns, and other pain-related parameters were also assessed.
Results: A total of 216 women (mean age 29.7 years) were randomized to the flexible regimen (n=108) or 28d regimen (n=108) and 212 were included in the full analysis sets (flexible regimen, n=105; 28d regimen, n=107). Women in the flexible-regimen group reported a mean of 3.4 fewer days with dysmenorrheic pain than women in the 28d-regimen group, with similar decreases in disease severity reported in both treatment groups. According to the investigators, 64.8% and 59.4% of women in the flexible-regimen and 28d-regimen treatment groups had “very much improved” or “much improved” disease, while 54.3% and 50.9% of patients reported being “very much satisfied” or “much satisfied” with their treatment, respectively.
Conclusion: In Japanese women with dysmenorrhea, a flexible extended regimen of ethinylestradiol/drospirenone decreased the number of days with dysmenorrheic pain versus the traditional 28d regimen.
Keywords: oral contraceptive, dysmenorrhea, ethinylestradiol/drospirenone, flexible extended regimen, pain relief
Introduction
Dysmenorrhea is characterized by menstrual pain, including lower abdominal pain, low back pain, and pain radiating to the legs, which starts around the beginning or just before the beginning of menstruation.1 In some cases, it is accompanied by systemic symptoms including nausea, headache, dizziness, insomnia, anxiety/irritability, diarrhea, and feeling depressed.1 Dysmenorrhea is common, affecting between 16% and 91% of menstruating women of any age.2 Pain is worse in women with long menstrual periods and those with poor self-rated health, and pain severity is associated with unhealthy lifestyles, such as smoking, and low levels of physical activity.3 In Japan, 50% of menstruating women report pain during menses.4
Dysmenorrhea is broadly classified into primary dysmenorrhea, which is the most common form and has no known cause, and secondary dysmenorrhea, which is associated with endometriosis, uterine fibroids, or other pelvic pathology. Primary dysmenorrhea without organic disease usually begins 2–3 years after menarche1 and affects predominantly young nulliparous women in their late teens to early twenties. Longitudinal data indicate that growing older and having a baby are associated with a decrease in primary dysmenorrhea severity.5 In contrast, secondary dysmenorrhea is more likely to affect older women.2
A survey of 4,230 Japanese women aged 20–49 years suggested that one-third of women needed medical treatment for symptoms of secondary dysmenorrhea.6 Three low-dose estrogen/progestin (EP) combination products have been approved for dysmenorrhea in Japan: a combination of ethinylestradiol 35 μg/norethisterone 1 mg, ethinylestradiol 20 μg/norethisterone 1 mg, and ethinylestradiol 20 μg/drosperinone 3 mg, and Japanese guidelines recommend EP combination products along with nonsteroidal anti-inflammatory drugs for first-line therapy of primary dysmenorrhea.7
Clinical trials have shown that a combination of ethinylestradiol and drospirenone significantly reduces the severity of symptoms in patients with dysmenorrhea,8,9 and has a well-characterized safety profile when used as an oral contraceptive over a long period of time.10–12 Generally, ethinylestradiol/drospirenone is administered on a 28-day cycle, with active pills taken for 24 consecutive days followed by a 4-day hormone-free interval to induce withdrawal bleeding. An alternative treatment schedule is the flexible extended regimen, in which the duration of active treatment is extended and, consequently, the frequency of withdrawal bleeding is reduced. As a result, the flexible extended regimen is expected to reduce not only the number of withdrawal bleeding days with associated pain (dysmenorrhea), but also the total number of bleeding days during the treatment.
