Back to Journals » Drug Design, Development and Therapy » Volume 12
Efficacy and safety of a fixed-dose combination of nimesulide/pantoprazole compared to naproxen/esomeprazole for pain relief in patients with osteoarticular diseases and dyspeptic symptoms
Authors Scheinberg M, Pott Júnior H , Macêdo EA, Bocchi de Oliveira MF, Ecclissato C , Amazonas RB
Received 24 April 2018
Accepted for publication 18 June 2018
Published 6 September 2018 Volume 2018:12 Pages 2775—2783
DOI https://doi.org/10.2147/DDDT.S172068
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Morton Scheinberg,1 Henrique Pott Júnior,2 Eduardo de Almeida Macêdo,2 Monalisa Fernanda Bocchi de Oliveira,2 Christina Ecclissato,2 Roberto Bleuel Amazonas2
1Clinical Research Center Hospital AACD, Hospital Israelita Albert Einstein, São Paulo, Brazil; 2Medical Affairs Department, EMS Pharma Inc., Hortolândia, Brazil
Purpose: This study investigated the safety and efficacy of fixed-dose combination tablets of naproxen/esomeprazole magnesium and nimesulide/pantoprazole to determine if both regimens are equally suited to relieve pain in patients with osteoarticular diseases and dyspeptic symptoms.
Methods: Patients were randomly assigned to receive either nimesulide/pantoprazole (100 mg/20 mg) twice daily or naproxen/esomeprazole magnesium (500 mg/20 mg) twice daily for 14 days. The primary endpoint was defined as the mean change in modified Western Ontario and McMaster Universities Osteoarthritis Index pain subscale. Secondary endpoints were mean visual analog scale score of dyspeptic symptoms (nausea, abdominal discomfort/pain, epigastric burning, postprandial fullness), mean visual analog scale score of individual dyspeptic symptoms, and individual score of dyspeptic symptoms according to patient diary. This study is registered at ClinicalTrials.gov: NCT01670552.
Results: A total of 490 patients were enrolled and randomized, and 399 completed treatment (naproxen/esomeprazole, n=201; nimesulide/pantoprazole, n=198). The difference in mean change in the modified Western Ontario and McMaster Universities Osteoarthritis Index pain score after 7 days of treatment between the two treatment groups was 2.33 mm (95% CI, -1.22 to 5.89 mm). After 14 days of therapy, the difference was 0.45 mm (95% CI, -3.29 to 4.19 mm). The most common adverse events in the pooled group were abdominal discomfort, abdominal distention, dyspepsia, and nausea, but none of these was deemed to be clinically meaningful.
Conclusion: The present study demonstrated noninferiority of a 14-day regimen with a fixed-dose combination of nimesulide/pantoprazole compared to naproxen/esomeprazole for the treatment of osteoarticular pain.
