Back to Journals » International Journal of General Medicine » Volume 15
Effects of Yunnan Baiyao on the Differentiation of HPDLFs on the Bio-Oss® Collagen Scaffold in vivo
Authors Yu X, Wang J, Han Q, Chu W, Lu S, Liu Y, Peng Y, Xu J, Shui Y
Received 7 February 2022
Accepted for publication 9 May 2022
Published 3 June 2022 Volume 2022:15 Pages 5395—5405
DOI https://doi.org/10.2147/IJGM.S359921
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Xiaohong Yu,1,* Jing Wang,1,* Qianqian Han,1,* Wen Chu,1 Shaowen Lu,1 Yu Liu,2 Yi Peng,1 Jie Xu,1 Yanqing Shui1
1Department of Periodontology, School and Hospital of Stomatology, Kunming Medical University, Yunnan, People’s Republic of China; 2Department of Oral and Maxillofacial Surgery, School and Hospital of Stomatology, Kunming Medical University, Yunnan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yanqing Shui, Department of Periodontology, Affiliated Stomatology Hospital of Kunming Medical University, Block C, Hecheng International, No. 1088 Haiyuan Middle Road, Wuhua District, Kunming, 650106, Yunnan, People’s Republic of China, Tel +86 15987150210, Email [email protected]
Background: Yunnan Baiyao, as a traditional Chinese herbal remedy with definite curative effect, has recently been proven can promote the proliferation of osteoblasts and differentiation of human periodontal ligament fibroblasts. Bio-Oss® scaffold is a porous bone graft material of natural and antigen-free bovine bone origin.
Methods: To observe the effect of Yunnan Baiyao on the differentiation of HPDLFs on the Bio-Oss® collagen scaffold in vivo, the HPDLFs-Bio-Oss® collagen complex was constructed in vitro, and Yunnan Baiyao aqueous solution was added, respectively. The complex was divided into Yunnan Baiyao group I (50 μg/mL), Yunnan Baiyao group II (100 μg/mL), positive control (rhBMP-2) group and sham group. HPDLFs were identified by immunocytochemistry and immunofluorescence. The compatibility of HPDLFs with Bio-Oss® collagen was observed by fluorescence microscope and scanning electron microscope. The specimens were taken for HE staining after 8 weeks since the complex was implanted into nude mice, and the expressions of osteocalcin (OC), bone sialoprotein (BSP), osteopontin (OPN), and collagen I (CON-I) were detected by immunohistochemistry and qRT-PCR.
Results: The number of capillaries and osteoblasts increased significantly after Yunnan Baiyao stimulation, and the expressions of BSP, OC, OPN and CON-I were increased after Yunnan Baiyao stimulation in a dose-dependent manner.
Conclusion: Yunnan Baiyao solution can promote the differentiation of HPDLFs and the generation of capillaries on Bio-Oss® collagen scaffold in a dose-dependent manner.
Keywords: Yunnan Baiyao, human periodontal ligament fibroblasts, Bio-Oss® collagen, differentiation
Introduction
Chronic periodontitis (CP) is a common bacterial infectious disease.1 It is an aggressive and extended form of gingivitis that has been present in the mouth continuously with deep periodontal tissue.2 The development of chronic periodontitis at an advanced stage can lead to bone loss in the periodontal area.3 Guided bone regeneration (GRB) is a proven and widely accepted method of bone augmentation that allows the growth of soft tissue fibroblasts around the barrier tissue to ensure that the slower migrating precursor osteoblasts have priority access to the bone defect area, allowing bone repair and regeneration in the defect area.4 HPDLFs are the main cells derived from periodontal ligament tissue. Studies have shown that HPDLFs can differentiate into cementum, alveolar bone, and periodontal ligament, which is conducive to tissue regeneration.5–7 Therefore, HPDLFs are considered as an ideal seed cell for periodontal tissue engineering. Bio-Oss® scaffold, as a bioactive material, is a porous bone graft material of natural and antigen-free bovine bone origin, whose natural structure determines that its physical and chemical properties are very similar to human bone, representing good biocompatibility.8 Bio-Oss® collagen is composed of Bio-Oss cancellous particles and 10% highly purified porcine collagen.9 It combines the advantages of bone and collagen. Collagen can support and protect cells, and at the same Bio-Oss® collagen is closely related to cell adhesion, growth, and phenotypic expression.10 The pressure of Bio-Oss® collagen has a pressure of 35 MPa and fine porosity (75–80% of volume), which can greatly raise its surface area. Such an increased surface area provides a matrix for the formation of blood vessels and a favorable scaffold condition for bone formation.11,12
Yunnan Baiyao is a traditional Chinese herbal remedy with definite curative effect.13 It is reported as a mixture of multiple herbs, such as borneol, stasis grass, bitter ginger, and geranium.14 The effect of Yunnan Baiyao on promoting tissue healing has been affirmed worldwide and has been widely applied in clinical practice.15 It is mainly used as an external or internal hemostatic drug since it enhanced prothrombin content and induces platelet aggregation and release.16 Also, it is prevalently used in treatment of bleeding,17 ulcer,18 infection,19 and inflammatory bowel disease.20 Recently, it was found that Yunnan Baiyao can promote the proliferation of osteoblasts and differentiation of human periodontal ligament fibroblasts (HPDLFs).21 Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2), as a mature cell growth factor, has achieved wide recognition from scholars all over the world.22 It has good osteoinductive activity and function of promoting cell differentiation. The addition of rhBMP-2 into fibroblast lines can evoke the expression of osteoblast phenotype, which stimulates the expression of osteocalcin and the activity of alkaline phosphatase.23 Thus far, rhBMP-2 has been proved by the US Food and Drug Administration to be used in localized alveolar ridge augmentations for tooth extraction defects.24 However, the high price limits the wide application of rhBMP-2 in clinical practice. Thus, Yunnan Baiyao, with its low cost and proven preparation process, is regarded as a promising substitute for clinical use in promoting cellular osteogenesis in patients with periodontitis with bone loss.