Phase III studies conducted in Europe and Canada showed that a flexible extended regimen of ethinylestradiol/drospirenone significantly reduced the number of days with bleeding during the treatment period compared with ethinylestradiol/drospirenone taken in 28-day cycles.13,14 In addition, the number of days with dysmenorrhea during the treatment period was reduced with the ethinylestradiol/drospirenone flexible regimen in patients with primary dysmenorrhea.14
Currently, all low-dose EP combination products in Japan are prescribed on a 28-day cyclic regimen. Therefore, the aim of this phase III study was to compare the safety and efficacy of a flexible extended regimen of ethinylestradiol/drospirenone (FlexibleMIB; YazFlex™; Bayer Yakuhin Ltd., Osaka, Japan) with those of the standard 28-day cyclic regimen of ethinylestradiol/drospirenone in Japanese women with primary or secondary dysmenorrhea.
Materials and methods
Study design
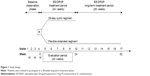
This was a multicenter, randomized, open-label, active-controlled, parallel-group study followed by a long-term single-arm treatment phase. The study consisted of a baseline observation phase consisting of two menstrual cycles during which patients attended three visits, followed by a 24-week treatment phase in which patients received either ethinylestradiol/drospirenone in the flexible extended regimen or as the standard 28-day cyclic regimen. At the end of the treatment phase, patients in the flexible extended regimen group were invited to participate in a 28-week long-term treatment phase in which they continued to receive their treatment for another 28 weeks (Figure 1). During the study, patients attended the clinic every 4 weeks to confirm they were not pregnant, be questioned about adverse events, and record the information from their patient diaries. At visit 10 or visit 17, patients came in for an end-of-treatment visit, or if the previous consecutive tablet-taking period was <24 days, the tablet taking was continued for the minimum period of 24 days (end of treatment). All patients returned for two post-treatment visits after the end of treatment (whether this was at 24 weeks or 52 weeks), the first visit after the last withdrawal bleeding, and the second visit after the recurrence of natural menstruation.
The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and the International Conference on Harmonization guideline E6: Good Clinical Practice, and met all local legal and regulatory requirements. The protocol and all protocol amendments were reviewed and approved by each study site’s Independent Ethics Committee/Institutional Review Board, namely, the committees/review boards of the Suzuran Clinic; Ikebukuro Clinic; Seijo Kinoshita Hospital; Hayakawa Clinic; New Medical Research System Clinic; Shirokane Ladies Clinic; Juno Vesta Clinic Hatta; and the Chayamachi Ladies Clinic. All patients provided written informed consent before entering the study. ClinicalTrials.gov identifier: NCT01892904.
Patients
Patients were eligible for inclusion if they were aged ≥20 years, had a regular 25- to 38-day menstrual cycle and a total dysmenorrhea score (the sum of two sub-scores describing severity of dysmenorrhea and use of analgesics) of ≥3 points in each of the two menstrual cycles during the baseline observation period, and experienced dysmenorrhea (as recorded in patient diaries during the baseline observation period). Dysmenorrhea was defined as any spasmodic pelvic pain or lower abdominal pain with possible radiation toward back or thighs recorded during withdrawal and/or a menstrual bleeding episode. All patients had to be willing to use a barrier (nonhormonal) method of contraception throughout the study, and women who smoked ≥15 cigarettes a day were only eligible for inclusion if they were <35 years of age.
Exclusion criteria included pregnancy, lactation, or desire to get pregnant within the intended treatment period, body mass index (BMI) >30 kg/m2, abnormal cervical smear requiring further follow-up, undiagnosed abnormal genital bleeding, organic disease requiring surgery, ovarian chocolate cysts with solid areas, or age ≥40 years and ovarian chocolate cysts of >10 cm longest diameter, contraindication to ethinylestradiol/drospirenone or hypersensitivity to any ingredient, and antiphospholipid antibody syndrome. Also excluded were patients who had undergone surgical treatment for endometriosis by laparotomy or laparoscopy within 2 months prior to the baseline observation phase and those needing analgesics regularly for therapeutic objectives other than relief from the pelvic pain associated with dysmenorrhea during this study (occasional use permitted). Any patient with a disease or condition that worsened under hormonal treatment according to the opinion of the investigator, including hypertension, valvular or coronary heart disease, cerebrovascular disease, thrombophlebitis, predisposition to thrombosis,15 hepatic dysfunction (including a history of liver disease or herpes during pregnancy), severe renal insufficiency, diabetes, dyslipidemia, estrogen-dependent tumors, migraine,16 otosclerosis, uncontrolled thyroid disorder, and clinical depression, or who were scheduled for imminent major surgery or who had any condition, or used any medication, that might interfere with the conduct of study or the interpretation of results, was excluded.
