Back to Journals » Drug Design, Development and Therapy » Volume 13
Effects Of Triptolide On Tooth Movement And Root Resorption In Rats
Authors Yang F, Wang XX, Ma D, Cui Q, Zheng DH, Liu XC, Zhang J
Received 31 May 2019
Accepted for publication 11 November 2019
Published 25 November 2019 Volume 2019:13 Pages 3963—3975
DOI https://doi.org/10.2147/DDDT.S217936
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Fan Yang,1,2 Xu Xia Wang,1,3 Dan Ma,1,2 Qun Cui,1,2 De Hua Zheng,1,2 Xiao Can Liu,1,2 Jun Zhang1,2
1Shandong Provincial Key Laboratory of Oral Tissue Regeneration & Shandong Engineering Laboratory for Dental Materials and Oral Tissue Regeneration, School of Stomatology, Shandong University, Jinan, Shandong Province, People’s Republic of China; 2Department of Orthodontics, School of Stomatology, Shandong University, Jinan, Shandong Province, People’s Republic of China; 3Department of Oral and Maxillofacial Surgery, School of Stomatology, Shandong University, Jinan, Shandong Province, People’s Republic of China
Correspondence: Jun Zhang
Department of Orthodontics, School of Stomatology, Shandong University, 44 Wenhua West Road, Jinan 250012, People’s Republic of China
Tel +86 139 5310 9816
Fax +86-531-88565657
Email [email protected]
Purpose: The aim of this study was to investigate the effects of triptolide on the tooth movement and root resorption in rats during orthodontic treatment.
Material and methods: A total of 48 male Wistar rats were divided into three groups of 16 each. The right maxillary first molars of rats were drawn mesially by closed coil nickel–titanium spring with a force of 50 g. The two experimental groups received intraperitoneal injections of triptolide for 14 days at a dose of 15 μg/kg/day and 30 μg/kg/day, respectively. The control group received vehicle injections. After 14 days, the rats were humanely killed. The amount of tooth movement was measured. Eight rats from each group were randomly chosen for analysis of the percentage of root resorption area by scanning electron microscopy. For the remaining eight rats in each group, the H&E staining, tartrate-resistant acid phosphatase (TRAP) staining and immunohistochemistry analysis were performed.
Results: The amount of tooth movement and the ratio of root resorption area were significantly decreased in the triptolide-treated rats. The number of TRAP-positive cells was significantly lower in triptolide-treated groups. Moreover, the expression of nuclear factor kappa B ligand (RANKL) was reduced. In contrast, the expression of osteoprotegerin was significantly up-regulated. In the tension side, the expressions of runt-related transcription factor 2 and osteocalcin were significantly enhanced by triptolide injection.
Conclusion: Triptolide injection could arrest orthodontic tooth movement and reduce root resorption in rats via inhibition of osteoclastogenesis. In addition, triptolide may exert a positive effect on osteoblastogenesis.
Keywords: triptolide, osteoclast, scanning electron microscope, tooth movement, bone formation
Introduction
Orthodontic tooth movement (OTM) is achieved by the remodeling of alveolar bone and periodontal ligament (PDL) with the application of sustained mechanical force exerted on teeth. During the orthodontic treatment, alveolar bone is resorbed at the pressure side while it is formed at the tension side. Bone resorption and bone formation are tightly coupled via the linkage between osteoclast and osteoblast. The force-induced strains alter the periodontal microenvironment releasing various cytokines, chemokines and inflammatory factors such as nuclear factor kappa B ligand (RANKL), osteoprotegerin (OPG), and tissue necrosis factor-α (TNF-α).1,2 These molecules participate in the regulation of this complex bone remodeling process. Several studies have been conducted to investigate the effect of pharmacological agents on OTM.3 A recent study found clodronate can arrest tooth movement following the dose- and time-dependent manners.4 Another study found parathyroid hormone could facilitate bone remodeling and accelerate tooth movement.3
Orthodontically induced inflammatory root resorption (OIRR) is one of the most common but unpredictable sequela of orthodontic treatment, which will lead to the permanent loss of tooth structure. Previous studies showed that approximately 90% of the patients who underwent orthodontic treatment presented a different extent of root resorption, and 3% of the patients had severe root resorption.5,6 The etiology of OIRR is multifactorial, including individual susceptibility, orthodontic force, type of OTM, and duration of orthodontic treatment.7 A recent study also found vitamin D may be associated with the pathology of root resorption.8 Although the exact mechanism of OIRR remains unclear, the process is considered as a part of hyaline elimination. During the removal of hyalinized tissues, the cementum and dentin are removed, resulting in root resorption.9,10 The local sterile inflammatory process induced by mechanical force is essential for OTM and it is also responsible for OIRR.2 The methods to predict and prevent root resorption are needed now.