Therefore, in this study, HPDLFs have been selected as seed cells and Bio-Oss® collagen acted as scaffold material. HPDLFs-Bio-Oss® collagen complex was prepared in vitro and transplanted into nude mice for culture. HE staining, immunohistochemistry and qRT-PCR were applied to investigate whether Yunnan Baiyao could induce HPDLFs to differentiate into osteoblasts in vivo.
Experimental Materials and Methods
In vitro Culture and Identification of HPDLFs
Three healthy bicuspids from patients for orthodontic treatment (aged from 12 to 25 years old) were harvested from The Second People’s Hospital of Yunnan Province in 2019. This was approved by the Scientific and Research Ethics Committee of The Second People’s Hospital of Yunnan Province (No. 20180764). Patients were fully informed about specimen usage and data retrieval before the acquisition of specimens. Informed consents were obtained from patients, or from a parent and/or legal guardian for subjects if the subjects were under 18 years old. The surgical area was disinfected before extraction. After extraction, the tooth was rinsed with physiological saline with the crown facing down and the root facing up. The teeth were immediately placed in sterile PBS solution (Beijing Solarbio Technology Co., Ltd., Beijing, China) after the bloodstain was washed, and primary HPDLFs were isolated by enzyme digestion method.25,26 To be brief, the teeth were immersed in α-MEM medium (Yan’an Hospital, Kunming, Yunnan Province, Yunnan, China) containing 10% fetal bovine serum (FBS, Life Technologies, Carlsbad, CA, US), and 1/3 of periodontal ligament tissue in the root was carefully scraped with a sharp blade in the culture dish. The scraped tissue was cut into 5 mm2 pieces and was evenly spread on the wall of the 25 mL culture bottle at 3 mm intervals, in which 4 mL α-MEM medium containing 20% FBS and Penicillin-Streptomycin Solution (100 U/mL penicillin + 100 U/mL streptomycin, Life Technologies, Carlsbad, CA, US) were added. The culture bottle was inverted placed in a CO2 incubator (Thermo, CA, USA) and set at 37°C, 5% CO2, and 100% humidity as the experimental conditions, flipped after 3–4 hours, and the fluid was changed every other day. When the cells reached 75–80% of the bottom, the culture medium was aspirated and rinsed 3 times with PBS to prevent the digestion of trypsin by FBS. The cells were digested with 0.25% trypsin (Beijing Solarbio Technology Co., Ltd., Beijing, China) for 1 min, observed under an inverted microscope (Nikon, Japan), and the digestion was terminated by adding medium containing serum when the cells were found to be shrunken and rounded, the cell bodies were translucent and the gap between cells was enlarged. After centrifugation (1000 r/min, 5 min), the supernatant was removed and the cells were resuspended by adding the appropriate amount of medium. The 4th to 6th generation cells were taken for subsequent experiments.
Identification of HPDLFs was performed by immunocytochemistry and immunofluorescence.26 To conduct immunocytochemistry, the well-grown cells at passage 4 were selected and inoculated into the culture dish with a cover glass. After the cells covered the slides inside, the slides were taken out and fixed with the fixed solution (95% ethanol: absolute methanol = 1:1) for 30 min. 0.3% TritonX-100 was dropped on the surface of the slides. 3% hydrogen peroxide was added into the incubator at 37°C for 20 min, and sheep serum was sealed at 37°C for 30 min. After incubated with the first antibody, including antibody against Vimentin (ab92547, 1:500, abcam, Cambridge, UK) and Keratin (ab222116, 1:100, abcam, Cambridge, UK), at 37°C for 2 hours, the second antibody was incubated at 37°C for 30 min. Then, Horseradish peroxidase complex was added at 37°C for 30 min. Color reaction was developed by DAB (3, 3-diaminobenzidine, Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) chromogen solution. The color reaction by DAB color development kit continued for 3 min. Images were acquired by microscopy.