Treatment
Patients were randomized 1:1 to a flexible extended regimen of ethinylestradiol/drospirenone or ethinylestradiol/drospirenone in 28-day cycles. Patients randomized to the flexible extended regimen received one tablet of oral ethinylestradiol 20 μg/drospirenone 3 mg each day for ≥24 days and up to 120 days, followed by a 4-day tablet-free interval (cycle of 28 up to 124 days). In the event of three consecutive days of spotting and/or bleeding during days 25-120 of the intake cycle, a tablet-free interval of 4 days was taken, followed by the start of the next intake cycle. Patients randomized to a 28-day cyclic regimen received one tablet of ethinylestradiol 20 μg/drospirenone 3 mg at the same time each day for 24 days, followed by 4 days of placebo tablets. Patients in this group received six cycles of treatment during the treatment phase.
In both groups, if a patient had forgotten to take the day’s tablet, she was expected to take that tablet as soon as she remembered it. If the dose of the previous day was missed, it was to be taken immediately and then the dose of the current day at the scheduled time. If the patient missed doses of two or more days, the dose from the previous day was taken immediately after she noticed the missed doses and then the dose of the current day was taken at the scheduled time; the regular dosing schedule was followed thereafter.
Assessments
At visit 1, all subjects were provided with a patient diary, in which they recorded menstrual patterns and any unscheduled vaginal bleeding, presence of dysmenorrhea and its severity, use of over-the-counter (OTC) analgesics for pelvic pain, and interference with daily activities caused by dysmenorrhea. Patient evaluation started on day 25 of treatment. If pain was present, patients rated it as mild (no need for analgesics and no impairment of daily activities), moderate (required OTC analgesics, but pain was effectively relieved during most cycles; discomfort interferes with usual daily activities), or severe (required analgesics, but achieved no consistent relief from OTC analgesia; discomfort causes inability to work or perform usual activities); patients also rated pain on a visual analog scale (VAS). Blood samples were taken at visits 3, 4, and 9 to determine levels of estrogen (visits 3 and 9) and progesterone (visits 4 and 9). Patients underwent transvaginal ultrasound at visits 4, 9, and 17 (or at discontinuation) to assess endometrial thickness.
End points
The primary efficacy variable was number of days of dysmenorrheic pain of at least mild intensity over 140 days of treatment, based on data recorded in the patients’ diaries; this variable was also analyzed independently in the primary and secondary dysmenorrhea subgroups. Secondary efficacy variables were change in dysmenorrhea score, change in VAS from baseline through the period of withdrawal bleeding, and other pain-related parameters, including number of days with at least moderate dysmenorrheic pain, number of days with pelvic pain (independent of occurrence of vaginal bleeding), number of days with dysmenorrheic pain associated with withdrawal bleeding or with unscheduled bleeding, number of days in which rescue medication was used to relieve dysmenorrhea or pelvic pain, and number of days in which dysmenorrheic pain interfered with daily activities. Also assessed were menstrual bleeding patterns, serum levels of estrogen and progesterone, endometrial thickness, clinical global impressions by patient and investigator (scale from 1= very much improved to 7= very much worse, where 4= no change), and patient satisfaction (scale from 1= very much satisfied to 7= very much dissatisfied, where 4= neither satisfied nor dissatisfied). Menstrual cycle length was evaluated from bleeding diary data. A withdrawal bleeding episode for a cycle either started on or after day 21 of the current cycle, and lasted at least until day 25 of the same cycle, or started on or after day 25 of the current cycle, but before day 25 of the next cycle. If more than one episode satisfied either of the above criteria, the first episode to occur was considered to be the withdrawal bleeding episode of the current cycle.