Triptolide, a diterpenoid trioxide isolated from the ancient Chinese herb Tripterygium wilfordii Hook F (TWHF), has been widely used to treat autoimmune and inflammatory diseases in China.11,12 As the most active and promising component of TWHF, triptolide exhibits a wide range of biological and pharmacological activities, such as antitumor, antioxidant, anti-inflammatory, and immunosuppressive properties.13–16 Researches have found triptolide could inhibit bone loss and up-regulate the bone mineral density.17 However, the underlying molecular and cellular mechanisms are not fully understood. Therefore, we hypothesized that triptolide may have a potential efficacy on the bone remodeling and root resorption in orthodontic treatment.
To the best of our knowledge, there is no study demonstrating if triptolide exhibits possible benefits or side effects on orthodontic treatment. Thus, in the present study, we investigated the effects of triptolide on tooth movement and root resorption during orthodontic treatment in rats and explored the underlying mechanisms.
Materials And Methods
Reagents
Triptolide was obtained from Solarbio Science & Technology (Beijing, China). The polyclonal anti-rabbit RANKL, anti-rabbit runt-related transcription factor 2 (RUNX2), anti-rabbit osteocalcin (OCN) were purchased from Proteintech (Wuhan, China). The polyclonal anti-rabbit OPG was obtained from Solarbio Science & Technology. The H&E staining kit and tartrate-resistant acid phosphatase (TRAP) staining kit were both purchased from Solarbio Science & Technology. The Biotin–Streptavidin HRP detection system is acquired from ZSGB-BIO (Beijing, China).
Ethical Statement
The animal experiments in this study were performed according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by the Institutional Animal Care and Use Committee of Shandong University.
Experimental Animals And Groups
Forty-eight 6-week-old male Wistar rats (260–280 g) were purchased from SPF Biotechnology (Beijing, China). The rats were pair-housed in standard plastic cages under specific pathogen-free conditions with controlled temperature (22±1°C), humidity (55±10%) and a 12-h light/dark cycle. The interior noise was controlled below 60 dB. They were provided with a powder diet and water ad libitum. The general condition and weight of each animal in this study were monitored during the experiment. All the animals were acclimated for 1 week before the start of the experiment.
The animals were randomly divided into three groups of 16 rats each as follows: one control group and two triptolide-treated groups (high-dose and low-dose groups). Triptolide was solubilized in 0.05% dimethyl sulfoxide then diluted in saline. The rats in the high-dose group were administrated with triptolide at a dose of 30 µg/kg/day by intraperitoneal injection for 14 days. The rats in the low-dose group were administrated with triptolide at a dose of 15 µg/kg/day by intraperitoneal injection for 14 days. The control group received the same volume of saline according to the same protocol.
Animal Model Establishment
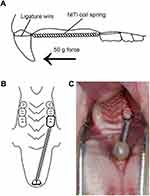
All the rats in three groups received orthodontic treatment. Induction of tooth movement was performed as described previously.18 The animals were anesthetized with 3% phenobarbital sodium at a dose of 35 mg/kg by intraperitoneal injection. After anaesthetization, the closed coil nickel–titanium spring (GRIMED Medical, Beijing, China) was inserted between the right maxillary first molar and incisors. A 0.5-mm-deep groove was prepared around the neck of the maxillary incisors. The closed coil nickel–titanium spring was fixed with a 0.20 mm stainless wire around the neck of teeth and the stainless wire was embedded into the groove to prevent the orthodontic appliance detaching from the incisors. In addition, two maxillary incisors were bonded together as a whole by light-curing composite resin (Charisma, Heraeus Kulzer, Germany) to enhance the anchorage. The force of the coil spring after activation is approximately 50 g verified by an ergometer (Westlake Biomaterial, Hangzhou, China). The right maxillary first molar was drawn mesially with the two incisors as anchorage and the anchorage loss was neglected. Figure 1 shows the orthodontic appliance used in this study. The monitoring of appliance breakage was performed daily.