As for immunofluorescence, the cells were inoculated in 24-well slides respectively. After grown to about 80%, cells were fixed by 4% polyformaldehyde for 30 min at room temperature and washed with PBS. After blocking with 5% normal goat serum, cells were stained with antibody against Vimentin (bs-8533R, 1:300, bioss, MA, USA) and Keratin (bs-16952R, 1:250, bioss, MA, USA) at 4°C overnight. Then the primary antibody was visualized with Affinity purified Antibody Daylight 488 Labeled Goat anti-Rabbit IgG (H+L (5230–0385, 1:1000, KPL, MD, USA). Cell nuclei were stained with DAPI. Fluorescence images were taken using a fluorescence microscope.
Preparation of HPDLFs- Bio-Oss® Collage Complex
Yunnan Baiyao extract was obtained from the Yunnan Baiyao group Co., Ltd., Yunnan, China, Bio-Oss® collagen was obtained from Geistlich, Switzerland, and rhBMP-2 was obtained from Beijing Yinqiao Shenzhou Biotechnology Co., Ltd., Beijing, China. The suspension of HPDLFs at passage 4 (at the concentration of 2×106 cells) was combined with Bio-Oss® collagen by the two-step precipitation method. Excess α-MEM medium was aspirated during pre-wetting of Bio-Oss® collagen scaffolds. Also, 100 μL HPDLFs cell suspension was slowly dropped into both sides of the Bio-Oss® collagen. Afterwards, it was placed in the incubator with 5% CO2 at 37°C for 30 min. Six-well plates were cultured in CO2 incubator for 4 h, and 2 mL culture medium containing 79% α-MEM, 10% FBS and 1% Penicillin-Streptomycin Solution were added to continue cultivation for 24 hours, and then the medium containing 1% FBS was added as new culture medium for 24 hours. Finally, the culture medium was changed according to four groups: (1) Sham group: HPDLFs- Bio-Oss® collagen complex + 1% FBS, (2) positive control group: HPDLFs- Bio-Oss® collagen complex + 100 ng/mL rhBMP-2, (3) Baiyao I: HPDLFs- Bio-Oss® collagen complex + 50 μg/mL Baiyao, (4) Baiyao II: HPDLFs- Bio-Oss® collagen complex + 100 μg/mL Baiyao. Yunnan Baiyao infusion was diffused and prepared as 100 mL of Baiyao solution with α-MEM medium at a concentration of 10 μg/mL. It was then diluted into α-MEM medium containing 50 μg/mL of 1% FBS and 100 μg/mL of Yunnan Baiyao when needed to be dosed. HPDLFs- Bio-Oss® collagen complex was cultured for 7 days, and the state of HPDLFs cells in the complex and the combination with scaffold materials was observed by scanning electron microscope (SEM) and laser scanning confocal microscope (LSCM).
Transplantation of HPDLFs- Bio-Oss® Collagen Complex
Eleven healthy male BALB/c-nu nude mice (six weeks old, weight: 17–23 g, Beijing Vital River Laboratory Animal Technology Co. Ltd., Beijing, China) were selected for operation at SPF standard environment. A longitudinal incision of about 6 mm was made along the spine, concretely, the center of the scapula and the center of the hind limbs. The mosquito forceps were bluntly separated along the two sides of the incision. The scaffold materials were implanted into the four parts of subcutaneous tissue, respectively, and then the incision was sutured tightly. All experimental procedures involving animals were performed in accordance with the guidelines of the Animal Experimentation Center of Kunming Medical University and were approved by the Animal Ethics Committee of Kunming Medical University (No. kmu-eac-20183416). All experimental animals were operated under anesthesia, and every effort was made to minimize pain and death.
The mice were monitored at 30°C until fully awakened. Then, the mice were housed in a single cage with new bedding in SPF standard environment. After 14 days, the stitches were removed, and after 8 weeks, all nude mice were euthanized and all specimens were removed and numbered.
HE Staining and Immunohistochemistry
Routine decalcification, dehydration, paraffin embedding and sectioning were performed. Subsequently, the slices were immersed in the hematoxylin and hydrochloride alcohol and washed with running water. Afterwards, slices were dehydrated with ethanol at gradient degree, and then transparented with dimethylbenzene. The tissue slices were observed using the microscope. The number of capillaries and osteoblasts was calculated.