Keywords: osteoarticular diseases, naproxen, esomeprazole, nimesulide, pantoprazole, randomized controlled trial
Introduction
Nonsteroidal antiinflammatory drugs (NSAIDs) are one of the most widely used drug classes in the world.1 They account for approximately 5%–10% of total prescriptions every year and are widely used for the treatment of various painful and inflammatory conditions.2 NSAIDs exert antiinflammatory, analgesic, and antipyretic effects primarily by inhibiting cyclooxygenase enzymes (COXs), which are essential in the synthesis of important inflammatory mediators, such as prostaglandins.3 However, evidence indicates that frequent and prolonged use of NSAIDs is associated with the occurrence of potentially limiting adverse events, especially in higher-risk groups such as the elderly.4,5 Most of these adverse events result from gastrointestinal toxicity, and roughly 10% of chronic NSAID users discontinue treatment due to gastrointestinal symptoms.6
The mechanisms underlying the gastrointestinal toxicity of NSAIDs are related both to their direct effect on the mucosa of the gastrointestinal tract and to systemic mechanisms related to the inhibition of COX-1.7,8 Major gastrointestinal symptoms include abdominal pain, heartburn, dyspepsia, nausea, and vomiting, which affect nearly 40% of patients.6,9 More severe complications include gastroduodenal peptic ulcers, bleeding, and gastroduodenal perforation.10 Gastroduodenal-related rates of hospitalization and mortality are estimated to be 47–87 hospitalizations per 100,000 patients and 15.3 deaths per 100,000 patients, respectively.10,11
Concomitant use of proton-pump inhibitors (PPIs) with NSAIDs, including fixed-dose combination regimens, have emerged as an alternative to NSAID use alone and provide equivalent pain control and minimize gastroduodenal complications, especially in patients at high risk of adverse effects.12,13 The main rationale for the use of a fixed-dose combination of NSAIDs and PPIs is the simplification of therapy and its convenience leading to better overall patient compliance. In addition, the presence of the PPI in the same NSAID tablet provides for prescribing physician the assurance that the proton pump inhibitor will be administered concomitantly to all NSAID intakes, increasing the safety of treatment with regard to the risk of gastrointestinal adverse events. This study investigated the safety and efficacy of fixed-dose combination tablets of naproxen/esomeprazole magnesium and nimesulide/pantoprazole to determine if both regimens are equally suited to relieve pain in patients with osteoarticular diseases and dyspeptic symptoms.
Materials and methods
Design overview
This was a multi-center, double-blind, double-dummy, active-controlled, parallel-group, noninferiority Phase III study to compare the efficacy and safety of fixed-dose combination tablets of naproxen/esomeprazole magnesium (500 mg/20 mg) and nimesulide/pantoprazole (100 mg/20 mg) for pain relief in patients with osteoarticular diseases and dyspeptic symptoms. Patients were followed for 14 days with an intermediate follow-up on day 7. The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki, and the protocol was approved by the ethics committees of all participating sites. All patients provided written informed consent to participate. The study is registered at ClinicalTrials.gov: NCT01670552.
Participants
Patients were enrolled if they met the following criteria: 1) >18 years of age with osteoarticular diseases and dyspeptic symptoms (defined as abdominal pain or discomfort, stunting, burning, or nausea) at the time of inclusion or within the last 2 weeks and 2) a score of 40 mm (scale, 0–100 mm) or higher on the visual analog scale (VAS) for at least three pain-related items in the VAS version of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC).14 The osteoarticular diseases included in the study were tendinitis, bursitis, low back pain, and acute exacerbations of osteoarthritis. Key exclusion criteria included history of bleeding or gastrointestinal ulcers, kidney disease, asthma, or allergic sensitivity to any NSAID; previous use of anticoagulants in the last month; previous use of NSAIDs, PPIs, histamine-2 receptor antagonists, or antacids in the last 7 days; and breastfeeding or pregnancy.
Randomization and intervention
The randomization list was generated electronically, in blocks of four, in the proportion of 1:1 (50% Test and 50% Comparator). The electronic tool (e-CRF) controlled medication consumption sequentially until the final number of evaluable participants in the research center was reached. The participant’s randomization number was recorded in the source document by the team member designated by the Principal Investigator. The confidentiality of the randomization list was guaranteed by the sponsor. There was no blind code break in this study.
Eligible patients were randomly assigned (1:1) to receive either nimesulide/pantoprazole (one tablet; 100 mg/20 mg) plus placebo twice daily or naproxen/esomeprazole magnesium (one tablet; 500 mg/20 mg) plus placebo twice daily according to a computer-generated randomization code. All therapies were given orally for 14 days.
