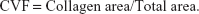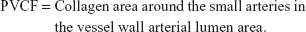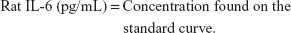Back to Journals » International Journal of Nanomedicine » Volume 14
Effects of triptolide and methotrexate nanosuspensions on left ventricular remodeling in autoimmune myocarditis rats
Authors Li W, Gong K, Ding Y , Chaurasiya B , Ni Y, Wu Y, Zhao P, Shen Y, Zhang Z, Webster TJ
Received 18 October 2018
Accepted for publication 27 December 2018
Published 29 January 2019 Volume 2019:14 Pages 851—863
DOI https://doi.org/10.2147/IJN.S191267
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Wei Li,1 Kaizheng Gong,1 Yuan Ding,2 Birenda Chaurasiya,2 Yue Ni,1 Yong Wu,1 Pei Zhao,1 Yan Shen,2 Zhengang Zhang,1 Thomas J Webster3
1Department of Cardiology, Affiliated Hospital of Yangzhou University, Yangzhou, Jiangsu 225000, China; 2Department of Pharmaceutics, School of Pharmacy, China Pharmaceutical University, Nanjing, Jiangsu 210009, China; 3Department of Chemical Engineering, Northeastern University, Boston, MA 02115, USA
Purpose: This study was carried out to investigate the effects of a triptolide (TP) nanosuspension and methotrexate (MTX) nanosuspension on left ventricular remodeling and cardiac function for autoimmune myocarditis (EAM) in rats. The regulating effects on inflammatory cytokines in the peripheral serum and related mechanisms are also discussed.
Methods: First, TP and MTX were prepared as a nanosuspension, and the EAM model was successfully established in rats with cardiac myosin. Then, the effect of TP and MTX suspensions was tested in an EAM model.
Results: Results revealed that both TP and MTX suspensions could reduce the degree of myocardial fibrosis and delay the remodeling process of the left ventricle which could further improve cardiac function. Finally, it was found that inflammatory cytokines in the peripheral serum were regulated by the nonspecific immune system and the inhibition of nuclear factor-κB signaling might have partly occurred due to this mechanism.
Conclusion: In summary, this study provided a complete foundation for EAM therapy of profound clinical relevance.
Keywords: inflammatory cytokines, heart failure, dilated cardiomyopathy, immunoregulation, suspension
Introduction
Currently, the rate of morbidity due to myocarditis is increasing, especially that due to dilated cardiomyopathy (DCM). It accounts for more than half of the causes of heart failure in America, Europe, and even some countries in Africa.1 The currently known pathogenesis for DCM includes genetic factors, continuous viral infection, the autoimmune response of myocardial epitopes, mechanical pressure, poisoning, etc.1 Except for genetic factors, recently, myocarditis (ecology virus infection or autoimmune inflammation) caused by DCM has attracted much attention.2,3
Myocarditis is a myocardial inflammatory disease and it is characterized by the degeneration, necrosis, and apoptosis of cardiomyocytes which also demonstrates inflammatory cell infiltration and myocardial interstitial remodeling. The activation of nuclear factor (NF)-κB signaling and the production of inflammatory cytokines (such as tumor necrosis factor [TNF]-α; IL-6, IL-1, IL-2, IL-4, and IL-10; monocyte chemoattractant protein [MCP]-1; interferon [IFN]-γ, etc) are the main factors for myocarditis. The inflammation causes T-cell immune-based persistent autoimmune injury to cardiomyocytes, thereby promoting cardiomyocyte apoptosis and left ventricular remodeling, which finally leads to DCM or even heart failure. Once the left ventricle gets remodeled, the collagen ratio changes and myocardium damage starts which can lead to heart failure.4 It has been reported that the treatment of myocarditis involves mainly anti-inflammatory and immunosuppressive agents such as cytokines,5–8 nitric oxide synthase inhibitors,9 immunoglobulin,10 angiotension-converting enzyme inhibitors,11 etc. According to reported results, immunomodulator therapy has shown good therapeutic effect against myocarditis.
