Back to Journals » Clinical Ophthalmology » Volume 11
Efficacy and safety of a 3-month loteprednol etabonate 0.5% gel taper for routine prophylaxis after photorefractive keratectomy compared to a 3-month prednisolone acetate 1% and fluorometholone 0.1% taper
Authors Mifflin MD, Betts BS, Frederick PA , Feuerman JM, Fenzl CR, Moshirfar M , Zaugg B
Received 29 March 2017
Accepted for publication 1 May 2017
Published 12 June 2017 Volume 2017:11 Pages 1113—1118
DOI https://doi.org/10.2147/OPTH.S138272
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mark D Mifflin,1 Brent S Betts,1 P Adam Frederick,2 Jason M Feuerman,3 Carlton R Fenzl,4 Majid Moshirfar,1,5 Brian Zaugg1
1Department of Ophthalmology and Visual Sciences, John A Moran Eye Center, University of Utah, Salt Lake City, UT, 2The Eye Center, Huntsville, AL, 3Eye Institute of Austin, Austin, TX, 4Eye Surgeons Associates, Bettendorf, IA, 5Hoopes Vision, Draper, UT, USA
Purpose: To compare the outcome of photorefractive keratectomy (PRK) and complications in patients treated with either loteprednol etabonate 0.5% gel or prednisolone acetate 1% suspension and fluorometholone (fml) 0.1% suspension.
Setting: John A Moran Eye Center, University of Utah, Salt Lake City, UT, USA.
Design: Prospective, randomized, partially masked trial.
Methods: PRK was performed on 261 eyes of 132 participants. Patients were randomized to a postoperative corticosteroid regimen of either loteprednol etabonate 0.5% gel (loteprednol) or prednisolone 1% acetate suspension followed by fluorometholone 0.1% suspension (prednisolone/fml). Primary outcome measures included incidence and grade of postoperative corneal haze and incidence of increased intraocular pressure of 10 mmHg above baseline, or any intraocular pressure over 21 mmHg. Secondary outcome measures included uncorrected distance visual acuity, best corrected distance visual acuity, and manifest refraction spherical equivalent.
Results: The incidence of haze in the first 3 months was 2.6% (3/114 eyes) in the loteprednol group and 4.8% (7/147 eyes) in the prednisolone/fml group and was not statistically significant between groups (P=0.37). The incidence of elevated intraocular pressure was 1.8% (2/114 eyes) in the loteprednol group and 4.1% (6/147 eyes) in the prednisolone/fml group, and was not statistically significant between the groups (P=0.12). The mean 3-month postoperative logMAR uncorrected visual acuity was −0.078±0.10 and −0.075±0.09 in the loteprednol and prednisolone/fml groups, respectively (P=0.83).
Conclusion: Postoperative corneal haze and elevated intraocular pressure were uncommon in both treatment arms. There was no statistically significant difference between each postoperative regimen. Refractive results were similar and excellent in both treatment arms. A tapered prophylactic regimen of loteprednol 0.5% gel is equally effective to prednisolone 1%/fml 0.1% after PRK.
Keywords: PRK, corticosteroid, fluorometholone, loteprednol, lotemax, wavefront optimized
Plain language summary
Topical corticosteroid drops are routinely used after photorefractive keratectomy (PRK) to prevent the formation of corneal haze, which can lead to decreased visual acuity. Loteprednol etabonate 0.5% gel, a medium strength topical corticosteroid, is noninferior to a standard postoperative PRK regimen of a strong topical corticosteroid (prednisolone acetate 1%) followed by a taper with a low-potency topical corticosteroid (fluorometholone 0.1%) in preventing corneal haze and minimizing intraocular pressure spikes. As health insurance medication coverage and drug availability change, PRK postoperative corticosteroid use can be tailored based on what is economically feasible for the patient with low risk of side effects.