Amount Of OTM
On the 14th day after orthodontic treatment, the distance between first and second molars as the amount of tooth movement was measured with a caliper (accuracy of 0.02 mm) (Measuring & Cutting Tool Works, Shanghai, China) from the distal contact area of the first molar to the mesial contact area of the second molar. All measurements were repeated three times by the same operator to ensure the consistency of our method.
Scanning Electron Microscope
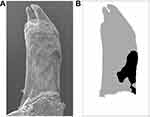
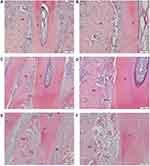
The eight rats in each group were sacrificed under anesthesia on the 14th day. For evaluating root resorption, the right maxillary first molars were extracted in toto. The teeth were soaked in 1% hypochlorite solution for 15 mins. Then, the PDL was carefully removed. Both distobuccal and distopalatal roots were exposed completely. Then, the teeth were dried and scanned under a scanning electron microscope (SEM, SU8010, HITACHI, Tokyo, Japan) at the same orientation and distance. The data of mesial surfaces of distobuccal and distopalatal roots were obtained and saved as digital photographs. The resorption areas on the two-thirds of the cervical side of both distobuccal and distopalatal roots were measured by ImageJ software. The resorption craters on the apical third region were ignored because the root apices of rats which covered by cellular cementum changed incessantly in shape and dimension by secondary cementum. We evaluated the root resorption by the percentage of the resorption area on the mesial side of distobuccal and distopalatal roots (Figure 2).
Tissue Preparation
The remaining eight rats of each group were generally anesthetized and perfused transcardially with 4% paraformaldehyde (pH 7.2–7.6) for fixation. Then, the maxilla of each rat including the first and second molars was carefully dissected and fixed in 10% buffered formalin (pH 7.2–7.6). Then, the specimens were decalcified in 10% ethylene diamine tetraacetic acidat 4°C for 8 weeks. Afterward, the samples were dehydrated through the ethanol series, rendered transparent by xylene, and embedded in paraffin wax. Serial sections with a thickness of 5 μm in a mesiodistal direction parallel to the long axis of the distopalatal root were cut.
Histological Evaluation
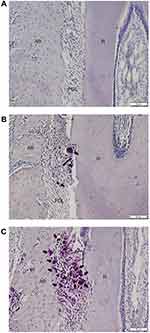
The sections were routinely stained with H&E to examine the remodeling of periodontal tissues and root resorption. To identify the osteoclastogenesis, the TRAP staining was performed according to the manufacturer’s instruction. The sections were counterstained with hematoxylin. The region of interest was the distopalatal root and adjacent alveolar bone, as the structure of distopalatal root was stable. The multinucleated TRAP-positive cells located around the alveolar bone were considered as osteoclasts and those located on root surface were considered as odontoclasts. Under high magnification (×200), the TRAP-positive cells within the periodontal area in the mesial side of distopalatal root were counted. The process was performed in five sections per animal, and the mean values were calculated.
Immunohistochemistry
Sections were deparaffinized and treated with 3% hydrogen peroxidase in methanol for 15 mins at room temperature to inhibit endogenous peroxidase activity. After washing in phosphate buffer solution (PBS), sections were preincubated in goat serum for 35 mins. Then, the sections were incubated with the polyclonal anti-rabbit RANKL (working dilution, 1:300), polyclonal anti-rabbit OPG (working dilution, 1:50), polyclonal anti-rabbit RUNX2 (working dilution, 1:400), and polyclonal anti-rabbit OCN (working dilution, 1:400) in a humid chamber for 16 hrs at 4°C. Sections were rinsed in PBS and stained with Biotin–Streptavidin HRP detection system according to the manufacturer’s instruction. All sections were counterstained with hematoxylin. The negative control was submitted to the same procedure as described above but incubated with PBS instead of primary antibodies.