Reports showed that anti-inflammatory drugs like statins can reduce the mortality of ECM mice; so, the application of nonspecific immunomodulatory drugs, such as triptolide (TP) and methotrexate (MTX), to this model is expected to improve the left ventricular remodeling of autoimmune myocarditis (EAM) from an immunological aspect. Clinically, the anti-foliate drug MTX is mainly used for the treatment of tumors and rheumatoid arthritis (RA). Researchers have found that MTX also has an immunomodulatory effect which can inhibit the production of Th1-type cytokines (such as TNF-α and IL-6) and can promote the secretion of Th2-type cytokines, such as IL-4, IL-5, and IL-10. Due to immunoregulation and the nonspecific anti-inflammatory properties of MTX, it has been widely used in the treatment of RA and psoriasis.12 Previously, our group has demonstrated the effect of MTX on congestive heart failure (CHF) patients and has found that MTX had a positive effect on heart function and improved the quality of life for patients. The New York Heart Association has also highlighted the anti-inflammatory effect of MTX and its effect on CHF patients.13
TP is a diterpene lactone compound isolated from Tripterygium wilfordii. It has potential pharmacological activity and has been used as an anti-inflammatory, antitumor, antifertility, and immunoregulatory agent. TP inhibits immune responses through various mechanisms. It also shows an inhibitory effect against many kinds of proinflammatory cytokines and adhesion molecules which are secreted by endothelial cells. It also has an ability to inhibit the expression and activity of NF-κB signaling. This further promotes cell apoptosis and prevents the occurrence and development of pathological immune responses.14 Our previous research in a hypertensive rat model proved that TP had obvious regulatory effects on the expression of inflammatory mediators to reduce myocardial fibrosis.
Nanosuspensions are colloidal dispersions formed by pure drug nanoparticles. The application of insoluble drugs in medicine is clearly limited due to their low solubility and low bioavailability. The preparation of nanosuspensions is simple, mild, and includes small adverse reactions which have improved the solubility of poorly soluble drugs, increased drug dissolution, and improved bioavailability. TP and MTX are both hydrophobic and insoluble in water; so, in order to improve the solubility and bioavailability of TP and MTX, a nanosuspension preparation was used in this study.
This study was designed to explore the effect of MTX and TP suspensions to treat EAM. According to the design of the experiments, the EAM model in rats was first developed. EAM induced by cardiac myosin (CM) was mainly used as an animal model to study the role of autoimmune mechanisms in the pathogenesis of myocarditis and DCM excluding other complex infectious factors. TP and MTX were then prepared as nanosuspensions before administration. Thereafter, the effects on left ventricle remodeling, cardiac function, and the nonspecific immunomodulatory effects of inflammatory cytokines in the peripheral blood were monitored after the treatment of the drugs. Furthermore, the mechanism of the negative regulation of NF-κB was studied to clarify the importance of the autoimmune mechanism and left ventricle remodeling in myocarditis and DCM, providing a more rational theoretical basis for delaying or preventing the transformation from myocarditis to DCM (Figure 1).
Materials and methods
Materials
CM (M-0531), Freund’s complete adjuvant (F-5881), as well as type I collagen monoclonal antibody and type III collagen monoclonal antibody were obtained from Sigma-Aldrich Co (St Louis, MO, USA). The Masson staining kit, cytokine ELISA kit, and NF-κB pathway activation kit were purchased from Shanghai Kangcheng Biological Company (Shanghai, China). TP with 98.7% purity was received from the Chinese Academy of Medical Sciences. MTX was purchased from Shanghai Pharmaceutical Company (Shanghai, China).
The preparation of a nanosuspension
First, TP and MTX were dispersed in water. Then, the dispersed TP (0.05 mg/mL) and MTX suspension (1.5 mg/mL) were sonicated (30 W, 15 minutes) to about 100 nm as the nanosuspension before administration. The size for these two nanosuspensions was determined on a Zetasizer Nano-ZS90 analyzer (Malvern Instruments, Malvern, UK).
Preparation of the EAM model
Preparation of the model drugs
Animal experiments were carried out in accordance with protocols approved by the Comparative Medicine Center of Yangzhou University (201829482). The model drugs were prepared from a CM stock solution by diluting from 11.7 to 10 mg/mL using sterile 0.01 mol/L PBS. A sterile 5-mL medical plastic syringe was used to aspirate 1.8 mL of the diluted CM solution, and another one to aspirate 1.8 mL of Freund’s complete adjuvant (containing 1 mg/mL of Mycobacterium tuberculosis H37Ra). Both syringes were connected with 2 cm of a sterile plastic infusion strip after removing air. Then, they were pushed back and forth for about 50 minutes so that the content became a water-in-oil emulsion with a CM concentration of 5 mg/mL.