Subcellular localization and expression of osteogenic related proteins, ie, Osteocalcin (OC), Bone sialoprotein (BSP), osteopontin (OPN), and Collagen I (CON-I), in HPDLFs-Bio-Oss® collagen complex was detected by immunohistochemistry. To be specific, the animal tissues were routinely cut into sections, and the primary antibody against Collagen I (1:1500, Epitomics, CA, US), OPN (1:300, Epitomics, CA, US), OC (1:100, Santa Cruz Biochemistry, CA, USA), and BSP (1:500, Santa Cruz Biochemistry, CA, USA) was added and sections were stained overnight at 4°C overnight. Then, a biotinylated second antibody (IgG, 1:1000, Hebei Bio-High Technology Development Co., Ltd., Hebei, China) was added on the slices at 37°C for 20 min. DAB reagent kit was used for color reaction enhancement. The results were analyzed by Image pro-plus 6.0.
qPCR
The expression difference of osteogenic related proteins in transcription level was detected by fluorescence quantitative PCR. Total RNA was extracted using a TRIzol reagent (Invitrogen, CA, USA). The RNA was reversely transcribed to cDNA according to the instructions of PrimeScrip RT Reagent kit (Takara Biomedical Technology (Dalian) Co., Ltd., Dalian, China), primer synthesis was conducted by Sangon Biotech (Shanghai) Co., Ltd., Shanghai, China. PCR reaction system was prepared using cDNA and SYBR Green Real-Time PCR Master Mixes (Thermo Fisher Scientific, USA). The samples were processed using an ABI StepOne. The PCR amplification included an initial denaturation at 95°C for 1 min, 35 cycles of denaturation at 95°C for 1 min, annealing at 60°C for 2 min, and extension for 30s, at 72°C. β-actin was applied as an internal reference gene for mRNA level detection. The data were analyzed by −2ΔΔCt method. The primer sequences amplified by fluorescent quantitative PCR are shown in Table 1.
 |
Table 1 Primer Sequences for Real-Time Quantitative PCR |
Statistical Analysis
SPSS 13.0 statistical software was used for statistical analysis, and the experimental data were expressed as mean ± standard deviation (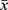 ± s). The comparison of difference significance between groups was conducted by one-way ANOVA, and further two-way comparisons were conducted by LSD-t-test, where P < 0.05 means there is statistical significance that exists.
± s). The comparison of difference significance between groups was conducted by one-way ANOVA, and further two-way comparisons were conducted by LSD-t-test, where P < 0.05 means there is statistical significance that exists.
Results
Identification of HPDLFs
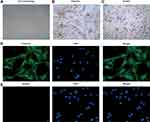
After passaged culture, most of the HPDLFs presented long shuttle or star-shaped morphology, and a few cells were flat and polygonal, resembling epithelioid cells (Figure 1A). Immunocytochemical staining showed that the vimentin was positively expressed in cytoplasm, and evenly distributed (Figure 1B). The keratin was negatively expressed in HPDLFs (Figure 1C). Identically, results of immunofluorescence showed that, vimentin was positively expressed in the membrane and cytoplasm of HPDLFs, while, Keratin was negatively expressed, indicating that the cultured cells were fibroblasts derived from mesoderm.
HPDLFs Have Good Compatibility with Bio-Oss® Collagen
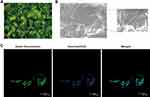
To establish the HPDLFs Bio-Oss® collagen complex, the suspension of HPDLFs at passage 4 was cultured with Bio-Oss® collagen for 7 days. Then, we observed the complex under the fluorescence microscope and scanning electron microscope (SEM) (Figures 2A and B). The spontaneous green fluorescence from Bio-Oss® collagen was observed under fluorescence microscope. Both fluorescence microscope and SEM results showed that, the human periodontal ligament fibroblasts were fully extended along the direction of collagen fibers, with long spindle, short spindle, or polygonal shapes. Small villi and folds were present on the cell surface. The cells are attached to the surface of Bio-Oss® collagen, and extended pseudopods adhered to and wrapped around the fibers. Some protrusions extended from the cell body and interconnected, some cells proliferate and join into multiple layers and arrange closely with each other, and some of these cells grow into the deep space. As shown in the laser scanning confocal microscope (LSCM) observation, Bio-Oss® collagen showed spontaneous green fluorescence in HPDLFs-Oss collagen complex, and the Hoechst33342-labeled nuclei showed blue fluorescence with uniformly sized nuclei that were uniformly attached to the surface and pores of the material. In some areas, cells and materials were completely fused together, indicating that HPDLFs have good compatibility with Bio-Oss® collagen (Figures 2C).