Statistical Analysis
Normality was assessed with Shapiro–Wilk test, and as data were found to be normally distributed, they were expressed as mean±SD and parametric statistical tests were used. One-way ANOVA followed by the Newman–Keuls' multiple comparison test was performed to analyze the statistical differences in the amount of tooth movement, number of TRAP-positive cells, the ratio of root resorption, and the mean optical density (MOD) values of RANKL, OPG, OCN, RUNX2 among the control and triptolide-treated groups using the SPSS software (22.0). The p value<0.05 was considered statistically significant.
Results
General Condition
No animals died during the experiment. No differences were observed in the body weights among different groups at the end of the experiment.
Distance Of Tooth Movement
After 14 days, the amounts of tooth movement were 0.51±0.04 mm and 0.40±0.10 mm in the 15 µg/kg and 30 µg/kg triptolide injection groups, respectively, which were significantly lower than the control group (0.71±0.04 mm) (Figure 3). The amount of tooth movement in the 30 µg/kg group was decreased significantly, compared with the 15 µg/kg group.
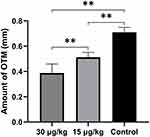 |
Figure 3 Amount of tooth movement. Notes: After 14-day orthodontic treatment, the tooth movement was significantly inhibited by triptolide injection. Data are expressed as mean±SD (** P<0.01). |
Root Resorption Analysis Via SEM
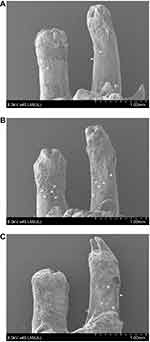
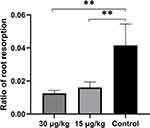
The compression side of distobuccal and distopalatal roots was observed under a SEM (Figure 4). The root surface of the control group was smoother than that of triptolide-treated groups (Figure 4A and B). There were more resorption pits showed in the control group and the extent of root resorption was more severe in the control group (Figure 4C). The percentage of resorption area was calculated. The mean root resorption area ratios were statistically reduced in triptolide-treated groups compared to the control group (Figure 5). The mean root resorption area ratio of the 30 µg/kg group was slightly lower than the 15 µg/kg group, but the difference was not significant (Figure 5).
Histologic Examination By H&E Staining
After 14-day tooth movement, resorption craters were detected on the surface of root and alveolar bone among different groups (Figure 6). The disorientation of PDL fibers was observed in the pressure side. There were more resorption pits detected on the root surface in the control group (Figure 6E). And the extent of resorption craters was decreased with increasing dose of triptolide (Figure 6B, D, F).
TRAP Staining Observation
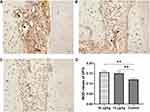
In the control group, numerous TRAP-positive multinuclear cells were observed in the pressure side of root surface and alveolar bone after 14 days of OTM (Figure 7). In the triptolide-treated groups, the TRAP-positive multinuclear cells were sparsely located on the alveolar bone and occasionally found on the root surface (Figure 7A and B).
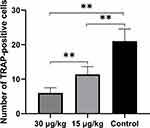
The number of TRAP-positive cells within the compression area was significantly lower in rats treated with triptolide compared to the control group (Figure 8). Moreover, the number of TRAP-positive cells was statistically reduced in the 30 µg/kg group compared with the 15 µg/kg group (Figure 8).
Immunohistochemistry Analysis
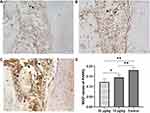
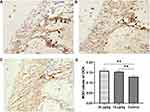
The immunoreactivity of RANKL was mainly observed within the compression side of periodontal area. The expression of RANKL was significantly higher in the control group than that of triptolide-treated groups (Figure 9). And the expression of RANKL in the 30 µg/kg group was statistically lower than that of the 15 µg/kg group (Figure 9D). Conversely, greater immunoreactivity of OPG was observed in the rats treated with triptolide (Figure 10). The MOD value of OPG was increased significantly in the rats administrated with triptolide (Figure 10D). Moreover, the expression of OPG in the 30 µg/kg group was higher than the 15 µg/kg group, but the difference was not significant (Figure 10D).