Modeling group
Lewis rats were numbered according to their body weight (BW). A random number generator was used to divide the group. Whole rats were randomly divided into two groups: a control group (n=8) and the EAM group (n=72).
Modeling method
Model was developed according to a previously described method.15 Briefly, rats in the EAM group were injected with 0.1 mL of an emulsion containing 1 mg CM under the skin in both the hind limbs on day 0 and with 0.2 mL of an emulsion containing the same amount of CM on day 7 at the same position where the first injection was given. In the control group, the rats were injected with the same volume of saline at the same time interval at the same position as in the EAM group to develop pseudo-immunity.
Evaluation of the model
General condition
The BW of the rats was recorded once a week and their food consumption activity was monitored on a regular basis. Simultaneously, the development of an ulcer in the limb was monitored on a regular basis.
The tail vein of both rat groups was punctured on day 28 and 0.5 mL of blood was collected to separate the serum, and the presence of an anti-myosin antibody was detected using indirect ELISA.
Animal grouping
After 29 days, all rats in the EAM group were randomly divided into five groups; the EAM group (n=13), a low-dose TP suspension group (TP-L, n=14), a high-dose TP suspension group (TP-H, n=14), a low-dose MTX suspension group (MTX-L, n=14), and a high-dose MTX suspension group (MTX-H, n=14).
Drug administration
For the drug interaction study, all preparations (such as TP-L: 5 μg·kg−1·d−1 of TP in saline; TP-H: 10 μg·kg−1·d−1 of TP in saline; MTX-L: 0.3 mg·kg−1·d−1 of MTX in saline, and MTX-H: 0.6 mg·kg−1·d−1 of MTX in saline) were prepared and injected intraperitoneally into the EAM group twice a week for 6 weeks. All of the suspensions were shaken before administration.
Dose adjustment
All medication doses were adjusted according to the BW of the rats.
Evaluation of cardiac structure and function
The cardiac structure and function of the treated rats were evaluated after 70 days of the experiment. All rats were fasted for 12 hours and were forbidden to drink for 2 hours before commencing the experiment. The rats were anesthetized with an intraperitoneal injection of 50 mg/kg ketamine and 5 mg/kg diazepam. Then, they were fixed on the operating plate in dorsal position and their chest was prepared for echocardiography (GE VIVID E9 [the probe frequency was 3–5 MHz]; GE Healthcare, Chicago, IL, USA). The scanning probe of the echocardiograph was placed on the left margin of the sternum and the size and movement of the heart were monitored. For proper functioning of the heart, the following parameters were measured: left ventricular end diastolic diameter (LVEDD), left ventricular end systolic diameter (LVESD), interventricular septal thickness (IVST), left ventricle posterior wall thickness (LVPWT), fractional shortening (FS), and left ventricular ejection fraction (LVEF). Variations in LVEDD and LVPWT revealed the changes in heart structure and IVEF and FS revealed the changes in left ventricular systolic function.
Observation of the specimen
Animals were placed on the dissecting board in a dorsal position and were dissected to open the chest of the animal. Animals were euthanatized to collect blood by puncturing the abdominal artery. The collected blood was centrifuged at 3,000 rpm for 10 minutes to collect the serum which was stored at −80°C for the following tests. A heart specimen was collected and was placed in an ice-containing dish immediately and was cleaned with ice-cold sterile saline for visual observation. The morphological condition of the harvested heart was determined based on the following scoring method:18 if the surface of the heart was smooth and no inflammatory lesions seen, then a score of “0” was given; if there was one gray lesion present on the surface of the heart, then a score of “1” was given; if more than one gray lesion was present but the covered area was <1/3 of the total heart surface area, then a score of “2” was given; if gray lesions were dispersed throughout the surface of the heart but the covered area was not more than 2/3, then a score of “3” was given; and if the gray lesions were distributed throughout the surface of the heart, then a score of “4” was given.