Yunnan Baiyao Can Promote the Differentiation of HPDLFs to Osteoblasts
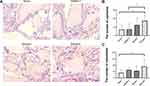
The cells in each group were implanted inside the scaffold. HE was conducted to observe the differentiation of HPDLFs into osteoblasts. In the sham group, the adhesion of cells to the scaffold was poor, and very few osteoblasts were arranged in layers. The material had capillary growth at the edges, but no blood vessels were seen in its center. It can be obviously noted that, in the Yunnan Baiyao I group, Yunnan Baiyao II group and positive control group, there are square or short columnar osteoblasts arranged in layers, and capillaries are seen growing into the edge and center of the material (Figure 3A). Five fields of view of each sample were randomly selected for capillary and osteoblast counts. The number of capillaries and osteoblasts in the Yunnan Baiyao II group was significantly different from that of sham group (Figure 3B and C). It indicates that Yunnan Baiyao can promote the differentiation of HPDLFs to osteoblasts.
Yunnan Baiyao Promote the Expression of Osteogenic Related Proteins
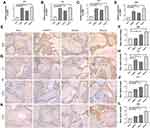
To further investigate the mechanism of differentiation of HPDLFs into osteoblasts, the expression of osteogenic related proteins was detected by qRT-PCR. The results showed that, compared with sham group, the mRNA expression of osteogenic related proteins, BSP, OC, OPN and CON-I were increase in HPDLFs-Bio-Oss collagen complex after stimulation of Yunnan Baiyao and rhBMP-2 (Figure 4A–D). Also, the results of immunohistochemistry showed that osteogenic related proteins were all expressed in the interstitium and cytoplasm of the cells. BSP was negatively expressed in sham group, and expression of BSP was significantly increased after Yunnan Baiyao stimulation compared to sham group (Figure 4E and F). OC was low-positively expressed in sham group, and positive expression was observed after Yunnan Baiyao and rhBMP-2 stimulation (Figure 4G and H) Positive expression of OPN was observed after Yunnan Baiyao and rhBMP-2 stimulation, mainly around osteoclasts, while OPN was negative in the sham group (Figure 4I and J). Positive expression of CON-I was observed in Yunnan Baiyao I groups and Yunnan Baiyao II groups, and low positive expression was observed in rhBMP-2 group, while negative expression was observed in sham group (Figure 4K and L). There was significant increase in mean optical density and integrated optical density value of osteogenic related protein after Yunnan Baiyao stimulation in dose dependence, indicating that Yunnan Baiyao, as a cell like substance, can promote the osteogenic differentiation of HPDLFs.
Discussion
In the experiment, the results of HE staining showed that Yunnan Baiyao could effectively promote the formation of new capillaries of HPDLFs on Bio-Oss® collagen scaffold, indicating that Yunnan Baiyao could effectively promote the formation of new capillaries in nude mice. The formation of capillaries provides the cells enough nutrition. Previous study showed that if there is no oxygen supply and nutrient supply vessels within 200μm around the living cells in vivo, the cells will have metabolic disorders and cannot function normally.27 Previous study has shown that Yunnan Baiyao can promote the expression of basic fibroblast growth factor (bEGF) and vascular endothelial growth factor (VEGF), thus accelerating the growth of blood vessels.28 VEGF is a significant regulator of angiogenesis and endothelial-cell-specific mitogen,29 and recent studies showed that exogenous VEGF in the conditioned medium of osteoblasts can stimulate the formation of calcium nodules in a dose-dependent manner.30 In this experiment, the formation of new capillaries may be promoted through this mechanism, and it is confirmed that 100 μg/mL Yunnan Baiyao is an effective concentration for promoting osteogenic differentiation and angiogenesis of HPDLFs and generation of new capillaries.
Both BSP and OC are markers of the late stage of osteogenic mineralization, and their appearance indicates the terminal differentiation of osteoblasts. BSP is a marker of bone metabolism, which can activate osteoblasts, dental osteocyte, or osteoblast like cells, and promote the differentiation of cells into osteoblasts, thus leading to bone mineralization.31,32 Due to the high expression of BSP in differentiated and mature osteoblasts, BSP can be used as a late marker of osteogenic differentiation.33 Our results showed that BSP was highly expressed in Yunnan Baiyao II group, which indicated that Yunnan Baiyao at concentration of 100 μg/mL could promote osteoblast maturation, mineralization, and deposition, and promote bone formation. In addition, BSP also played an important role in the process of angiogenesis, and the endothelial cell surface of new capillary endothelial cells could express and recognize BSP, which could support vascular growth in bone matrix calcification,34 therefore, BSP is closely related to the growth of blood vessels during the bone formation process.
OC mainly occurs in the bone mineralization stage, whose expression boosts during bone growth and hydroxyapatite deposition, which is a biochemical marker of late differentiation of osteoblasts, which can reflect the activity of osteoblasts and bone turnover level,35 and has been applied as one of the markers for osteoblasts identification. The results showed that OC was highly expressed in Yunnan Baiyao groups, indicating that the osteoblast activity of Yunnan Baiyao II group was significant, which therefore contributed to the formation of bone mineralization. OC can also promote the combination of Ca2+ and Hydroxyapatite (HA), inhibiting the formation of abnormal HA crystals, inhibiting the mineralization rate of growing cartilage, and promoting a series of normal mineralization processes of bone tissues such as the mineral deposition of bone tissue.36 In this experiment, HE staining results showed that no cartilage was found. Therefore, it is speculated that OC may inhibit the formation of cartilage growth factors and only form a channel to promote osteogenesis and mineralization.