Introduction
The corneal wound resulting after photorefractive keratectomy (PRK) induces keratocyte transformation to myofibroblasts.1 Myofibroblasts produce disorganized collagen, causing the appearance of corneal haze and leading to increased light scattering and decreased visual acuity.1 The mainstay in prevention of visually significant corneal haze involves decreasing the corneal inflammatory response with anti-inflammatory medications such as topical corticosteroids or other immunomodulatory medications.2 Previous studies have demonstrated topical corticosteroids to be beneficial in modulating post-PRK corneal inflammatory response, as well as reducing regression.3,4 Rabbit models have also shown topical steroids to be superior in reducing “peak haze” amounts compared with another ophthalmic immunomodulatory agent, cyclosporine A 0.05% (Restasis).5
Loteprednol etabonate 0.5% (loteprednol) is an ester-based corticosteroid that exerts local therapeutic effects and is then quickly broken down into inactive metabolites by nonspecific esterases found in the cornea.5,6 The relatively fast metabolism of loteprednol gives it a lower side effect profile than other steroids, including a smaller effect on intraocular pressure (IOP).7,8 In the ophthalmic literature, there is currently no consensus on a standard post-PRK corticosteroid regimen.
A previous retrospective study from our department demonstrated a similar incidence of haze and increased IOP in patients treated with loteprednol 0.5% suspension or flourometholone (fml) 0.1% suspension after both groups received prednisolone acetate 1% suspension for the first 3–4 weeks postoperatively after PRK.9 Given the known lower incidence of increase in IOP with loteprednol relative to traditional corticosteroids,7,8 in this study, we aimed to compare the rates of this complication in patients using a taper of loteprednol with that of patients using prednisolone tapered to fluorometholone 0.1% (prednisolone/fml) after PRK. We also hypothesized that loteprednol will demonstrate comparable efficacy to prednisolone/fml in preventing corneal haze after PRK. We performed a prospective, randomized, partially masked study to test these hypotheses.
Materials and methods
Study approval was obtained from the Institutional Review Board of University of Utah, and the study was conducted according to the ethical principles originating from the Declaration of Helsinki. The trial is registered clinicaltrials.govt NCT03123614. Patients underwent our standard refractive screening examination with 1 of 5 surgeons (MDM, PAF, JMF, CRF, and BZ), during which their candidacy for refractive surgery was determined, and the risks and benefits of all potential refractive procedures for which they medically qualify, which may or may not include PRK, were discussed. Eligible patients gave informed consent to participate in the study. Patients with a history of prior refractive surgery, keratoconus, forme fruste keratoconus, inferior steepening, and baseline untreated IOP of over 21 mmHg were excluded. Monovision eyes or near-targeted eyes were excluded from the study as well.
Corneal epithelium was gently debrided after exposure to ethanol 20% in balanced salt solution for 35–40 seconds and placed in an 8.0 mm well. Laser ablation was performed using the Alcon Allegretto Wavelight® EX500 excimer laser (Alcon Laboratories, Fort Worth, TX, USA) according to the surgeon-optimized nomogram.10 Mitomycin C 0.02% (MMC) was used in 62 eyes (24 loteprednol eyes and 38 prednisolone eyes) whose ablation depth was greater than 60 microns after the laser ablation. Immediately after ablation or MMC, the ocular surface was rinsed with chilled saline for 30 seconds. This was followed by one drop of prednisolone acetate 1%, a topical fluoroquinolone, a topical nonsteroidal anti-inflammatory drug, and a soft bandage contact lens.
Patients were randomly assigned to 1 of the 2 postoperative steroid regimens. Group 1 used loteprednol 0.5% gel in both eyes, starting at a frequency of 4 times per day for the first week, and then tapered to 3 times per day for 3 weeks, then 2 times per day for 1 month, and finally daily for 1 month. Group 2 used prednisolone acetate 1% suspension in both eyes, starting at a frequency of 4 times per day for the first week, then 2 times per day for 3 weeks, and subsequently converted to fml 0.1% suspension. Fml was used 3 times per day for 1 month, then 2 times per day for 1 month, and then stopped. Group 2 either received name brand or generic corticosteroid based on insurance coverage.
The primary outcome measures were incidence and grade of postoperative corneal haze and incidence of increased IOP ≥10 mmHg above baseline or any IOP over 21 mmHg. Examiners were masked to the treatment arm when obtaining measurements of IOP and grading corneal haze. Secondary outcome measures included uncorrected distance visual acuity, corrected distance visual acuity, and manifest refraction spherical equivalent. Study outcome measures were evaluated for each treated eye of each subject at each study time point. The surgeon had the liberty to alter routine clinical management if IOP was elevated sufficiently to require medical intervention, if there was significant postoperative corneal haze, or if there were other postoperative complications. If enhancement surgery was deemed necessary and performed during the 1-year postoperative period, study data collection ended for that eye at the time of the enhancement.