Determination of left ventricular mass and left ventricular mass index (LVMI)
After determination of morphological changes of the heart by the above-mentioned scoring method, the atrium and the right ventricle were removed carefully without the interventricular septum and the left ventricle was weighted (LVW, mg) using an electronic balance (Mettler AJ100; Mettler Toledo, Columbus, OH, USA) and the LVMI (mg/g) was calculated as LVW/BW.
Collection of rat heart specimen
The harvested heart was placed on the ice dish immediately. After the blood vessels were trimmed, the heart was weighed and recorded. The heart was sliced horizontally into three parts using a dissecting blade. The middle part of the heart was frozen for type I and III collagen examination and the lower section was placed in paraffin for HE and Mason staining. The frozen section was sent to the pathology department, while the upper section of the heart was kept for cryopreservation in liquid nitrogen.
Paraffin section check
The middle section of the heart was imbedded into a vial with 10% formalin and kept in a refrigerator at 4°C for 24 hours. Thereafter, the section was dehydrated and embedded into a paraffin block form. The paraffin block was sliced into 4-μm-thick sections for the following examination.
HE staining
The presence of inflammation in the heart was determined by the presence of myocardial infiltration and the severity of inflammation was evaluated by the following scoring method:16 if no infiltration was present, a score of “0” was given; if the infiltration covered <25% of the total area, then it was scored a “1”; if infiltration covered between 25% and 50%, then it was scored a “2”; and if the infiltration area was between 50% and 75%, then it was scored a “3.”
The degree of myocardial fibrosis was recorded and its severity was evaluated by the following scoring method:16 if no infiltration was present, then it was scored “0”; if the infiltration covered <25% of the total area, then it was scored a “1”; if the infiltration covered between 25% and 50%, then it was scored a “2”; and if the infiltration area was between 50% and 75%, then it was scored a “3.”
The infiltration area was measured by the eyepiece scale.
Masson staining
In Masson staining sections, the nucleus stained black, the myofibers stained red, and the collagen fibers stained bright green. Semi-quantitative investigation of the myocardial collagen was performed by an image analysis system under a 200× visual field. Then, the collagen volume fraction (CVF) and the perivascular collagen area (PVCA) were calculated as follows (the collagen area did not include PVCA): five fields were randomly chosen and averaged in order to analyze the percentage of collagen. In each specimen, five small arteries in the vessel were chosen to be measured and averaged.
|
|
Immunohistochemical staining of type I and III collagen and calculation of the collagen ratio
Immunohistological staining of type I and III collagen was determined by using a primary antibody. The primary antibodies were the type I collagen monoclonal antibody and the type III collagen monoclonal antibody (Sigma-Aldrich Co). The optimal dilution ratio of the antibody used was 800. For the negative control, collagen was stained with PBS and for the positive control, collagen was stained with a primary antibody. The presence of color after dyeing was determined by using an image analysis software system and the presence of collagen was determined by the ratio of the integrated optical density.
Content of TNF-α in rat serum with a sandwich ELISA
A standard curve was developed using OD as a vertical coordinate and the concentration as a horizontal coordinate. The concentration of the sample was found with the curve according to its OD. Each group had three repetitions:
|
Content of IL-6 in rat serum with a sandwich ELISA
A standard curve was developed using OD as a vertical coordinate and the concentration as a horizontal coordinate. The concentration of the sample was found with the curve according to its OD. Each group had three repetitions. The content of IL-1 in rat serum and the content of IL-10 and MCP-1 in rat serum were both determined according to this method.
|
ELISA assay to determine NF-κB
Avidin peroxidase was coated on a 96-well ELISA plate and then was washed with PBS with Tween 20 (PBST) and blocked with 3% skim milk solution. The plate was incubated for 1 hour at room temperature with 1 μg/mL of a DNA oligonucleotide sequence containing an NF-κB binding motif. After 1 hour of incubation, the DNA oligonucleotide sequence was washed with PBS, and then NF-κB antibodies were added to it. After three washes in PBST/0.1% Tween 20, the corresponding AP-coupled secondary antibody was added. AP activity was then detected by the addition of a p-nitrophenyl phosphate solution. After 10 minutes of further incubation, the reaction was stopped by the addition of H2SO4. OD was determined at 405 nm using an ELISA reader.