OPN is a negatively charged secreted phosphorylated glycoprotein, most of which are synthesized by pro-osteoblasts, osteoblasts, and osteocytes.35 OPN can induce the differentiation and mineralization of osteoblasts, promoting the maturation of bone matrix, regulating the formation of HA crystals, promoting the formation and reconstruction of mineralized bone tissue, which plays an important role in the mineralization and absorption of bone matrix.37 Therefore, OPN is also considered as one of the main markers of osteoblast maturation and differentiation.38 Our results indicated that Yunnan Baiyao could promote the synthesis of OPN in osteoblasts. The high expression of OPN could regulate the growth of hydroxyapatite crystals and promote the mineralization of osteoblasts.
The secretion of CON-I began at the early stage of osteoblast differentiation, and gradually increased with the maturation of the matrix.39 The results showed that CON-I is highly expressed after Yunnan Baiyao stimulation, indicating that Yunnan Baiyao continues to promote osteoblast differentiation. CON-I is one of the main components of the bone matrix and plays a vital role in the differentiation of osteoblasts, and the normal structure and quantity of CON-I are the basis for such functions.40–42 Studies have shown that Yunnan Baiyao can promote the mRNA expression of OC, OPN, CON-I, OSX, RUNX2 and ALP during tooth formation and osteogenic process of apical papilla stem cells.43,44 Wang et al44 showed that Yunnan Baiyao can promote the production of BMP and TGF-β.
In recent years, some researches have shown that Yunnan Baiyao can promote the repair of bone defects and promote guided bone regeneration.14,43,45 Yang et al46 have proved that in the early and middle stages of fracture healing, Yunnan Baiyao can improve the gene expression of VEGF and CD31 in callus tissue after fracture, accelerating the reconstruction of microvascular at the broken end, improving the blood supply of fracture sites, which accelerates fracture healing. Wang et al44 showed that different components of Yunnan Baiyao could, respectively, increase the mRNA expression of OC, OPN and BSP, and the results of this experiment were consistent with it.
In conclusion, Yunnan Baiyao group can effectively promote the osteogenic expression of HPDLFs, and the effect of Yunnan Baiyao with the concentration of 100 μg/mL was more obvious. This provides a theoretical basis for Yunnan Baiyao as a cell-like substance to promote the osteogenic differentiation of HPDLFs and the generation of capillaries, and verified the promising value of Yunnan Baiyao to be used in clinical applications to promote cellular osteogenic differentiation and help patients with periodontitis who have bone defects.
Data Sharing Statement
All data, models, or code generated or used during the study are available from the corresponding author by request.
Consent for Publication
Some or all data, models, or code generated or used during the study are available from the corresponding author by request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Yunnan Provincial Department of Science and Technology-Kunming Medical University Fund(202001AY070001-254; 2010CD214; 202101AY070001-193).
Disclosure
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
References
1. Cardoso EM, Reis C, Manzanares-Céspedes MC. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad Med. 2018;130(1):98–104. doi:10.1080/00325481.2018.1396876
2. Li C, Lv Z, Shi Z, et al. Periodontal therapy for the management of cardiovascular disease in patients with chronic periodontitis. Cochrane Database Syst Rev. 2017;11(11):Cd009197. doi:10.1002/14651858.CD009197.pub3
3. Wang J, Wang B, Lv X, et al. NIK inhibitor impairs chronic periodontitis via suppressing non-canonical NF-κB and osteoclastogenesis. Pathog Dis. 2020;78(7). doi:10.1093/femspd/ftaa045.
4. Johnson TB, Siderits B, Nye S, et al. Effect of guided bone regeneration on bone quality surrounding dental implants. J Biomech. 2018;80:166–170. doi:10.1016/j.jbiomech.2018.08.011
5. Nohutcu RM, McCauley LK, Koh AJ, et al. Expression of extracellular matrix proteins in human periodontal ligament cells during mineralization in vitro. J Periodontol. 1997;68(4):320–327. doi:10.1902/jop.1997.68.4.320
6. Ebe Y, Nakamura T, Hasegawa-Nakamura K, et al. Effect of interleukin-1β on bone morphogenetic protein-9-induced osteoblastic differentiation of huma n periodontal ligament fibroblasts. Eur J Oral Sci. 2021;129(4):e12792. doi:10.1111/eos.12792
7. Zhao. C, Chen Q, Yu S, et al. Effect of interleukin-22 on osteogenic differentiation and the osteoclastogenic response of human per iodontal ligament fibroblasts in vitro. J Periodontol. 2020;91(8):1085–1097. doi:10.1002/JPER.19-0470
8. Spector M. Anorganic bovine bone and ceramic analogs of bone mineral as implants to facilitate bone regeneration. Clin Plast Surg. 1994;21(3):437–444. doi:10.1016/S0094-1298(20)31021-X
9. Li Q, Yu T, Wang F, et al. Endothelial progenitor cells with stem cells enhance osteogenic efficacy. Am J Transl Res. 2020;12(6):2409–2424.