The immunoreactivities of RUNX2 and OCN were apparently observed in the PDL area and on the surface of alveolar bone of the tension side (Figures 11 and 12). The expression of RUNX2 was remarkably higher in the triptolide-treated group compared to the control group (Figure 11). Besides, the expression of OCN was significantly enhanced by the injection of triptolide (Figure 12). The expressions of both RUNX2 and OCN were increased with increasing dose of triptolide, but the difference in the expression of OCN between the 15 µg/kg and 30 µg/kg groups was not significant (Figures 11 and 12).
Discussion
In the present study, Wistar rats were used to establish the orthodontic model, which are considered as an adequate model with translational potential and widely used in the study of OTM and pharmacology.19–21 In order to exclude the influence of estrogen, only male rats were chosen. The optimum force for the tooth movement of rats may be less than 10 g as previously suggested.22 And it is believed that the heavy force would aggravate the root resorption.7 In this study, 50 g force was applied to move the first molar of rat mesially for 14 days to induce the tooth movement and root resorption.
The OTM results from the bone resorption at the compression side induced by osteoclast under mechanical stimuli. Osteoclast, the only cell with bone resorption function in vivo, plays a critical role in bone remodeling. Triptolide was found to exhibit a regulatory effect on bone turnover. A previous study showed triptolide had a protective effect on age-related bone loss,23 and it was demonstrated to attenuate titanium-induced osteolysis and osteoclast formation.24 In the present study, the TRAP-positive cells were observed on the surface of alveolar bone and the root of compression side after 14 days under orthodontic force. The number of TRAP-positive cells including osteoclasts and odontoclasts was significantly decreased in the triptolide-treated groups. In the triptolide-treated groups, the TRAP-positive cells were hardly detected. Our findings provided further evidence on the inhibitory effect of triptolide on osteoclast activity. As a result of that, the OTM of rats administrated with triptolide was arrested. This result could raise a clinical concern. As triptolide was widely used to treat a variety of diseases, the tooth movement would be hindered in patients who take triptolide.
In general, what both patient and orthodontist pursue is to accelerate OTM and diminish the duration of orthodontic treatment. Several studies had been performed on these purposes.25–27Whereas the inhibition of tooth movement may also imply its clinical use. The study showed strontium ranelate can improve tooth anchorage and inhibit root resorption in the orthodontic treatment of rats.28 Another study found that aspirin can inhibit tooth movement and block orthodontic relapse.29 The present study was performed as an explorative experiment. Our result indicated triptolide may have the potential to be used for these aims. The correlative studies would be performed in the future.
The mechanism of OIRR is similar to that of osteoclast-induced bone resorption. Several effector cells are implicated in the root resorption process – specifically, the odontoclast, the morphologic and functional characteristics of which are extremely similar to those of the osteoclast.10 In order to evaluate the root resorption, the pressure sides of distobuccal and distopalatal roots were scanned by SEM. The clear micrographs of the surface of distobuccal and distopalatal roots were obtained. There were more resorption pits located on the surface of distobuccal and distopalatal roots in the control group. The amount of root resorption area was significantly reduced in the triptolide-treated groups. Our finding indicated triptolide injection may be able to inhibit root resorption during orthodontic treatment.