OD of the negative control was used to compare values. When the OD of the sample was ≥2.1 times of the negative control, it was judged as positive. Then, the OD of NF-κB was analyzed quantitatively.
Statistics
All data were calculated for ANOVA using SPSS (v11.0; SPSS Inc, Chicago, IL, USA). All results were expressed as the mean ± SD. t-test was used to compare the obtained data from the two groups. F-test was used to compare the obtained data from more than two groups. A difference was accepted as very significant at P<0.01 and as significant at P<0.05.
Results
Size of TP and MTX nanosuspensions
The TP (0.05 mg/mL) and MTX suspensions (1.5 mg/mL) were sonicated for 15 minutes and the final size was 104±2.1 nm for the TP suspension and 107±1.9 nm for the MTX suspension. Before administration, the suspension was shaken for 30 seconds for uniform mixing.
General condition
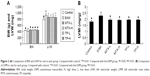
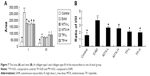
After injection of CM, rats were kept under observation for 1 week. After 1 week, all of the EAM rats showed various abnormalities like burnout, irritability, less movement, and fluffy fur compared with the control group and three of them died. At the same time, foot ulcers began to appear in some of the EAM rats. After 4 weeks, all the EAM rats had foot ulcers and 18 of them also had ankle swelling for up to 10 weeks. However, the foot ulcers on the EAM rats were healed and scabbed after treatment with the TP and MTX suspensions and the ankle swelling also obviously reduced. For the control group, the BW increased from 142.92±4.31 to 437.38±10.17 g while the BW for the EAM group increased from 142 to 407.11 g, which was relatively slower than the control group. However, the BW for the TP-L, TP-H, MTX-L, and MTX-H group was 446.06±24.51, 442.47±27.70, 448.00±21.13, and 443.23±14.52 g, respectively. The BW of the treatment group was obviously higher than that of the EAM group. There were no significant differences of BW between the treated groups, which included the MTX-L, MTX-H, TP-L, and TP-H groups (Figure 2A).
Both the LVW and LVMI of the EAM group increased significantly (P<0.01) in comparison to those of the control group. The LVW of the MTX-L group and the MTX-H group decreased less (P<0.05) than the LVMI (P<0.01) compared to that of the EAM group. There were no significant changes seen for the low-dose and high-dose groups. A similar pattern was seen for the TP-L group and TP-H group (Figures 1 and 2B).
Effect of TP and MTX on left ventricular remodeling and cardiac function
Heart structure and function
Compared with the control group, the LVEDD and LVESD of the EAM group increased significantly (P<0.01) and the LVEF (P<0.01) and FS (P<0.05) also declined significantly. Though a downward trend was observed in LVPWT and IVST, there was no statistical significance seen. Compared with the EAM group, there was an obvious decline in LVEDD and LVESD (P<0.01) in the TP-L group and TP-H group. The LVEF of these two groups significantly increased (P<0.01), but the FS, LVPWT, and IVST did not show any significant changes. In addition, compared with the EAM group, the LVEDD and LVESD of the MTX-L group and MTX-H group were reduced (P<0.01) and the LVEF increased significantly (P<0.01), but there was no significant difference in FS, LVPWT, and IVST. The ultrasonic parameters showed no statistical significant difference between the TP group and MTX group (Table 1).
Observation of the heart specimens by the naked eye
Hearts in the control group were smooth and had no inflammatory lesion; but in the EAM group, they became larger and looked more gloom with some gray lesions distributed on the surface. In the four treatment groups, the hearts became smaller again and the lesions were less or even disappeared. However, there were no obvious changes in the size and lesions in the TP-L, TP-H, MTX-L, and MTX-H groups (Table 2, Figure 3).
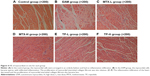
HE staining
No remarkable change in the arrangement of the myocardial parenchymal cells was observed in the control group, while for the EAM group, there appeared to be many sparse and degenerative cells. Besides these, myocardial interstitial fibroses and mild inflammatory infiltrations were also observed in the EAM group. In all four treated groups, the degree and intensity of the infiltration were significantly reduced (Figure 4).