The incidence of treatment-emergent adverse events (TEAEs) and drug-related adverse events was summarized by treatment group using the MedDRA (version 18.0) preferred term and the primary system organ class. Other safety variables were summarized descriptively by treatment group.
Statistical analyses
A 1:1 randomization of 204 patients was planned, based on a sample size estimate of 81 per group and a dropout rate of 20%. The sample size estimate was based on the assumption that patients receiving ethinylestradiol/drospirenone 28-day cycles would experience a mean ± standard deviation (SD) of 14.9±8.9 days of dysmenorrhea, and those receiving the flexible extended regimen would experience a mean ± SD of 10.6±7.8 days of dysmenorrhea, at a power of 90% and a significance level of α=5% (two-sided). All analyses were conducted on the full analysis set (FAS; defined as all randomized patients who received ≥1 dose of study drug).
The primary efficacy variable was compared between the ethinylestradiol/drospirenone flexible extended regimen and the 28-day cyclic regimen groups. The flexible extended regimen was considered to be superior to the 28-day cyclic regimen if the estimated mean number of days with dysmenorrheic pain over 140 days of the evaluation period in the flexible extended regimen group was less than in the 28-day cyclic regimen group and the two-sided P-value of the t-test was ≤0.05. For the difference between the test and the reference treatment the two-sided 95% confidence interval (CI) was calculated. Statistical analyses were performed using SAS 9.2 or higher (SAS Institute Inc., Cary, NC, USA).
Results
Patients
The study was conducted between July 2013 and September 2015; a total of 335 patients were enrolled from eight centers in Japan, and 216 patients were randomized to treatment. However, four patients (three in the flexible extended group and one in the 28-day cycle group) did not receive study medication. Therefore, the FAS consisted of 212 patients, 105 randomized to the flexible extended group and 107 to the 28-day cycle group (Figure 2). The treatment phase was completed by 98 (90.7%) patients in the flexible extended group and 84 (77.8%) patients in the 28-day cycle group. Reasons for discontinuation were patient choice (n=17), adverse events (n=8), protocol violation (n=3), protocol-driven decision (n=3), loss to follow-up (n=2), and physician decision (n=1). Of the 98 patients who completed the treatment phase in the flexible extended group, 59 decided to continue with the long-term treatment phase, and all but one of these patients completed 52 weeks of treatment (Figure 2).
  | Figure 2 CONSORT diagram of enrolled patients. |
Patient characteristics were similar between treatment groups (Table 1). All patients in the FAS were Asian, with a mean ± SD age of 29.7±6.4 years (range 20-46 years), and mean BMI was 20.6±2.5 kg/m2. Most patients (78.3%) had never smoked, 24 (11.3%) were current smokers, and 22 (10.4%) were former smokers. Menstrual cycle length from the analysis of diary data following the evaluation method described above ranged from 4 to 38 days (mean ± SD 29.2±4.5 days). Excluding the tablet-free interval, compliance during the treatment phase was 99.9%±0.4% in the flexible extended group and 99.8%±1.2% in the 28-day cycle group.
  | Table 1 Patient baseline characteristics |
Efficacy
Over 140 days of treatment, women taking the flexible extended regimen reported on average 3.4 fewer days with dysmenorrheic pain than those allocated to the 28-day cycle regimen (95% CI −6.5 to −0.3; P=0.03; Table 2). In the subgroup analysis, the number of days with dysmenorrheic pain over the 140 days of the evaluation period was 11.0±8.8 days in the flexible extended regimen group and 14.3±11.0 days in the 28-day cyclic group in women who had primary dysmenorrhea, compared with 13.8±10.7 and 17.8±13.4 days in the flexible extended regimen group and 28-day cyclic group in women with secondary dysmenorrhea.