Study assessments
Efficacy
Symptoms were evaluated by the investigator at baseline, after 7 days of treatment, and after 14 days of treatment. The primary endpoint was defined as the mean change in WOMAC pain subscale.14 The WOMAC is a self-administered questionnaire consisting of 24 items divided into three dimensions: pain (5 items), stiffness (2 items), and physical function (17 items). We used the VAS version of the pain subscale as a measure of osteoarticular pain (scale, 0–100 mm), where 0 represents “no pain at all” and the upper limit of 100 represents “the worst pain ever possible.” Secondary endpoints were mean VAS score of dyspeptic symptoms (nausea, abdominal discomfort/pain, epigastric burning, postprandial fullness), mean VAS score of individual dyspeptic symptoms, and individual score evolution of individual dyspeptic symptoms according to patient diary. Dyspeptic symptoms were evaluated once daily for 14 days with the VAS. Each patient self-administered the questionnaire, filling out the form before the beginning of the treatment and then daily according to symptoms in the previous 24 hours.
Safety
Serum urea, creatinine, and full blood counts were performed at admission and before outpatient discharge. Decisions regarding discharge were made on an individual basis after substantial improvement of symptoms.
Statistical analysis
For all efficacy endpoints, statistical analyses were performed in the per-protocol population that included patients who were compliant with the protocol.
Sample size was estimated to detect noninferiority between nimesulide/pantoprazole and naproxen/esomeprazole for the difference in the mean change in modified WOMAC pain subscale of participants reaching the primary endpoint: we presumed that the true difference in mean change in modified WOMAC pain subscale between treatment groups is 2 mm, and we assumed that the SD is 22.25 mm. Assuming a dropout rate of 10% after randomization, 203 randomized patients per group (406 total patients) was estimated to provide at least 80% power to exclude a noninferiority margin of −3.5 mm.
All analyses were conducted using R 3.4.3 software (The R Project for Statistical Computing, RStudio Inc., Boston, MA, USA).15
Results
A total of 490 patients were randomized and started treatment between February and October of 2016. Of these, 91 patients discontinued treatment (Figure 1). Common reasons for discontinuation included lost to follow-up (nimesulide/pantoprazole, n=10; naproxen/esomeprazole, n=2), withdrawal of consent (nimesulide/pantoprazole, n=4; naproxen/esomeprazole, n=4), and protocol violation (nimesulide/pantoprazole, n=27; naproxen/esomeprazole, n=29). Other reasons included adverse events (nimesulide/pantoprazole, n=5; naproxen/esomeprazole, n=6) and investigator’s decision (nimesulide/pantoprazole, n=1; naproxen/esomeprazole, n=3).
  | Figure 1 Flowchart of trial design. |
There were no clinically meaningful differences among treatment groups in patient characteristics at baseline (Table 1). Most of the patients were female (88.8%), and the median age was 49 years (range, 19–79 years). Overall mean baseline-modified WOMAC pain score was 64.41±12.99 mm; the mean baseline scores for the nimesulide/pantoprazole and naproxen/esomeprazole groups were 64.66±12.36 and 64.15±13.60 mm, respectively (Figure 2). Mean baseline VAS scores of dyspeptic symptoms for the nimesulide/pantoprazole and naproxen/esomeprazole groups were 50.57±20.42 and 49.44±22.32 mm, respectively.
  | Figure 2 Mean baseline WOMAC pain score for the intent-to-treat population. |
Efficacy
After the baseline assessment, symptoms were evaluated by the investigator after 7 and 14 days of treatment. Mean changes in the modified WOMAC pain score are presented in Table 2 for the entire sample and for each group. A detailed view of the evolution of the global modified WOMAC pain score is presented in Figure 3.
  | Table 2 Mean changes in the WOMAC pain score in the per-protocol population |
  | Figure 3 Daily mean WOMAC pain score for both treatment groups. |
The difference in mean change in the modified WOMAC pain score after 7 days of treatment between groups was 2.33 mm (95% CI: −1.22 to 5.89 mm). After 14 days, the difference was 0.45 mm (95% CI: −3.29 to 4.19 mm). Therefore, noninferiority of nimesulide/pantoprazole compared to naproxen/esomeprazole was verified according to this analysis.