Eyes were evaluated with the following procedures:
- IOP (by masked observer using applanation tonometry): preoperative and postoperative week 1 (±3 days), month 1 (±10 days), month 2 (±10 days), month 3 (±14 days), month 6 (±1 month), and month 12 (±1 month)
- Grade of corneal haze (by masked ophthalmologist using Fantes scale11): postoperative month 1 (±10 days), month 2 (±10 days), month 3 (±14 days), month 6 (±1 month), and month 12 (±1 month)
- Manifest refraction (sphere, cylinder, and axis): preoperative and postoperative month 1 (±10 days), month 2 (±10 days), month 3 (±14 days), month 6 (±1 month), and month 12 (±1 month)
- Best corrected visual acuity: preoperative and postoperative month 1 (±10 days), month 2 (±10 days), month 3 (±14 days), month 6 (±1 month), and month 12 (±1 month)
- Uncorrected visual acuity (UCVA): preoperative and postoperative day 1 (±1 day), week 1 (±3 days), month 1 (±10 days), month 2 (±10 days), month 3 (±14 days), month 6 (±1 month), and month 12 (±1 month).
Standard statistics were calculated and used to describe the treatment groups in terms of all study variables, primarily IOP, presence or absence and degree of postoperative corneal haze, manifest refraction, uncorrected and corrected visual acuity, and incidence of other postoperative complications. A P-value of ≤0.05 was considered statistically significant. Data were analyzed using Microsoft Excel (Microsoft Corp, Redmond, WA, USA).
Results
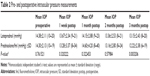
A total of 261 eyes of 132 participants with 3-month data were included in the study (Table 1). There were 114 eyes in the loteprednol group (60% male, 40% female) and 147 eyes in the prednisolone/fml group (52% male, 48% female). The mean age was 34.6 years in the loteprednol group and 35.3 years in the prednisolone/fml group. The mean preoperative spherical equivalent for the loteprednol group was −3.72 D ±2.12 and −4.03 D ±1.99 for the prednisolone/fml group (P=0.245). Average preoperative pachymetry readings were 537.2 and 545.4 in the loteprednol and prednisolone/fml groups, respectively.
MMC was used in 24 loteprednol eyes and 38 prednisolone eyes. There was no statistically significant difference in preoperative refraction or 3-month visual acuity outcomes in patients who had adjunctive MMC in either group.
Haze occurred in 3 out of 114 (3%) loteprednol eyes and in 7 out of 147 (5%) prednisolone/fml eyes (P=0.43). Haze was not seen in any loteprednol eyes with adjunctive MMC but was seen in 3 prednisolone/fml eyes with adjunctive MMC. There was significantly more haze in prednisolone/fml eyes treated with MMC than those without MMC (P=0.05), but there was no significant difference in lotemax eyes that received adjunctive MMC compared to those who did not receive MMC (P=0.12). Overall, when all eyes treated with MMC were compared to eyes without adjunctive MMC, there was no significant difference in haze formation (P=0.13). Despite the presence of haze, 2 loteprednol eyes and all prednisolone/fml eyes had a UCVA of better than 20/20 at the 3-month visit. One loteprednol eye with 1+ haze lost 1 line of UCVA 3 months postoperatively.
Mean pre- and postoperative IOP measurements are shown in Table 2. The mean preoperative IOP for the loteprednol arm was 14.4±2.11 mmHg and 14.3±2.13 mmHg in the prednisolone arm (P=0.76). At 3 months, the mean IOP was 13.15±2.43 mmHg in the loteprednol group and 12.22±2.38 mmHg in the prednisolone/fml group (P=0.003). Clinically significant elevation of IOP only occurred in 8 eyes of 4 patients. These patients were slightly older than the average patient with a mean age of 42.8 years. Two eyes were in the loteprednol arm and 6 eyes were in the prednisolone/fml arm. All IOP elevations occurred at or before the 1 month visit and were controlled with topical ocular antihypertensives and tapering of topical steroids. No surgical intervention was required for IOP elevation during the study period.