  | Table 2 Primary and secondary efficacy parameters during the first 140 days of treatment |
The flexible extended regimen was also associated with greater reductions in the number of days with at least moderate dysmenorrheic pain, with pelvic pain independent of vaginal bleeding, with dysmenorrhea associated with withdrawal bleedings, with dysmenorrhea associated with unscheduled bleeding, when rescue analgesic medication was taken, or with dysmenorrheic pain interfering with daily activities (Table 2).
A decrease of dysmenorrhea severity from the baseline observation phase (visit 3) was observed in both the flexible extended regimen group and the 28-day cyclic regimen group, with similar magnitudes of change across the two groups (Figure 3). In patients who continued to take the flexible extended regimen during the long-term extension phase, the reduction in pain severity achieved by week 24 was maintained throughout follow-up, so that at 52 weeks the VAS score was at the same level as it was at 24 weeks (Figure 3).
  | Figure 3 Pelvic pain, assessed using a visual analog scale (VAS), and reported during menstrual or withdrawal bleeding. |
Effects on bleeding
Women receiving the flexible extended regimen had cycles ranging from 28 to 124 days versus the 28-day cycle in women in the 28-day cyclic regimen group. In the flexible extended regimen group, the number of days with bleeding (excluding spotting-only days) over the treatment phase was smaller than in the 28-day cyclic regimen group, whereas the number of days with spotting was greater than in the 28-day cyclic regimen group. Consequently, the number of days with bleeding and spotting over the treatment phase was not different between the two groups (Table 3). While women in the flexible extended regimen group tended to have fewer bleeding episodes than those in the 28-day cyclic regimen group, the duration of bleeding episodes was longer. When assessed by 90-day increments, the mean number of withdrawal bleeding episodes was lower during treatment with the flexible extended regimen than with the 28-day cyclic regimen (Figure 4).
  | Table 3 Bleeding patterns during the 140-day observation phase |
  | Figure 4 Number of withdrawal bleeding episodes assessed per 90-day periods of treatment. |
In patients who continued to take the flexible extended regimen after week 24, over the 52-week extended phase the mean ± SD number of days with bleeding and spotting was 95.3±39.2 days, with bleeding (excluding spotting) being 50.6±20.1 days, and with spotting only being 44.7±31.3 days.
The duration of bleeding and/or spotting episodes during the long-term treatment period was similar to the duration of these episodes during the first 24 weeks of treatment with the flexible extended regimen.
Effects on serum hormones and endometrial thickness
Serum levels of estradiol and progesterone decreased in both groups between the baseline observation period and the end of the evaluation period (visit 9). The proportion of patients with serum estradiol level ≥27.2 pg/mL, which suggested follicular maturation, was >85% in both groups at visit 3, but was 6% in the flexible extended regimen group and 19% in the 28-day cyclic regimen group at visit 9. The proportion of patients with serum progesterone level ≥1.57 ng/mL, which suggested ovulation, was around 80% in both groups at visit 4 while it was <5% of patients in both groups at visit 9.
Mean ± SD endometrial thickness as assessed by transvaginal ultrasound was 12.1±3.8 mm in the flexible extended regimen group and 11.1±3.1 mm in the 28-day cyclic regimen group at visit 4. At visit 9, it had decreased in both groups by ~7 mm to 4.4±2.1 mm in the flexible extended regimen group and 3.8±1.7 mm in the 28-day cyclic regimen group.