Secondary outcomes
The baseline VAS score for dyspeptic symptoms was 50.57±20.42 mm in the group that used naproxen+esomeprazole and 49.44±22.32 mm in the group that used nimesulide+pantoprazole, and there was no statistically significant difference between them (P=0.120).
Mean changes in the VAS score for dyspeptic symptoms (nausea, abdominal discomfort/pain, epigastric burning, postprandial fullness) are presented in Table 3 for the entire sample and for each group. A detailed view of the evolution of the global VAS score of dyspeptic symptoms is presented in Figure 4.
  | Table 3 Mean reductions in VAS scores for individual dyspeptic symptoms in the per-protocol population |
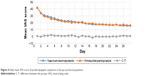  | Figure 4 Daily mean VAS score of pooled dyspeptic symptoms in the per-protocol population. |
Safety
Adverse events that occurred during the treatment period were consistent with those reported in previous clinical trials of nimesulide/pantoprazole and naproxen/esomeprazole. No additional or unique adverse events occurred.
Overall frequencies of adverse events were similar in the two groups during the entire treatment period. There were 733 episodes of adverse events: 366 episodes (50%) in the nimesulide/pantoprazole group and 367 episodes (50%) in the naproxen/esomeprazole group (P=0.526). The most common adverse events were nausea (12.6%), abdominal distention (11.3%), dyspepsia (11.1%), headache (7.5%), and abdominal discomfort (7.1%), but none of these was deemed to be clinically meaningful (Table 4).
  | Table 4 Overall frequencies of adverse events in the entire group and in each treatment group |
All adverse events reported or observed during the study were mild, transient, and did not result in any clinical impact. No clinically significant laboratory abnormalities were identified in any patient.
Discussion
Musculoskeletal pain is one of the most frequent reasons for medical consultation in the world.16 This symptom may be due to a variety of causes, including osteoarthritis, periarthritis, and autoimmune diseases, and each cause has its specific treatment regimen. However, the use of NSAIDs is common to many of the regimens used for a variety of conditions and disease states. One of the main limiting factors for the use of NSAIDs, especially for prolonged periods, is the gastrointestinal morbidity related to these medications. In this context, the concomitant use of PPIs has been shown to be effective in minimizing the risk of gastrointestinal complications secondary to NSAIDs.13,17 The present study investigated the efficacy and safety of a fixed-dose combination of nimesulide/pantoprazole (100 mg/20 mg) compared to naproxen/esomeprazole (500 mg/20 mg). In both treatment groups, there was a marked predominance of female patients (90.2% in the nimesulide/pantoprazole group and 87.3% in the naproxen/esomeprazole group). The mean age was close to 50 years (48.14±13.63 years in the nimesulide/pantoprazole group and 49.37±14.34 years in the naproxen/esomeprazole group). The mean body mass index indicated overweight in both groups (29.98±5.27 kg/m2 in the nimesulide/pantoprazole group and 29.80±6.57 kg/m2 in the naproxen/esomeprazole group). None of the characteristics were significantly different between groups (Table 1). These characteristics of the study sample correspond to those of the population subgroups in which most of the musculoskeletal diseases that cause osteoarticular pain, especially osteoarthritis and periarthritis, occur most frequently.19
The mean modified WOMAC pain score and mean VAS for dyspeptic symptoms at baseline were similar between the two groups (Table 1 and Figure 2). In both groups, progressive reductions of the mean modified WOMAC pain score were observed on days 7 and 14, with final mean scores of 22.46±18.18 mm in the nimesulide/pantoprazole group and 23.00±17.93 mm in the naproxen/esomeprazole group (P=0.765; Table 2). Both treatment regimens, therefore, showed a considerable reduction in the modified WOMAC pain score after 7 and 14 days, and nimesulide/pantoprazole was noninferior to naproxen/esomeprazole in terms of pain control. The reduction of the modified WOMAC score was more pronounced in the first 7 days in both groups, with a slight reduction in the mean score between days 7 and 14 of treatment (Figure 3).
