Back to Journals » OncoTargets and Therapy » Volume 9
Efficacy and toxicities of adding molecular targeted agents to first-line chemotherapy in the treatment of advanced biliary tract cancer: a systematic review and meta-analysis
Authors Zhao S, Miao Y, Wang R, Guo H, Jin F, Guo X, Luo T
Received 20 April 2016
Accepted for publication 13 July 2016
Published 28 October 2016 Volume 2016:9 Pages 6695—6700
DOI https://doi.org/10.2147/OTT.S110926
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Li
Sheng Zhao,1 Yanping Miao,2 Ruijun Wang,2 Haidong Guo,2 Feng Jin,2 Xiuling Guo,2 Tianyou Luo1
1Department of Radiology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, 2Department of Radiology, The First Affiliated Hospital of Inner Mongolia Medical University, Huhhot Inner Mongolia, People’s Republic of China
Purpose: The purpose of this study was to assess the efficacy and toxicities of adding molecular targeted agents (MTAs) to first-line chemotherapy in the treatment of advanced biliary tract cancer (BTC).
Methods: An extensive search for relevant clinical trials was conducted in electronic databases (PubMed, Web of Science, and Cochrane) and abstracts presented at meetings. Prospective randomized controlled trials (RCTs) reporting the efficacy and toxicity of chemotherapies with or without MTAs in advanced BTC were selected. The endpoints were overall survival (OS), progression-free survival (PFS), and grade 3 or 4 toxicities. The results were expressed as hazard ratio or relative risk (RR), with their corresponding 95% confidence intervals.
Results: The final analysis included a total of 855 advanced BTC patients from six RCTs. Compared with chemotherapy alone, the combination of MTAs with chemotherapy significantly improved overall response rate (ORR) (RR 1.68, 95% confidence interval: 1.28–2.19, P<0.001). And there was also a tendency to improve PFS in the combination regimens (hazard ratio 0.89, 95% confidence interval: 0.78–1.02, P=0.097) but not for OS (hazard ratio 1.01, 95% confidence interval: 0.90–1.13, P=0.93). Subgroup analysis according to targeted agents indicated that the addition of anti-epidermal growth factor receptor agents to chemotherapy significantly improved ORR and PFS, but it did not translate into OS benefits. Additionally, equivalent frequencies of grade 3 or 4 neutropenia, anemia, thrombocytopenia, nausea, and vomiting were found between the two groups excepting for diarrhea.
Conclusion: The present study indicates that the addition of anti-epidermal growth factor receptor agents to first-line chemotherapy in advanced BTC offers an improved ORR and PFS, but not for OS. Further RCTs with larger samples are warranted to confirm our findings.
Keywords: biliary tract cancer, randomized controlled trials, molecular targeted agents, meta-analysis
Introduction
Biliary tract cancers (BTCs) are a heterogeneous group of malignancies including cholangiocarcinoma, gallbladder adenocarcinoma, and cancers of the ampulla of Vater.1 The potentially curative options are complete surgical resection.2 Unfortunately, this disease is often diagnosed at an advanced stage when surgical resection is no longer feasible. The treatment strategy for advanced BTC patients is systemic chemotherapy.3–6 Currently, gemcitabine plus cisplatin is the standard chemotherapy regimen for advanced BTCs due to a single positive randomized trial conducted by Valle et al in 2010, which showed that gemcitabine plus cisplatin significantly improved overall survival (OS) in comparison with gemcitabine alone (hazard ratio [HR] 0.64, 95% confidence interval [CI]: 0.52–0.80, P<0.001).7–9 However, the prognosis for advanced BTCs remains poor, with the median survival <1 year. Thus, development of a more effective treatment strategy is clearly desired.
During the past decades, several studies have been conducted to clarify the mechanism underlying the onset and proliferation of BTCs, accompanied by efforts directed at the development of molecular-targeted drugs for the treatment of this cancer.10–12 Until now, inhibition of epidermal growth factor receptor (EGFR) or vascular endothelial growth factor (VEGF) signal pathways are two potentially effective treatment strategy for advanced BTCs.13–16 In fact, several randomized controlled trials (RCTs) have been conducted to assess the efficacy and toxicities of molecular targeted agents (MTAs) in the treatment of advanced BTCs, but the results are controversial. As a result, we conduct this meta-analysis of RCTs to assess the role of MTAs as first-line treatment for advanced BTCs.