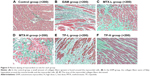
Masson staining of myocardial collagen fibers
In the control group, only a small amount of collagen fibers is found (dyed in green color) around the red muscle bundle and the small blood vessels. However, in the EAM group, the collagen fibers were of flaky hyperplasia and the CVF and PVCA increased significantly (P<0.01) compared with the control group. Then, hyperplasia of the collagen fibers decreased and the CVF (P<0.01) and PVCA (P<0.05) also reduced in the MTX-L group and the MTX-H group compared with those in the EAM group (Table 3). This phenomenon also found in the TP-L group and the TP-H group was similar to that observed in the MTX-L group and the MTX-H group (Figure 5).
Immunohistochemical staining of collagen fibers
In the control group, there were some brown collagen fibers (type I and III) around the myocardial cells and the blood vessels. Compared to the control group, in the EAM group, type I and III collagen were significantly higher in hyperplasia (P<0.01). Furthermore, type I collagen was more than type III and the ratio of I/III also increased (P<0.01) in the EAM group. In both the MTX groups, type I collagen significantly decreased (P<0.05) and type III collagen decreased slightly (P>0.05), but the ratio of I/III was still significantly (P<0.01) reduced. In both TP groups, type I collagen decreased significantly (P<0.01), but type III collagen increased (P<0.05), with a dramatic decline in the ratio of I/III (P<0.01). The amount of type I and III collagen and the ratio of I/III showed no significant differences between the MTX groups and the TP groups (Figures 6 and 7).
Concentration change of cytokines in the peripheral serum
The concentration of TNF-α, IL-1, IL-6, and MCP-1 significantly increased (P<0.01) in the EAM group and were markedly (P<0.05) decreased in the MTX-L, MTX-H, TP-L, and TP-H groups. However, the concentration of IL-10 in the EAM group did not show any difference with the control group. In addition, the results showed that the inhibition of cytokines in all of the four treatment groups was dose-dependent (Figure 8).
Changes in NF-κB activity in different groups of myocardial tissue
In the control group, the activity of NF-κB in myocardial tissue was less, whereas in EAM, MTX-L, MTX-H, TP-L, and TP-H groups, the activities of NF-κB and phosphorylated NF-κB were much higher (P<0.01). In the case of both MTX and TP groups, the amounts of activated NF-κB and phosphorylated NF-κB were lower than those of the EAM group (P<0.05, P<0.01). However, comparing the MTX and TP groups, no significant differences were observed in the activity of NF-κB (Figure 9).
Discussion
In this work, a nanosuspension was applied to increase the solubility and bioavailability of TP and MTX. In the nanosuspension preparation, stabilizers, suspending agents, and solubilizers were not used because the excipients may have caused an unpredictable impact on the result which may have increased the heart burden or caused toxicity to the heart. We found, for the first time, that left ventricular remodeling plays an important role in the transformation of myocarditis to DCM. Nowadays, there are various models for myocarditis, including coxsackievirus vaccination, polypeptide induction, and CM immunization. Among these models, the CM-induced EAM model is mainly used to study the function of the autoimmune system in the incidence and progress of myocarditis. The EAM model is an artificially induced autoimmune model for the myocardium; it can exclude other effects of complex infections, and it is easy to prepare. As a result, our study adopted the left ventricular remodeling of autoimmune myocarditis as model.