Previous studies have evaluated nimesulide and naproxen alone (ie, not in combination with PPIs) for efficacy and safety in the treatment of osteoarticular pain.20–24 A randomized, double-blind study compared the efficacy of these two NSAIDs in osteoarthritis-related pain in a total of 370 patients followed for a 6-month period; the results were similar between groups.24 Another study compared nimesulide/beta-cyclodextrin and naproxen for 2 weeks in patients with osteoarthritis; the drugs were shown to be equivalent in terms of pain control and functional capacity.23 Quattrini et al also demonstrated similar efficacy between nimesulide and naproxen for pain control in 120 patients with hip osteoarthritis.21 Similar results were described by Lecomte et al after 14 days of treatment for osteoarticular pain secondary to tendinitis and bursitis22 and by Calligaris et al in 660 patients with minor traumatic injuries related to sports practice.25 Thus, even though this study evaluated NSAIDs in combination with PPIs, the present results support previous findings of the equivalence of efficacy between nimesulide and naproxen alone for the treatment of osteoarticular pain.
NSAIDs exert their antiinflammatory and analgesic effects by inhibiting the synthesis of prostanoids, a group of lipids with diverse biological functions: among these is an important role in inhibiting inflammatory reactions. This inhibition results from the blockade of COX-1 and COX-2, which are key enzymes in the conversion of arachidonic acid to prostanoids.17 The antiinflammatory effects of NSAIDs largely derive from its inhibition of COX-2; COX-1 inhibition is responsible for some of the adverse effects of these drugs. The various available NSAIDs differ in terms of pharmacokinetic characteristics but also in the degree of selectivity for inhibition of COX-1 and COX-2, which has implications for efficacy and tolerability.26–28 Nimesulide is an NSAID with preferred action on COX-2; it has a short half-life and is widely used in the treatment of musculoskeletal pain conditions.29,30 Studies in patients with osteoarthritis have demonstrated significant concentrations of nimesulide in the synovial fluid after oral intake of the drug, as well as reduced levels of inflammatory cytokines in this fluid, pointing to antiinflammatory actions at the joint level.31 The efficacy of this NSAID in the treatment of osteoarticular pain caused by osteoarthritis and other musculoskeletal diseases has been demonstrated in several studies previously.32–36
In both treatment groups, a progressive reduction of the mean VAS of dyspeptic symptoms was observed throughout the 14 days of treatment, and no statistically significant differences were found between the groups in any of the evaluated symptoms (Table 3 and Figure 3). Thus, both treatment regimens showed a protective effect on the gastrointestinal tract and, in this regard, nimesulide/pantoprazole was deemed to be noninferior to naproxen/esomeprazole.
The strategy of coprescribing PPIs with NSAID treatment regimens has demonstrated efficacy in reducing the gastrointestinal toxicity of these medications.12,37–39 NSAID-related gastrointestinal morbidity stems from both the systemic effects of COX-1 inhibition and local actions on the gastrointestinal mucosa.3 Several mechanisms related to the protection of the gastrointestinal mucosa are affected by NSAIDs, including secretion of mucus and bicarbonate ions. In addition, NSAIDs have a negative effect on the blood flow in the mucosa and on the cells of the immune system, which interferes with normal tissue repair processes. Finally, a topical irritative effect of NSAIDs on the gastrointestinal mucosa may also contribute to the local toxicity of these drugs.18,40–42 PPIs block gastric acid secretion through irreversible inhibition of H+/K+ATPase, thus inhibiting gastric acid secretion and raising intragastric pH, which decreases the potential for NSAID damage on the gastric mucosa.13,43
Several studies have comparatively evaluated the efficacy of different PPIs for the treatment of various types of gastrointestinal disease. In general, esomeprazole has been shown to be superior to pantoprazole in the control of gastric acid secretion and in the relief of symptoms of conditions such as gastroesophageal reflux disease and esophagitis.44–50 A study that specifically evaluated patients taking NSAIDs also showed superiority in the control of gastric acid secretion of esomeprazole over pantoprazole.51 In the present study, however, the nimesulide/pantoprazole fixed-dose combination regimen demonstrated noninferiority to treatment with naproxen/esomeprazole in terms of control of dyspeptic symptoms during 14 days of treatment. The occurrence of adverse events was similar between the two groups, with gastrointestinal symptoms such as discomfort, abdominal distention, dyspepsia, and nausea being the most frequently reported (Table 4).