Material and methods
Selection of studies
For this meta-analysis, we searched for published RCTs in PubMed, Embase, and the Cochrane Library databases from January 2000 to March 2016 which met the following inclusion criteria: 1) phase II and III randomized controlled trails; 2) designed to compare chemotherapy in combination with a MTA versus chemotherapy alone for the treatment of advanced BTCs; and 3) had sufficient efficacy and toxicity data for extraction.
The following terms were used in the search: “bevacizumab,” “avastin,” “aflibercept,” “VEGFR-TKIs,” “sorafenib,” “nexavar,” “sunitinib,” “sutent,” “SU1248,” “vandetanib,” “caprelsa,” “ZD6474,” “axitinib,” “pazopanib,” “votrient,” “GW786034,” “regorafenib,” “apatinib,” “ramucirumab,” “nintedanib,” “BIBF1120,” “thalidomide,” “lenalidomide,” “angiogenesis inhibitors,” “cetuximab,” “panitumumab,” “erlotinib,” “gefitinib,” “afatinib,” “randomized,” “biliary tract cancer.” We also searched abstracts presented at the American Society of Clinical Oncology (http://www.asco.org/ASCO) conferences for relevant trials (from January 2004 to June 2015).
Data extraction and clinical endpoint
Data were extracted by two independent investigators according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement.17 All eligible articles underwent full-text review for relevancy and reporting outcomes of interest. The following information was extracted from study: name of first author, publication year, trial phase, number of enrolled patients, treatment regimens, median age, and primary endpoints. The five-item Jadad scale was used to roughly assess the quality of reports of clinical trials.18
Data analysis
Statistical analysis of the overall HR for OS and PFS, and the relative risk (RR) of overall response rate (ORR), and grade 3 or 4 toxicities were calculated using comprehensive meta-analysis software version 2.0 (Biostat, Englewood, NJ, USA). A statistical test with a P-value <0.05 was considered significant. HR >1 reflected more deaths or progression in MTA-containing regimen group, and RR >1 indicated more toxicities, ORR in MTA-containing regimen, and vice versa. Between-study heterogeneity was estimated using the χ2-based Q statistic.19 The I2 statistic was also calculated to evaluate the extent of variability attributable to statistical heterogeneity between trials. If heterogeneity existed, data were analyzed using a random effects model. In the absence of heterogeneity, a fixed effects model was used. Finally, the presence of publication bias was evaluated by using the Begg’s and Egger’s tests.20
Results
Search results
In the literature search, a total of 105 relevant studies were identified. After initial review, 12 recordings underwent additional review for the assessment of eligibility. Finally, a total of six published RCTs met the eligibility criteria and were included in the meta-analysis,21–26 while the remaining six trials were ineligible and so were excluded (Figure 1). Table 1 shows the baseline characteristics of each trial. A total of 855 patients were available for the meta-analysis. The quality of each included study was roughly assessed according to Jadad scale, and two trials had Jadad score of 5, and four trials had Jadad scores of 3.
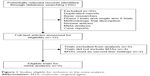  | Figure 1 Studies eligible for inclusion in the meta-analysis. |
  | Table 1 Baseline characteristic of included 6 trials for analysis |
OS
All six trials reported OS data in BTC patients. Our results indicated that the addition of MTAs to first-line chemotherapy did not improve OS in comparison with chemotherapy alone (HR 1.01, 95% CI: 0.90–1.13, P=0.93; Figure 2) using a fixed effects model (I2=0%, P=0.48). Similar results were observed in subgroup analysis according to MTAs, which showed that both AIs and anti-EGFR agents did not significantly improve OS when compared to controls (HR 0.99, 95% CI: 0.73–1.33, P=0.92, and HR 1.01, 95% CI: 0.89–1.14, P=0.89, respectively, Figure 2).
Progression-free survival
All six trials reported progression-free survival (PFS) data. The pooled HR for PFS showed that the addition of MTAs to chemotherapies in advanced BTCs had a tendency to improve PFS (HR 0.89, 95% CI: 0.78–1.02, P=0.097, Figure 3) when compared with chemotherapy alone. No significant heterogeneity was found (I2=32%, P=0.20), and the pooled HR for PFS was performed by using fixed effects model. The subgroup analysis was then performed according to targeted agents and found that the use of anti-EGFR agents was associated with a 27% reduced risk of disease progression compared to chemotherapy alone (HR 0.83; 95% CI: 0.70–0.99, P=0.036) in advanced BTC patients, while the addition of angiogenesis inhibitors (AIs) to chemotherapy in these patients did not significantly improve PFS (HR 1.00; 95% CI: 0.81–1.23, P=0.97).