10. Alekseeva IS, Rachinskaia OA, Volkov AV, et al. [A comparative evaluation of bone tissue formation by tissue scaffold and osteoplastic material «Bio-Oss» transplantation in the maxillary sinus floor]. Stomatologiia. 2012;91(6):41–44. Russian.
11. Hallman M, Cederlund A, Lindskog S, et al. A clinical histologic study of bovine hydroxyapatite in combination with autogenous bone and fibrin g lue for maxillary sinus floor augmentation. Results after 6 to 8 months of healing. Clin Oral Implants Res. 2001;12(2):135–143. doi:10.1034/j.1600-0501.2001.012002135.x
12. Ying Y, Li B, Liu C, et al. A biodegradable gelatin-based nanostructured sponge with space maintenance to enhance long-term osteogenesis in maxillary sinus augmentation. J Biomater Appl. 2021;35(6):681–695. doi:10.1177/0885328220964446
13. Ren JL, Dong H, Han Y, et al. Network pharmacology combined with metabolomics approach to investigate the protective role and detoxification mechanism of Yunnan Baiyao formulation. Phytomedicine. 2020;77:153266. doi:10.1016/j.phymed.2020.153266
14. Ren X, Zhu Y, Xie L, et al. Yunnan Baiyao diminishes lipopolysaccharide-induced inflammation in osteoclasts. J Food Biochem. 2020;44(6):e13182. doi:10.1111/jfbc.13182
15. Ren X, Zhang M, Chen L, et al. The anti-inflammatory effects of Yunnan Baiyao are involved in regulation of the phospholipase A2/arachidonic acid metabolites pathways in acute inflammation rat model. Mol Med Rep. 2017;16(4):4045–4053. doi:10.3892/mmr.2017.7104
16. Chen L, Jiang H, Xing G, et al. Effects of Yunnan Baiyao adjunct therapy on postoperative recovery and clinical prognosis of patients with traumatic brain injury: a randomized controlled trial. Phytomedicine. 2021;89:153593. doi:10.1016/j.phymed.2021.153593
17. Rådmark O, Werz O, Steinhilber D, et al. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim Biophys Acta. 2015;1851(4):331–339. doi:10.1016/j.bbalip.2014.08.012
18. Ladas EJ, Karlik JB, Rooney D, et al. Topical Yunnan Baiyao administration as an adjunctive therapy for bleeding complications in adolescents with advanced cancer. Support Care Cancer. 2012;20(12):3379–3383. doi:10.1007/s00520-012-1598-1
19. Yang B, Xu Z-Q, Zhang H, et al. The efficacy of Yunnan Baiyao on haemostasis and antiulcer: a systematic review and meta-analysis of randomized controlled trials. Int J Clin Exp Med. 2014;7(3):461–482.
20. Li R, Alex P, Ye M, et al. An old herbal medicine with a potentially new therapeutic application in inflammatory bowel disease. Int J Clin Exp Med. 2011;4(4):309–319.
21. Chu W, Lei Y, Shui Y. Effects of Yunnan Baiyao with different concentration gradients on the proliferation of HPDLFs cultured in vitro and the expression of ALP and OC. J Dent Periodontol. 2009;19(10):
22. Um IW, Ku J-K, Kim Y-K, et al. Histological review of Demineralized Dentin Matrix as a carrier of rhBMP-2. Tissue Eng Part B Rev. 2020;26(3):284–293. doi:10.1089/ten.teb.2019.0291
23. Um IW. Demineralized Dentin Matrix (DDM) As a carrier for recombinant human Bone Morphogenetic Proteins (rhB MP-2). Adv Exp Med Biol. 2018;1077:487–499.
24. Nguyen PD, Lin CD, Allori AC, et al. Scaffold-based rhBMP-2 therapy in a rat alveolar defect model: implications for human gingivoperiosteoplasty. Plast Reconstr Surg. 2009;124(6):1829–1839. doi:10.1097/PRS.0b013e3181bf8024
25. Tang L, Li X, Bai Y, et al. MicroRNA-146a negatively regulates the inflammatory response to Porphyromonas gingivalis in human periodontal ligament fibroblasts via TRAF6/p38 pathway. J Periodontol. 2019;90(4):391–399. doi:10.1002/JPER.18-0190
26. Wan W, He C, Du C, et al. Effect of ILK on small-molecule metabolism of human periodontal ligament fibroblasts with mechanical stretching. J Periodontal Res. 2020;55(2):229–237. doi:10.1111/jre.12706
27. Colton CK. Implantable biohybrid artificial organs. Cell Transplant. 1995;4(4):415–436. doi:10.1177/096368979500400413
28. Yu M. Modern pharmacological effects and new clinical uses of Yunnan Baiyao. Chin J Ethnomed Ethnopharm. 2009;18(09):64–65.