Conclusion
The present study demonstrated noninferiority of a 14-day regimen with a fixed-dose combination of nimesulide/pantoprazole compared to naproxen/esomeprazole for the treatment of osteoarticular pain. The results of our study show that the gastrointestinal adverse effects related to NSAID use may be reduced by the use of a fixed-dose combination of nimesulide/pantoprazole, which demonstrated tolerability and sustained efficacy.
The short follow-up time is a limitation to our study. The efficacy and safety seen during 14 days of treatment may not be sustained if the study was conducted for a longer period. In most patients with severe osteoarticular disease, relapses are frequent and the need for prolonged NSAID use may increase the risk of gastrointestinal complications of treatment.
Acknowledgments
The authors thank BioMed Proofreading LLC. The study was funded by EMS Pharma Inc., Hortolândia, SP, Brazil.
Disclosure
Morton Scheinberg reports no conflicts of interest in this work. Henrique Pott Júnior, Eduardo de Almeida Macêdo, Monalisa Fernanda Bocchi de Oliveira, Christina Ecclissato, and Roberto Bleuel Amazonas are full-time employees at EMS Pharma Inc. The authors report no other conflicts of interest in this work.
References
Fine M. Quantifying the impact of NSAID-associated adverse events. Am J Manag Care. 2013;19(14 Suppl):S267–S272. | ||
Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9(1):143–150. | ||
Vane JR, Botting RM. New insights into the mode of action of anti-inflammatory drugs. Inflamm Res. 1995;44(1):1–10. | ||
Griffin MR. Epidemiology of nonsteroidal anti-inflammatory drug-associated gastrointestinal injury. Am J Med. 1998;104(3A):S23–S29. | ||
Brooks P. Use and benefits of nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104(3A):9S–13S. | ||
Brun J, Jones R. Nonsteroidal anti-inflammatory drug–associated dyspepsia: the scale of the problem. Am J Med. 2001;110(1A):12S–13S. | ||
Schoen RT, Vender RJ. Mechanisms of nonsteroidal anti-inflammatory drug-induced gastric damage. Am J Med. 1989;86(4):449–458. | ||
Scheiman JM. NSAIDs, gastrointestinal injury, and cytoprotection. Gastroenterol Clin North Am. 1996;25(2):279–298. | ||
Armstrong CP, Blower AL. Non-steroidal anti-inflammatory drugs and life threatening complications of peptic ulceration. Gut. 1987;28(5):527–532. | ||
Sostres C, Gargallo CJ, Arroyo MT, Lanas A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2010;24(2):121–132. | ||
Lanas A, García-Rodríguez LA, Polo-Tomás M, et al. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. 2009;104(7):1633–1641. | ||
Scheiman JM. The use of proton pump inhibitors in treating and preventing NSAID-induced mucosal damage. Arthritis Res Ther. 2013;15(Suppl 3):S5. | ||
Laine L. Proton pump inhibitor co-therapy with nonsteroidal anti-inflammatory drugs – nice or necessary? Rev Gastroenterol Disord. 2004;4(Suppl 4):S33–S41. | ||
Bellamy N. WOMAC osteoarthritis index user guide. Version V, Brisbane, Australia; 2002. | ||
R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. Available from: http://www.r-project.org/. Accessed October 1, 2017. | ||