RANKL is a crucial factor for osteoclast and odontoclast formation and activation. It exerts its effect by binding to the RANK receptor expressed on osteoclast lineage cells. This binding initiates the rapid differentiation of hematopoietic osteoclast precursors to mature osteoclasts. OPG acts as the decoy receptor and competes with RANK for RANKL binding, resulting in inhibition of terminal stages of osteoclast differentiation, suppression of activation of matrix osteoclasts, and induction of apoptosis. The multifunctional roles of RANK, OPG, and RANKL may provide an important link between bone remodeling, OTM, and root resorption.30 Osteoclast and odontoclast differentiations in root resorption areas are critically regulated by RANKL, which is produced as a local factor by PDL cells in response to heavy orthodontic force.31 In addition, the deletion of RANKL in PDL and bone lining cells greatly reduces osteoclastogenesis in response to mechanical force.32 Therefore, we examined the expression of RANKL and OPG to further elucidate the underlying mechanism of inhibitory effect of triptolide on osteoclastgenesis. After 14-day tooth movement, the RANKL was observed in the compressive side of PDL around the alveolar bone and root. The expression of RANKL was significantly decreased in rats injected with triptolide. The finding was in agreement with the previous study that demonstrated triptolide inhibited osteoclastogenesis by suppressing RANKL-induced NF-κB activation.33 On the other hand, the OPG expression was greatly up-regulated in the triptolide-treated groups, which could contribute to the inhibition of osteoclastogenesis. The suppression of tooth movement and root resorption in rats administrated with triptolide could result from the decreasing expression of RANKL and increasing expression of OPG. Furthermore, another in vitro study showed triptolide inhibited osteoclast differentiation by promoting the production of IL-10 and TGF-β.34 The further study of the molecular mechanism of triptolide on osteoclast is needed.
Bone formation results from complex events that involve differentiation of osteoblast precursor cells from primitive mesenchymal cells, maturation of osteoblasts, and matrix formation, followed by its mineralization.1 With regard to the effect of triptolide on osteoblastogenesis, literature has emerged that offers contradictory findings. An in vitro study demonstrated the suppressive effect of triptolide on osteoblast proliferation and mineralization.35 Nevertheless, in another in vitro study, triptolide was found to be able to reverse TNF-α-associated suppression of osteoblast differentiation.36 The process of bone formation plays an important role in OTM, which could sustain the structural integrity of alveolar bone. In the present study, the expressions of OCN and RUNX2, which are regarded as the late and early markers of osteoblastogenesis, respectively, were examined. The positive expressions of RUNX2 and OCN were observed within the periodontal area in the tension side along the alveolar bone. The MOD values of both RUNX2 and OCN were significantly increased in the triptolide-treated groups. Our result suggested that triptolide may exert a positive effect on osteoblastogenesis.
It is notable that the triptolide is reported to exhibit adverse reactions, involving hepatotoxicity, renal cytotoxicity, and reproductive toxicity. A variety of studies showed triptolide-induced toxicity depends on dosage and administration.37 And the minimal toxic does of triptolide for Wistar rats was 150 µg/kg/day for 28 days.38 The dosages we used were 15 µg/kg/day and 30 µg/kg/day for 14 days, which were much lower than the minimal toxic dose. Moreover, there was no difference observed in the body weight among the triptolide-treated groups and the control group. Meanwhile, no adverse reaction was observed during the experiment. Thus, the triptolide was well tolerated by rats in this study.
The limitation of this study was that we only examined the short-time effect of triptolide on orthodontic treatment in rats. But OTM refers to a complex process. The long-term effect of triptolide on orthodontic treatment should be evaluated in the future.
Conclusion
The present study demonstrated that triptolide could significantly arrest OTM and reduce root resorption via inhibition of osteoclastogenesis. In addition, triptolide may exert a positive effect on osteoblastogenesis.
Acknowledgments
This work was supported by the Shandong Province Natural Science Foundation (ZR2019MH113).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006;129(4):
2. Li Y, Jacox LA, Little SH, et al. Orthodontic tooth movement: the biology and clinical implications. Kaohsiung J Med Sci. 2018;34(4):207–214. doi:10.1016/j.kjms.2018.01.007
3. Thaleia K, Christos K, Stephan VG. The potential use of pharmacological agents to modulate Orthodontic Tooth Movement (OTM). Front Physiol. 2017;8:67. doi:10.3389/fphys.2017.00067
4. Nakaš E, Lauc T, Tiro A, et al. Dose- and time-dependent effects of clodronate on orthodontic tooth movement. Bosn J Basic Med Sci. 2017;17(1):23–28. doi: 10.17305/bjbms.2017.1715
5. Kaley J, Phillips C. Factors related to root resorption in edgewise practice. Angle Orthod. 1991;61(2):125–132. doi:10.1043/0003-3219(1991)061<0125:FRTRRI>2.0.CO;2
6. Lupi JE, Handelman SC. Prevalence and severity of apical root resorption and alveolar bone loss in orthodontically treated adults. Am J Orthod Dentofacial Orthop. 1996;109(1):28–37. doi:10.1016/S0889-5406(96)70160-9
7. Lopatiene K, Dumbravaite A. Risk factors of root resorption after orthodontic treatment. Stomatologija. 2008;10(3):89–95.