Global impressions of treatment
In the evaluation by investigators, the proportion of patients considered “very much improved” or “much improved” was 64.8% in the flexible extended regimen group and 59.4% in the 28-day cyclic regimen group (Figure 5A). When treatment satisfaction was evaluated by patients, the proportion who considered themselves “very much satisfied” or “much satisfied” was 54.3% and 50.9%, respectively (Figure 5B).
  | Figure 5 Subjective evaluation of treatment by (A) investigators and (B) patients. |
Safety
Overall, TEAEs were reported in 96 of 105 patients (91.4%) in the flexible extended regimen group and 97 of 107 patients (90.7%) in the 28-day cyclic regimen group. The rates of drug-related TEAEs were also comparable between the two groups (73/105 [69.5%] in the flexible extended regimen group vs 76/107 [71.0%] in the 28-day cyclic regimen group). Most TEAEs were mild (89% and 87% in the flexible extended and 28-day cyclic regimen groups, respectively) or moderate (3% and 4% in the flexible extended and 28-day cyclic regimen groups, respectively) in severity. One patient in the flexible extended regimen group and six patients in the 28-day cyclic regimen group discontinued treatment because of TEAEs. Only one serious TEAE occurred during the study, with one patient having an epileptic seizure while receiving the 28-day cyclic regimen, but this was not considered to be related to the study drug. The most frequently reported TEAEs in both groups were genital hemorrhage, nasopharyngitis, headache, and increased plasminogen in both groups (Table 4). The most frequently reported drug-related TEAEs were genital hemorrhage (35.2%), increased plasminogen levels (20.0%), and menorrhagia (11.4%) in the flexible extended regimen group, and genital hemorrhage (26.2%), headache (20.6%), increased plasminogen levels (17.8%), and menorrhagia (11.2%) in the 28-day cyclic regimen group. The TEAE profile of the flexible extended regimen during the long-term extension phase was similar to that during the initial 24 weeks (Table 4).
  | Table 4 Most common treatment-emergent adverse events (reported in ≥5% of patients in any treatment group) |
Discussion
To our knowledge, these are the first published data on the use of a flexible extended regimen of ethinylestradiol/drospirenone for the treatment of dysmenorrhea in Japanese women. Our data show that the flexible extended regimen significantly reduces the number of days with dysmenorrheic pain relative to the 28-day cyclic regimen, by a mean of 3.4 days for every 140 days of treatment. Based on these results, the number of days with dysmenorrheic pain at an annualized rate is estimated to be ~31 days in the flexible extended regimen group and ~40 days in the 28-day cyclic regimen group, suggesting that the number of days with dysmenorrheic pain is ~9 days shorter per year in the flexible extended group than in the 28-day cycle group.
Our data are consistent with the findings of a similar European study in which the flexible extended regimen of ethinylestradiol/drospirenone reduced the number of days with dysmenorrheic pain by a mean of 4.2 days over 140 days of treatment, relative to a 28-day cyclic regimen.14 Current data suggest that 27.3% of Japanese women have to take time off work or school because of symptoms associated with menstruation,6 and 51.5% use OTC analgesics for pain management.17 In addition, a survey conducted in 2013 in ~20,000 Japanese women found that 17.25% had experienced menstrual symptoms that interfered with their work in the past 3 months.4 The use of medication and absenteeism related to dysmenorrhea correlates with pain severity3 and Japanese women with dysmenorrhea use significantly more OTC analgesics than women without dysmenorrhea.17
The annual burden of symptoms associated with menstruation in Japan is estimated to be ¥683 billion or $US 8.6 billion.4 Therefore, reducing the number of days with dysmenorrheic pain even by 1 day can substantially alleviate the burden of dysmenorrhea on both patients and society. Consistent with the reduced number of days with dysmenorrheic pain, our study also demonstrated a significant reduction in the need for OTC analgesics in patients taking the flexible extended regimen of ethinylestradiol/drospirenone compared with those taking the 28-day cyclic regimen.