Picavet HS, Hazes JM. Prevalence of self reported musculoskeletal diseases is high. Ann Rheum Dis. 2003;62(7):644–650. | ||
Day RO, Graham GG. Non-steroidal anti-inflammatory drugs (NSAIDs). Br Med J. 2013;346:f3195. | ||
Laine L. Gastrointestinal effects of NSAIDs and coxibs. J Pain Symptom Manage. 2003;25(2):32–40. | ||
Vincent TL, Watt FE. Osteoarthritis. Medicine (United Kingdom). 2018;46:187–195. | ||
Fossaluzza V, Montagnani G. Efficacy and tolerability of nimesulide in elderly patients with osteoarthritis: double-blind trial versus naproxen. J Int Med Res. 1989;17(3):295–303. | ||
Quattrini M, Paladin S. A double-blind study comparing nimesulide with naproxen in the treatment of osteoarthritis of the hip. Clin Drug Investig. 1995;10(3):139–146. | ||
Lecomte J, Buyse H, Taymans J, Monti T. Treatment of tendinitis and bursitis: a comparison of nimesulide and naproxen sodium in a double-blind parallel trial. Eur J Rheumatol Inflamm. 1994;14(4):29–32. | ||
Fioravanti A, Storri L, di Martino S, et al. A randomized, double-blind, multicenter trial of nimesulide-beta-cyclodextrin versus naproxen in patients with osteoarthritis. Clin Ther. 2002;24(4):504–519. | ||
Kriegel W, Korff KJ, Ehrlich JC, et al. Double-blind study comparing the long-term efficacy of the COX-2 inhibitor nimesulide and naproxen in patients with osteoarthritis. Int J Clin Pract. 2001;55(8):510–514. | ||
Calligaris A, Scaricabarozzi I, Vecchiet L. A multicentre double-blind investigation comparing nimesulide and naproxen in the treatment of minor sport injuries. Drugs. 1993;46(Suppl 1):187–190. | ||
Bacchi S, Palumbo P, Sponta A, Coppolino MF. Clinical pharmacology of non-steroidal anti-inflammatory drugs: a review. Antiinflamm Antiallergy Agents Med Chem. 2012;11(1):52–64. | ||
Sandilands EA, Bateman DN. Non-steroidal anti-inflammatory drugs. Medicine. 2016;44(3):185–186. | ||
Bateman DN. Non-steroidal anti-inflammatory drugs. Medicine. 2012;40(3):140. | ||
Valentovic M. Nimesulide. In: Enna SJ, Bylund DB, editors. xPharm: The Comprehensive Pharmacology Reference. New York: Elsevier; 2007:1– 5. | ||
Rainsford KD. Nimesulide – Actions and Uses. Berlin, Germany: Springer; 2005:1–433. | ||
Bianchi M, Broggini M, Balzarini P, Franchi S, Sacerdote P. Effects of nimesulide on pain and on synovial fluid concentrations of substance P, interleukin-6 and interleukin-8 in patients with knee osteoarthritis: comparison with celecoxib. Int J Clin Pract. 2007;61(8):1270–1277. | ||
Huskisson EC. Nimesulide, a balanced drug for the treatment of osteoarthritis. Clin Exp Rheumatol. 2001;19(Suppl 22):S21–S25. | ||
Bianchi M, Broggini M. A randomised, double-blind, clinical trial comparing the efficacy of nimesulide, celecoxib and rofecoxib in osteoarthritis of the knee. Drugs. 2003;63(Suppl 1):37–46. | ||
Wober W, Rahlfs VW, Büchl N, Grässle A, Macciocchi A. Comparative efficacy and safety of the non-steroidal anti-inflammatory drugs nimesulide and diclofenac in patients with acute subdeltoid bursitis and bicipital tendinitis. Int J Clin Pract. 1998;52(3):169–175. | ||
Sarzi-Puttini P, Santandrea S, Boccassini L, Panni B, Caruso I. The role of NSAIDs in psoriatic arthritis: evidence from a controlled study with nimesulide. Clin Exp Rheumatol. 2001;19(1 Suppl 22):S17–S20. | ||