8. Fontana ML, de Souza CM, Bernardino JF, et al. Association analysis of clinical aspects and vitamin D receptor gene polymorphism with external apical root resorption in orthodontic patients. Am J Orthod Dentofacial Orthop. 2012;142(3):339–347. doi:10.1016/j.ajodo.2012.04.013
9. Feller L, Khammissa RA, Thomadakis G, et al. Apical external root resorption and repair in orthodontic tooth movement: biological events. Biomed Res Int. 2016;2016(2):1–7. doi:10.1155/2016/4864195
10. Jager A, Kunert D, Friesen T, et al. Cellular and extracellular factors in early root resorption repair in the rat. Eur J Orthod. 2008;30(4):336–345. doi:10.1093/ejo/cjn012
11. Chen SR, Dai Y, Zhao J, et al. A mechanistic overview of triptolide and celastrol, natural products from Tripterygium wilfordii Hook F. Front Pharmacol. 2018;9:104. doi:10.3389/fphar.2018.00104
12. Li W, Gong K, Ding Y, et al. <p>Effects of triptolide and methotrexate nanosuspensions on left ventricular remodeling in autoimmune myocarditis rats. Int J Nanomedicine. 2019;14:851–863. doi:10.2147/IJN
13. Zou Y, Hu W. Investigation of gene expression profiles in a rat adjuvant arthritis model suggests an effective role of triptolide via PI3K-AKT signaling. Exp Ther Med. 2019;17(5):3999–4006. doi:10.3892/etm.2019.7425
14. Ding YY, Li JM, Guo FJ, et al. Triptolide upregulates myocardial forkhead helix transcription factor p3 expression and attenuates cardiac hypertrophy. Front Pharmacol. 2016;7:471. doi:10.3389/fphar.2016.00471
15. Kim ST, Kim SY, Lee J, et al. Triptolide as a novel agent in pancreatic cancer: the validation using patient derived pancreatic tumor cell line. BMC Cancer. 2018;18(1):1103. doi:10.1186/s12885-018-4995-0
16. Han Y, Huang W, Liu J, et al. Triptolide inhibits the AR signaling pathway to suppress the proliferation of enzalutamide resistant prostate cancer cells. Theranostics. 2017;7(7):1914–1927. doi:10.7150/thno.17852
17. Fan D, Guo Q, Shen J, et al. The effect of triptolide in rheumatoid arthritis: from basic research towards clinical translation. Int J of Mol Sci. 2018;19(2):376. doi:10.3390/ijms19020376
18. Li J, Wang X, Li N, et al. Short-term effects of nicotine on orthodontically induced root resorption in rats. Angle Orthod. 2016;86(2):199–205. doi:10.2319/101014-727.1
19. Dolci GS, Ballarini A, Gameiro GH, et al. Atorvastatin inhibits osteoclastogenesis and arrests tooth movement. Am J Orthod and Dentofacial Orthop. 2018;153(6):872–882. doi:10.1016/j.ajodo.2017.09.021
20. Li F, Li G, Hu H, et al. Effect of parathyroid hormone on experimental tooth movement in rats. Am J Orthod and Dentofacial Orthop. 2013;144(4):523–532. doi:10.1016/j.ajodo.2013.05.010
21. Arias OR, Marquez-Orozco MC. Aspirin, acetaminophen, and ibuprofen: their effects on orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2006;130(3):364–370. doi:10.1016/j.ajodo.2004.12.027
22. Gonzales C, Hotokezaka H, Yoshimatsu M, et al. Force magnitude and duration effects on amount of tooth movement and root resorption in the rat molar. Angle Orthod. 2008;78(3):502–509. doi:10.2319/052007-240.1
23. Luo D, Ren H, Zhang H, et al. The protective effects of triptolide on age-related bone loss in old male rats. Biomed Pharmacother. 2018;98:280–285. doi:10.1016/j.biopha.2017.12.072