Visual acuity outcomes are represented in Figure 1. The mean 3-month postoperative logMAR UCVA was −0.078±0.10 and −0.075±0.09 in the loteprednol and prednisolone groups, respectively (P=0.83). The mean postoperative logMAR best corrected visual acuity at 3 months was −0.120±0.059 and −0.114±0.03 for the loteprednol and prednisolone arms, respectively (P=0.41). The mean spherical equivalent at 3 months was −0.10 D ±0.29 and −0.11 D ±0.30 for the loteprednol and prednisolone arms, respectively (P=0.72). Snellen visual acuity was excellent in both groups. In the loteprednol arm, 101 (89%) eyes achieved UCVA of 20/20 or better and 81 (71%) eyes achieved UCVA of 20/15 or better at 3 months. Similarly, 134 (91%) prednisolone eyes achieved a UCVA of 20/20 or better and 100 (68%) achieved a UCVA of 20/15 or better at 3 months.
  | Figure 1 Pre- and postoperative visual acuity expressed in logMAR. |
Discussion
Recent data from The European Registry of Quality Outcomes for Cataract and Refractive Surgery (EUREQUO) demonstrate that PRK is becoming increasingly popular compared to LASIK as a refractive surgical option.12 In the changing healthcare insurance environment, many insurance plans are beginning to alter coverage for commonly used ophthalmic steroid drops. This has the potential to create an extra cost burden on PRK patients who need treatment for several months after their laser ablations. We have seen this in our practice with unpredictable drug pricing, variable insurance coverage, and availability of some steroid medications depending on numerous factors, which make it difficult for patients to obtain the necessary medicines in a timely and affordable manner.
Our results show an equal efficacy and a low incidence of side effects of loteprednol taper versus a prednisolone/fml taper with respect to haze formation and clinically significant IOP elevation. These findings support the use of either postoperative steroid regimen in patients after PRK.
Corneal haze formation was not significantly different in either group. Overall, 3.8% of patients had haze, but only 1 eye (0.3%) had visually significant haze at 3 months. Other studies have found rates of haze formation after PRK to be anywhere from 2%–21%, and our study found similar results.13–15 Even when mild corneal haze was present, patients were often able to maintain an excellent UCVA as the haze slowly resolved. Both steroid regimens were shown to be effective in preventing haze development while avoiding common intraocular side effects. Five eyes (3 loteprednol and 2 prednisolone/fml) developed trace, late haze between months 3 and 6 that was visually insignificant. There was significantly more haze formation in prednisolone/fml eyes with adjunctive MMC compared to those without adjunctive MMC. Given the small number of patients who received MMC in our study, it is difficult to draw definitive conclusions from this trend. Eyes with adjunctive MMC after PRK may warrant closer observation of eyes without adjunctive MMC.
IOP elevation due to steroid response was uncommon in our study and was seen at or before the 1 month visit in all patients regardless of which drop they were using. At 3 months, patients in the loteprednol arm had statistically significant higher mean IOP than those who were at the end of the fml taper. This was clinically insignificant as no eyes in either group had abnormal IOP readings at the 3-month time point. These findings confirm the results of previous studies; the incidence of increase in IOP is low with medium (loteprednol)7,8 and low strength (fml)16 topical corticosteroids. Our previously published retrospective chart review of loteprednol versus fluorometholone after PRK found very similar results with regard to IOP elevation after PRK.9 Busool et al reported that there was a correlation between high IOP after PRK and corneal haze formation.17 In their study, 16.98% of eyes with corneal haze versus 4.25% of eyes without corneal haze had IOP elevation. Only 1 eye with haze in our study also had high IOP.
Visually significant haze was exceedingly uncommon in our patient population (1 eye, 0.3% of eyes). In addition, visually insignificant haze occurred in 3.8% of eyes (10 eyes). The long-term significance of nonvisually significant haze or even visually significant haze that resolves is unclear. We plan to follow patients up to a year after PRK to see if haze or elevated IOP has any effect on outcomes compared to patients without these findings.
Visual and refractive results from our study were excellent in both groups, with nearly all eyes achieving a UCVA of 20/20 or better by 3 months. Differences in logMAR visual acuity, preoperative refractive errors, postoperative residual refractive errors, and UCVA were statistically insignificant at all time points.
We have concluded that topical treatment with a loteprednol 0.5% taper after PRK is clinically noninferior to a prednisolone acetate 1%/fluorometholone 0.1% taper with respect to haze prevention, IOP elevation, and refractive results.
Conclusion
To our knowledge, this is the first prospective randomized study directly comparing a postoperative loteprednol taper to a prednisolone and fml taper in patients after PRK. We hope this study will continue to build evidence for multiple prophylactic steroid regimens that can be used after PRK, and that this will help physicians and patients achieve safe, optimal results when undergoing this procedure as it continues to grow in popularity worldwide.