29. Wu L, Gu Y, Liu L, et al. Hierarchical micro/nanofibrous membranes of sustained releasing VEGF for periosteal regeneration. Biomaterials. 2020;227:119555. doi:10.1016/j.biomaterials.2019.119555
30. Street J, Bao M, deGuzman L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99(15):9656–9661. doi:10.1073/pnas.152324099
31. Wade-Gueye NM, Boudiffa M, Vanden-Bossche A, et al. Absence of bone sialoprotein (BSP) impairs primary bone formation and resorption: the marrow ablation model under PTH challenge. Bone. 2012;50(5):1064–1073. doi:10.1016/j.bone.2012.02.014
32. Valverde P, Tu Q, Chen J. BSP and RANKL induce osteoclastogenesis and bone resorption synergistically. J Bone Miner Res. 2005;20(9):1669–1679. doi:10.1359/JBMR.050511
33. Vijaykumar A, Dyrkacz P, Vidovic-Zdrilic I, et al. Expression of BSP-GFPtpz Transgene during osteogenesis and reparative dentinogenesis. J Dent Res. 2020;99(1):89–97. doi:10.1177/0022034519885089
34. Dong C, Goldschmidt-Clermont PJ. Bone sialoprotein and the paradox of angiogenesis versus atherosclerosis. Circ Res. 2000;86(8):827–828. doi:10.1161/01.RES.86.8.827
35. Bailey S, Karsenty G, Gundberg C, et al. Osteocalcin and osteopontin influence bone morphology and mechanical properties. Ann NY Acad Sci. 2017;1409(1):79–84. doi:10.1111/nyas.13470
36. Hunter GK, HAUSCHKA PV, POOLE RA, et al. Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. Biochem J. 1996;317(Pt 1):59–64. doi:10.1042/bj3170059
37. Qin X, Yan M, Wang X, et al. Cancer-associated fibroblast-derived IL-6 promotes head and neck cancer progression via the osteopontin-NF-kappa B signaling pathway. Theranostics. 2018;8(4):921–940. doi:10.7150/thno.22182
38. McKee MD, Nanci A. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: ultrastruc tural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc Res Tech. 1996;33(2):141–164. doi:10.1002/(SICI)1097-0029(19960201)33:2<141::AID-JEMT5>3.0.CO;2-W
39. Rasheeda K, Fathima NN. Trigonelline hydrochloride: a promising inhibitor for type I collagen fibrillation. Colloids Surf B Biointerfaces. 2018;170:273–279. doi:10.1016/j.colsurfb.2018.06.030
40. Lynch MP, Stein JL, Stein GS, et al. The influence of type I collagen on the development and maintenance of the osteoblast phenotype in pr imary and passaged rat calvarial osteoblasts: modification of expression of genes supporting cell gr owth, adhesion, and extracellular matrix mineralization. Exp Cell Res. 1995;216(1):35–45. doi:10.1006/excr.1995.1005
41. Yamauchi M, Sricholpech M, Terajima M, et al. Glycosylation of Type I collagen. Methods Mol Biol. 2019;1934:127–144.
42. Yamazaki S, Higuchi Y, Ishibashi M, et al. Collagen type I induces EGFR-TKI resistance in EGFR-mutated cancer cells by mTOR activation through A kt-independent pathway. Cancer Sci. 2018;109(6):2063–2073. doi:10.1111/cas.13624
43. Pang X, Wang Y, Wu J, et al. Yunnan baiyao conditioned medium promotes the odonto/osteogenic capacity of stem cells from apical papilla via nuclear factor kappa B signaling pathway. Biomed Res Int. 2019;2019:9327386. doi:10.1155/2019/9327386
44. Wang T. Study on the bone formation function of the main components of Yunnan Baiyao. Kunming Medical University; 2007.
45. He H, Ren X, Wang X, et al. Therapeutic effect of Yunnan Baiyao on rheumatoid arthritis was partially due to regulating arachidon ic acid metabolism in osteoblasts. J Pharm Biomed Anal. 2012;59:130–137. doi:10.1016/j.jpba.2011.10.019
46. Yang QQ, Hu ZM, Pu B, et al. Effects of major components in Yunnan Baiyao on angiogenesis during the process of fracture healing. J Yunnan Univ Tradit Chin Med. 2011;34(05):
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.