24. Huang J, Zhou L, Wu H, et al. Triptolide inhibits osteoclast formation, bone resorption, RANKL-mediated NF- қB activation and titanium particle-induced osteolysis in a mouse model. Mol Cell Endocrinol. 2015;399:346–353. doi:10.1016/j.mce.2014.10.016
25. Hashimoto F, Kobayashi Y, Mataki S, et al. Administration of osteocalcin accelerates orthodontic tooth movement induced by a closed coil spring in rats. Eur J Orthod. 2001;23(5):535–545. doi:10.1093/ejo/23.5.535
26. Jing D, Xiao J, Li X, et al. The effectiveness of vibrational stimulus to accelerate orthodontic tooth movement: a systematic review. BMC Oral Health. 2017;17(1):143. doi:10.1186/s12903-017-0437-7
27. Yi J, Yan B, Li M, et al. Caffeine may enhance orthodontic tooth movement through increasing osteoclastogenesis induced by periodontal ligament cells under compression. Arch Oral Biol. 2016;64:51–60. doi:10.1016/j.archoralbio.2015.12.009
28. Kirschneck C, Wolf M, Reicheneder C, et al. Strontium ranelate improved tooth anchorage and reduced root resorption in orthodontic treatment of rats. Eur J Pharmacol. 2014;744:67–75. doi:10.1016/j.ejphar.2014.09.039
29. Liu Y, Zhang T, Zhang C, et al. Aspirin blocks orthodontic relapse via inhibition of CD4(+) T lymphocytes. J Dent Res. 2017;96(5):586–594. doi:10.1177/0022034516685527
30. Yamaguchi M. RANK ⁄ RANKL ⁄ OPG during orthodontic tooth movement. Orthod Craniofac Res. 2009;12:113–119. doi:10.1111/j.1601-6343.2009.01444.x
31. Nakano Y, Yamaguchi M, Fujita S, et al. Expressions of RANKL/RANK and M-CSF/c-fms in root resorption lacunae in rat molar by heavy orthodontic force. Eur J Orthod. 2011;33(4):335–343. doi:10.1093/ejo/cjq068
32. Yang CY, Jeon HH, Alshabab A, et al. RANKL deletion in periodontal ligament and bone lining cells blocks orthodontic tooth movement. Intl J Oral Sci. 2018;10(1):31–39. doi:10.1038/s41368-017-0004-8
33. Liu C, Zhang Y, Kong X, et al. Triptolide prevents bone destruction in the collagen-induced arthritis model of rheumatoid arthritis by targeting RANKL/RANK/OPG signal pathway. Evid Based Complementary Alternat Med. 2013;2013:1–12. doi:10.1155/2013/626038
34. Xu H, Zhao H, Lu C, et al. Triptolide inhibits osteoclast differentiation and bone resorption in vitro via enhancing the production of IL-10 and TGF- β 1 by regulatory T cells. Mediators Inflamm. 2016;2016(4):1–10. doi:10.1155/2016/8048170
35. Ji W, Liu S, Zhao X, et al. Triptolide inhibits proliferation, differentiation and induces apoptosis of osteoblastic MC3T3-E1 cells. Mol Med Rep. 2017;16(5):7391–7397. doi:10.3892/mmr.2017.7568
36. Liu SP, Wang GD, Du XJ, et al. Triptolide inhibits the function of TNF-α in osteoblast differentiation by inhibiting the NF-κB signaling pathway. Exp and Ther Med. 2017;14(3):2235–2240. doi:10.3892/etm.2017.4749
37. Li XJ, Jiang ZZ, Zhang LY. Triptolide: progress on research in pharmacodynamics and toxicology. J Ethnopharmacol. 2014;155(1):67–79. doi:10.1016/j.jep.2014.06.006
38. Wang J, Jiang Z, Ji J, et al. Gene expression profiling and pathway analysis of hepatotoxicity induced by triptolide in Wistar rats. Food and Chem Toxicol. 2013;58:495–505. doi:10.1016/j.fct.2013.04.039
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.