Acknowledgments
This study was supported in part by an unrestricted grant from Research to Prevent Blindness, Inc., NY, USA, to the Department of Ophthalmology and Visual Sciences, University of Utah, Salt Lake City, UT, USA.
Disclosure
None of the authors have a financial or proprietary interest in any material or method mentioned. The authors report no conflicts of interest in this work.
References
Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999;18(3):311–356. | ||
Margo JA, Munir WM. Corneal haze following refractive surgery: a review of pathophysiology, incidence, prevention, and treatment. Int Ophthalmol Clin. 2016;56(2):111–125. | ||
Baek SH, Chang JH, Choi SY, Kim WJ, Lee JH. The effect of topical corticosteroids on refractive outcome and corneal haze after photorefractive keratectomy. J Refract Surg. 1997;13(7):644–652. | ||
Vetrugno M, Maino A, Quaranta GM, Cardia L. The effect of early steroid treatment after PRK on clinical and refractive outcomes. Acta Ophthalmol Scand. 2001;79(1):23–27. | ||
Nien CJ, Flynn KJ, Chang M, Brown D, Jester JV. Reducing peak corneal haze after photorefractive keratectomy in rabbits: prednisolone acetate 1.00% versus cyclosporine A 0.05%. J Cataract Refract Surg. 2011;37(5):937–944. | ||
Noble S, Goa KL. Loteprednol etabonate: clinical potential in the management of ocular inflammation. BioDrugs. 1998;10(4):329–339. | ||
Holland EJ, Bartlett JD, Paterno MR, Usner DW, Comstock TL. Effects of loteprednol/tobramycin versus dexamethasone/tobramycin on intraocular pressure in healthy volunteers. Cornea. 2008;27(1):50–55. | ||
Bartlett JD, Horwitz B, Laibovitz R, Howes JF. Intraocular pressure response to loteprednol etabonate in known steroid responders. J Ocul Pharmacol. 1993;9(2):157–165. | ||
Mifflin MD, Leishman LL, Christiansen SM, Sikder S, Hsu M, Moshirfar M. Use of loteprednol for routine prophylaxis after photorefractive keratectomy. Clin Ophthalmol. 2012;6:653–659. | ||
Labiris G, Sideroudi H, Giarmoukakis A, Koukoula S, Pagonis G, Kozobolis VP. Evaluation of the difference between intended and measured ablation and its impact on refractive outcomes of the wavefront optimize profile and the S001 Wellington nomogram in myopic spherocylindrical corrections. Clin Exp Ophthalmol. 2012;40(2):127–133. | ||
Fantes FE, Hanna KD, Waring GO 3rd, Pouliquen Y, Thompson KP, Savoldelli M. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990;108(5):665–675. | ||
Lundström M, Manning S, Barry P, Stenevi U, Henry Y, Rosen P. The European registry of quality outcomes for cataract and refractive surgery (EUREQUO): a database study of trends in volumes, surgical techniques and outcomes of refractive surgery. Eye Vis (Lond). 2015;2:8. | ||
Parekh P, Davis EA. Prevention and treatment of haze in refractive surgery. Int Ophthalmol Clin. 2008;48(1):29–40. | ||
Sia RK, Ryan DS, Edwards JD, Stutzman RD, Bower KS. The U.S. Army Surface Ablation Study: comparison of PRK, MMC-PRK, and LASEK in moderate to high myopia. J Refract Surg. 2014;30(4):256–264. | ||
Ang BC, Foo RC, Lim EW, et al. Risk factors for early-onset corneal haze after photorefractive keratectomy in an Asian population: outcomes from the Singapore Armed Forces Corneal Refractive Surgery Programme 2006 to 2013. J Cataract Refract Surg. 2016;42(5):710–716. | ||
Vetrugno M, Quaranta GM, Maino A, Cardia L. A randomized, comparative study of fluorometholone 0.2% and fluorometholone 0.1% acetate after photorefractive keratectomy. Eur J Ophthalmol. 2000;10(1):39–45. | ||
Busool Y, Mimouni M, Vainer I, et al. Risk factors predicting steroid-induced ocular hypertension after photorefractive keratectomy. J Cataract Refract Surg. 2017;43(3):389–393. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.