Al-Abd AM, Al-Abbasi FA, Nofal SM, et al. Nimesulide improves the symptomatic and disease modifying effects of leflunomide in collagen induced arthritis. PLoS One. 2014;9(11):e111843. | ||
Scheiman JM. Prevention of NSAID-induced ulcers. Curr Treat Options Gastroenterol. 2008;11(2):125–134. | ||
Vonkeman HE, Fernandes RW, van der Palen J, van Roon EN, van de Laar MA. Proton-pump inhibitors are associated with a reduced risk for bleeding and perforated gastroduodenal ulcers attributable to non-steroidal anti-inflammatory drugs: a nested case-control study. Arthritis Res Ther. 2007;9(3):R52. | ||
Lazzaroni M, Porro GB. Management of NSAID-induced gastrointestinal toxicity: focus on proton pump inhibitors. Drugs. 2009;69(1):51–69. | ||
Fiorucci S, Antonelli E, Morelli A. Mechanism of non-steroidal anti-inflammatory drug-gastropathy. Dig Liver Dis. 2001;33(Suppl 2):S35–S43. | ||
Lichtenberger LM. The hydrophobic barrier properties of gastrointestinal mucus. Annu Rev Physiol. 1995;57:565–583. | ||
Peskar BM, Maricic N. Role of prostaglandins in gastroprotection. Dig Dis Sci. 1998;43(9 Suppl):S23–S29. | ||
Becker JC, Domschke W, Pohle T. Current approaches to prevent NSAID-induced gastropathy – COX selectivity and beyond. Br J Clin Pharmacol. 2004;58(6):587–600. | ||
Röhss K, Wilder-Smith C, Nauclér E, Jansson L. Esomeprazole 20 mg provides more effective intragastric acid control than maintenance-dose rabeprazole, lansoprazole or pantoprazole in healthy volunteers. Clin Drug Investig. 2004;24(1):1–7. | ||
Röhss K, Lind T, Wilder-Smith C. Esomeprazole 40 mg provides more effective intragastric acid control than lansoprazole 30 mg, omeprazole 20 mg, pantoprazole 40 mg and rabeprazole 20 mg in patients with gastro-oesophageal reflux symptoms. Eur J Clin Pharmacol. 2004;60(8):531–539. | ||
Armstrong D, Bair D, James C, Tanser L, Escobedo S, Nevin K. Oral esomeprazole vs. intravenous pantoprazole: a comparison of the effect on intragastric pH in healthy subjects. Aliment Pharmacol Ther. 2003;18(7):705–711. | ||
Labenz J, Armstrong D, Lauritsen K, et al. A randomized comparative study of esomeprazole 40 mg versus pantoprazole 40 mg for healing erosive oesophagitis: the EXPO study. Aliment Pharmacol Ther. 2005;21(6):739–746. | ||
Miehlke S, Madisch A, Kirsch C, et al. Intragastric acidity during treatment with esomeprazole 40 mg twice daily or pantoprazole 40 mg twice daily – a randomized, two-way crossover study. Aliment Pharmacol Ther. 2005;21(8):963–967. | ||
Zheng RN. Comparative study of omeprazole, lansoprazole, pantoprazole and esomeprazole for symptom relief in patients with reflux esophagitis. World J Gastroenterol. 2009;15(8):990–995. | ||
Miner P, Katz PO, Chen Y, Sostek M. Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five-way crossover study. Am J Gastroenterol. 2003;98(12):2616–2620. | ||
Goldstein JL, Miner PB, Schlesinger PK, Liu S, Silberg DG. Intragastric acid control in non-steroidal anti-inflammatory drug users: comparison of esomeprazole, lansoprazole and pantoprazole. Aliment Pharmacol Ther. 2006;23(8):1189–1196. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