The mean number of withdrawal bleeding episodes was lower during treatment with the flexible extended regimen than the 28-day cyclic regimen in this study, and this probably accounts for the lower number of days with dysmenorrheic pain. The effects of the flexible extended regimen on bleeding patterns observed in this study are consistent with those previously reported, that is, fewer withdrawal bleeding episodes but a small increase in the number of days of intracyclic bleeding.13,14,18 Based on these data, one can predict that women receiving ethinylestradiol/drospirenone as a flexible extended regimen would have 6–7 withdrawal bleeding episodes per year, whereas those receiving ethinylestradiol/drospirenone as a 28-day cyclic regimen would have 10–11 episodes, suggesting 3–5 fewer withdrawal bleeding episodes with the flexible extended regimen.
In this study, the type and number of adverse events was similar with the flexible extended and the 28-day cyclic regimen. In addition, the safety profile of the ethinylestradiol/drospirenone flexible extended regimen was similar to that previously reported with other regimens of ethinylestradiol/drospirenone.13,14,19,20 All combined hormonal contraceptives increase the risk of thromboembolism relative to nonhormonal contraceptives,12 but whether there is any modulating effect on the risk of venous thromboembolism by the progestin component of combined oral contraceptives is still debated.10–12 One study has found that relative to other combined oral contraceptives, those containing drospirenone are associated with a lower risk of arterial thromboembolism.12
There are some limitations to this study, including the open-label nature of the study. However, while open-label comparisons can introduce bias, this was unavoidable given the widely differing regimens involved. Another limitation to this study was that less than a third of patients presented with secondary dysmenorrhea, which means that the results of this study are not generalizable in this patient population. Further studies in women with secondary dysmenorrhea are warranted.
Conclusion
This study demonstrated that a flexible extended regimen of ethinylestradiol/drospirenone decreased the number of days with dysmenorrheic pain compared with a 28-day cyclic regimen of ethinylestradiol/drospirenone in Japanese women and was well tolerated.
Acknowledgments
We would like to thank Catherine Rees who drafted the outline of this manuscript on behalf of inScience Communications and Cécile Duchesnes of inScience Communications who drafted the first draft of this manuscript. Sheridan Henness of inScience Communications provided assistance with revisions following peer review, including English language editing. This medical writing assistance was funded by Bayer Yakuhin Ltd.
This study was supported by Bayer Yakuhin Ltd who participated in the trial design and managed all operational aspects of the study.
Author contributions
The authors (Mikio Momoeda and Tasuku Harada) and employees of the sponsor (Masami Kondo, Masanobu Yasuda, Shigetomo Yamamoto, and Joerg Elliesen) interpreted the data and prepared, reviewed, and approved the manuscript. All authors participated in manuscript revision and vouch for the accuracy and completeness of the data reported.
Disclosure
The sponsor, Bayer Yakuhin Ltd, provided financial support for the conduct of the research and preparation of the article. The sponsor designed and conducted the study including collection, management, and analysis of data. Mikio Momoeda and Tasuku Harada received a medical advisory fee from Bayer Yakuhin Ltd when the study was planned and conducted. Masami Kondo, Masanobu Yasuda, and Shigetomo Yamamoto are employees of Bayer Yakuhin Ltd and Joerg Elliesen is an employee of Bayer Pharma AG. The authors report no other conflicts of interest in this work.