ORR
As shown in Figure 4, the addition of MTAs to chemotherapies in BTCs significantly improved ORR when compared to chemotherapy alone (RR 1.96, 95% CI: 1.40–2.76, P<0.001), and there was no significant heterogeneity among included trials (I2=0%, P=0.37). The subgroup analysis was also performed according to MTAs, and the results showed that the addition of both anti-EGFR agents (RR 1.75, 95% CI: 1.19–2.56, P=0.005) and AIs (RR 3.07, 95% CI: 1.46–6.48, P=0.003) to first-line chemotherapy significantly improved ORR when compared to chemotherapy alone (Figure 4).
Safety
Equivalent frequencies of grade 3–4 neutropenia (RR 1.41, 95% CI: 0.89–2.24, P=0.14), thrombocytopenia (RR 1.36, 95% CI: 0.74–2.49, P=0.32), anemia (RR 1.12, 95% CI: 0.56–2.23, P=0.32), nausea (RR 1.01, 95% CI: 0.41–2.47, P=0.98), or vomiting (RR 0.71, 95% CI: 0.31–1.60, P=0.41) were found between groups excepting for diarrhea (RR 2.48, 95% CI: 1.20–5.10, P=0.014, Table 2). No significant inter-study heterogeneity was observed.
  | Table 2 Outcomes of grade 3 or 4 toxicities between the two groups |
Publication bias
Funnel plots did not observe any significant asymmetry (data not shown). Begg’s and Egger’s tests also did not detect publication bias (OS: P=0.71 and 0.55; PFS: P=0.71 and 0.77; and ORR: P=0.71 and 0.81, respectively).
Discussion
BTCs are considered to follow the sequence of dysplasia followed by hyperplasia of the bile duct epithelium. To date, several molecular pathways, such as EGFR and VEGF signal pathway, have been suggested to be involved in the onset and proliferation of BTCs. These pathways are expected to serve as potential targets for the treatment of BTCs. Indeed, several RCTs have been conducted to assess the efficacy and toxicities of MTAs in advanced BTCs, but the results are controversial, and the role of MTAs in advanced BTCs remains unknown.
A total of 855 patients from six RCTs are included for analysis, and the pooled results show that the addition of MTAs to chemotherapy in advanced BTC patients significantly improves ORR, and there is also a tendency to improve PFS in the combination regimens, but it does not translate into survival benefits. Subgroup analysis according to MTAs shows that the addition of anti-EGFR agents to first-line chemotherapy significantly improves ORR and PFS, while no significant OS benefits have been observed in anti-EGFR agents plus chemotherapy group. According to our results, the combination of anti-EGFR agents plus first-line chemotherapy could be suggested as first-line treatment for advanced BTC patients because of its improved ORR and PFS, but more evidence is still needed to identify patients who will most likely benefit for the specific anti-EGFR agents plus chemotherapy. In addition, the data are immature to arrive at an exact conclusion about the role of AIs in this setting, because only two RCTs assessing the efficacy of AIs plus chemotherapy in advanced BTCs are included for analysis.
To our best knowledge, this is the first and most comprehensive meta-analysis pooling well-designed RCTs to assess the efficacy and toxicities of adding MTAs to first-line chemotherapy in advanced BTCs patients. No publication bias is detected in this meta-analysis, and no single study would affect the pooled results. All the abovementioned statistical analyses show that the results are robust. However, several limitations should be acknowledged. First, this study is not a meta-analysis of individual patient data, which might provide further insight into the efficacy of specific MTAs in advanced BTCs patients. Second, different targeted agents for analysis were included, which would increase the clinical heterogeneity among included trials, which also make the interpretation of a meta-analysis more problematic, although we pool subgroup analysis according to targeted agents. Finally, the total sample size of trials included in this study is relatively small.
Conclusion
In conclusion, the addition of anti-EGFR agents to first-line chemotherapy significantly improves ORR and PFS, but not the OS. And the combined regimen also increases toxicity of diarrhea. With the present available data from randomized clinical trials, we could not clearly set the role of MTAs in the firstline treatment for advanced BTC patients. Additional RCTs with larger samples are warranted to confirm these findings.
Disclosure
The authors report no conflicts of interest in this work.
References
Chan E, Berlin J. Biliary tract cancers: understudied and poorly understood. J Clin Oncol. 2015;33(16):1845–1848. | ||
Jarnagin WR, Shoup M. Surgical management of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):189–199. | ||
Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30(16):1934–1940. | ||
Park JO, Oh DY, Hsu C, et al. Gemcitabine plus cisplatin for advanced biliary tract cancer: a systematic review. Cancer Res Treat. 2015;47(3):343–361. | ||
Fiteni F, Nguyen T, Vernerey D, et al. Cisplatin/gemcitabine or oxaliplatin/gemcitabine in the treatment of advanced biliary tract cancer: a systematic review. Cancer Med. 2014;3(6):1502–1511. | ||
Sasaki T, Isayama H, Nakai Y, Koike K. Current status of chemotherapy for the treatment of advanced biliary tract cancer. Korean J Intern Med. 2013;28(5):515–524. | ||
Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–1281. | ||
Liu H, Zhang QD, Li ZH, Zhang QQ, Lu LG. Efficacy and safety of gemcitabine-based chemotherapies in biliary tract cancer: a meta-analysis. World J Gastroenterol. 2014;20(47):18001–18012. | ||
Ghosn M, Kourie HR, El Rassy E, et al. Optimum chemotherapy for the management of advanced biliary tract cancer. World J Gastroenterol. 2015;21(14):4121–4125. | ||
Mathema VB, Na-Bangchang K. Current insights on cholangiocarcinoma research: a brief review. Asian Pac J Cancer Prev. 2015;16(4):1307–1313. | ||
Bizama C, Garcia P, Espinoza JA, et al. Targeting specific molecular pathways holds promise for advanced gallbladder cancer therapy. Cancer Treat Rev. 2015;41(3):222–234. | ||
Okusaka T, Ojima H, Morizane C, Ikeda M, Shibata T. Emerging drugs for biliary cancer. Expert Opin Emerg Drugs. 2014;19(1):11–24. | ||
Thomas MB. Systemic and targeted therapy for biliary tract tumors and primary liver tumors. Surg Oncol Clin N Am. 2014;23(2):369–381. | ||
Noel MS, Hezel AF. New and emerging treatment options for biliary tract cancer. Onco Targets Ther. 2013;6:1545–1552. | ||
Santoro A, Gebbia V, Pressiani T, et al. A randomized, multicenter, phase II study of vandetanib monotherapy versus vandetanib in combination with gemcitabine versus gemcitabine plus placebo in subjects with advanced biliary tract cancer: the VanGogh study. Ann Oncol. 2015;26(3):542–547. | ||
Gruenberger B, Schueller J, Heubrandtner U, et al. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a phase 2 study. Lancet Oncol. 2010;11(12):1142–1148. | ||
Moher D LA, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. | ||
Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352(9128):609–613. | ||
Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28(2):123–137. | ||
Vandenbroucke JP. Bias in meta-analysis detected by a simple, graphical test. Experts’ views are still needed. BMJ. 1998;316(7129):469–470; author reply 470–461. | ||
Chen JS, Hsu C, Chiang NJ, et al. A KRAS mutation status-stratified randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with cetuximab in advanced biliary tract cancer. Ann Oncol. 2015;26(5):943–949. | ||
Leone F, Marino D, Cereda S, et al. Panitumumab in combination with gemcitabine and oxaliplatin does not prolong survival in wild-type KRAS advanced biliary tract cancer: a randomized phase 2 trial (Vecti-BIL study). Cancer. 2016;122(4):574–581. | ||
Moehler M, Maderer A, Schimanski C, et al. Gemcitabine plus sorafenib versus gemcitabine alone in advanced biliary tract cancer: a double-blind placebo-controlled multicentre phase II AIO study with biomarker and serum programme. Eur J Cancer. 2014;50(18):3125–3135. | ||
Lee J, Park SH, Chang HM, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012;13(2):181–188. | ||
Malka D, Cervera P, Foulon S, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014;15(8):819–828. | ||
Valle JW, Wasan H, Lopes A, et al. Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC-03): a randomised phase 2 trial. Lancet Oncol. 2015;16(8):967–978. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