References
Deligeoroglou E. Dysmenorrhea. Ann N Y Acad Sci. 2000;900:237–244. | ||
Ju H, Jones M, Mishra G. The prevalence and risk factors of dysmenorrhea. Epidemiol Rev. 2014;36:104–113. | ||
Teperi J, Rimpela M. Menstrual pain, health and behaviour in girls. Soc Sci Med. 1989;29(2):163–169. | ||
Tanaka E, Momoeda M, Osuga Y, et al. Burden of menstrual symptoms in Japanese women: results from a survey-based study. J Med Econ. 2013;16(11):1255–1266. | ||
Weissman AM, Hartz AJ, Hansen MD, Johnson SR. The natural history of primary dysmenorrhoea: a longitudinal study. BJOG. 2004;111(4):345–352. | ||
Taketani Y. Investigating the prevention, diagnosis and treatment of conditions including endometriosis from the perspective of reproductive health. Report for the 2000 fiscal year. Japanese Ministry of Health, Labor and Welfare; 2001. | ||
Minakami H, Maeda T, Fujii T, et al. [Japan Society of Obstetrics and Gynecology, Japan Association of Obstetricians and Gynecologists. Guidelines for gynecological practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2014 edition.] J Obstet Gynaecol Res. 2014;40(6):1469–1499. Japanese. | ||
Harada T, Momoeda M, Taketani Y, Hoshiai H, Terakawa N. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2008;90(5):1583–1588. | ||
Momoeda M, Mizunuma H, Taketani Y. Treatment of functional and organic dysmenorrhea: efficacy and safety of drospirenone/ethinylestradiol combination tablet: Sanka to Fujinka. Obstet Gynecol. 2010;77:977–988. | ||
Bateson D, Butcher BE, Donovan C, et al. Risk of venous thromboembolism in women taking the combined oral contraceptive: a systematic review and meta-analysis. Aust Fam Physician. 2016;45(1):59–64. | ||
Dinger J, Bardenheuer K, Heinemann K. Cardiovascular and general safety of a 24-day regimen of drospirenone-containing combined oral contraceptives: final results from the International Active Surveillance Study of Women Taking Oral Contraceptives. Contraception. 2014;89(4):253–263. | ||
Dinger J, Mohner S, Heinemann K. Cardiovascular risks associated with the use of drospirenone-containing combined oral contraceptives. Contraception. 2016;93(5):378–385. | ||
Klipping C, Duijkers I, Fortier MP, Marr J, Trummer D, Elliesen J. Contraceptive efficacy and tolerability of ethinylestradiol 20 mug/drospirenone 3 mg in a flexible extended regimen: an open-label, multicentre, randomised, controlled study. J Fam Plann Reprod Health Care. 2012;38(2):73–83. | ||
Strowitzki T, Kirsch B, Elliesen J. Efficacy of ethinylestradiol 20 mug/drospirenone 3 mg in a flexible extended regimen in women with moderate-to-severe primary dysmenorrhoea: an open-label, multicentre, randomised, controlled study. J Fam Plann Reprod Health Care. 2012;38(2):94–101. | ||
Practice Committee of the American Society for Reproductive Medicine. Combined hormonal contraception and the risk of venous thromboembolism: a guideline. Fertil Steril. 2017;107(1):43–51. | ||
Chiofalo B, Lagana AS, Imbesi G, et al. Is oral contraceptive-induced headache dependent on patent foramen ovale? Clinical dynamics, evidence-based hypothesis and possible patient-oriented management. Med Hypotheses. 2016;94:86–88. | ||
Ohde S, Tokuda Y, Takahashi O, Yanai H, Hinohara S, Fukui T. Dysmenorrhea among Japanese women. Int J Gynaecol Obstet. 2008;100(1):13–17. | ||
Machado RB, de Melo NR, Maia H Jr. Bleeding patterns and menstrual-related symptoms with the continuous use of a contraceptive combination of ethinylestradiol and drospirenone: a randomized study. Contraception. 2010;81(3):215–222. | ||
Klipping C, Duijkers I, Fortier MP, Marr J, Trummer D, Elliesen J. Long-term tolerability of ethinylestradiol 20 mug/drospirenone 3 mg in a flexible extended regimen: results from a randomised, controlled, multicentre study. J Fam Plann Reprod Health Care. 2012;38(2):84–93. | ||
Seidman DS, Yeshaya A, Ber A, et al. A prospective follow-up of two 21/7 cycles followed by two extended regimen 84/7 cycles with contraceptive pills containing ethinyl estradiol and drospirenone. Isr Med Assoc J. 2010;12(7):400–405. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.