In our study, the EAM model was developed by using CM immunized rats. During the experiment, the EAM rats were moving less and were more tired and irritable and their weight gain was obviously slower than rats in the control groups. Then, foot ulcers appeared gradually and some of them also experienced ankle swelling, and on day 28, the myosin antibody was observed in the serum. Accordingly, it could be determined that the acute period of remodeling in the EAM model was successful. After day 70, the hearts of rats in the EAM group turned gray with poor elasticity and experienced increased heart volume, LVW, and LVMI. Color Doppler ultrasound on day 70 revealed that rats in the EAM group had left ventricular enlargement, increased LVESD and LVEDD, and the weakened myocardial contraction and the reduction in function values such as FS and LVEF were in line with the transformation of myocarditis to DCM in humans. In the histopathology analysis, HE staining showed that the cardiomyocytes in EAM rats were degenerating and necrotic. In the myocardial interstitium, collagen fibers were disorderly arranged and the hyperplasia was obvious, with a mild lymphocytic infiltration. At the same time, Masson staining showed that in EAM rats the myocardial interstitium and collagen around blood vessels demonstrated great hyperplasia and CVF and PVCA increased. In immunohistochemical staining, the amount of type I and III collagen and the ratio of I/III both increased, which inferred that left ventricular remodeling took place in the EAM rats. We also found that, in EAM rats, the proinflammatory cytokines increased, including TNF-α, IL-6, IL-1, MCP-1, and IL-6. However, the amount of the anti-inflammatory cytokine IL-10 tended to increase but it was not overly obvious. The increase of LVW and LVMI confirmed the results of Horii et al17 mainly because of a decline in the BW of the rats and increased heart weight because of inflammation and fibrosis. As a result, after day 70, left ventricular remodeling existed in EAM rats, which was in line with the transformation from myocarditis to DCM in humans, thus laying the foundation for later experiments.
Based on the above results, as nonspecific immune medicines, chemical TP and natural MTX were utilized here in the EAM model, and the effects to reduce left ventricular remodeling in the EAM model were investigated. The immunosuppressant MTX is broadly applied in the treatment of tumors, RA, and psoriasis. Recent research showed that the mechanism of MTX involves not only cytotoxicity but also has more of an effect on cytokine production.18 It can inhibit the function of B cells and macrophages and regulate the production of IL-1 and superoxide ions. Furthermore, it can prevent IL-lβ from combining with the receptors on peripheral blood cells, inducing cytosine release to resist inflammation.12 Researchers revealed that MTX can enhance the expression of IL-4 and IL-10 and reduce the expression of IL-1 and IFN-γ, thus inhibiting cytokine inflammation and increasing the effects of anti-inflammatory cytokines to control RA. This could be one of the mechanisms of MTX treatment on RA.
TP is a highly active epoxidized diterpene lactone compound extracted from Celastraceae called Tripterygium and is one of the main active ingredients in Tripterygium. Its related potency is 100–200 times higher than that of Tripterygium glycosides. Pharmacological tests and clinical trials revealed that TP has immunosuppressive, anti-inflammation, antifertility, and antitumor properties. TP targets TNF-α when treating RA and it can inhibit the production of IL-6 by preventing the production of TNF-α and then eliminating inflammation.19 Other studies showed that TP can lower the expression of proinflammatory cytokines such as IL-1, IL-6, IL-8, and TNF-α. It can also decrease the activity of NF-κB and increase apoptosis, resulting in the prevention of the pathological immunological reaction. TP not only regulates Th1 and Th2 cytokines but also affects other cytokines.17
Because of the nonspecific immune modulation of MTX, we chose it to treat EAM. We found that both the MTX-L group and MTX-H group showed weak local arthrocele and improved ulcer healing. In both groups, LVEDD and LVESD decreased and LVEF increased, with the improvement of heart function. Simultaneously, CVF, PVCA, type I collagen, and the ratio of type I/III collagen decreased, and type III collagen was slightly reduced. Inflammatory cytokines in the peripheral serum (such as TNF-α, IL-1, MCP-1, and IL-6) decreased. Though the comparison between the MTX group and the control group showed no statistical significance on IL-10, it still tended to increase. This is probably related to the fact that MTX can enhance adenosine release and thus improve the expression of IL-10.
Because TP can nonspecifically regulate many cytokines, we also used it to treat EAM. In this research, both the TP-L group and TP-H group showed weakened local arthrocele and improved ulcer healing compared to the EAM group. Their inflammatory response decreased, causing a decrease in LVW, LVMI, LVEDD, LVESD, and LVEF. Immunohistochemistry staining showed that CVF and PVCA decreased. Further, it was also found that type I collagen decreased while type III collagen increased; so, the ratio of type I/III collagen decreased. The inflammatory cytokines in the peripheral serum (such as TNF-α, IL-1, MCP-1, and IL-6) also decreased, but for IL-10, it just reduced slightly with no statistical significance. It is possible that TP can obviously inhibit Thl and slightly inhibit Th2 at the same time, thus improving the balance of Th1/Th2.
In this research, both MTX and TP nonspecifically inhibited inflammatory cytokines and reduced myocarditis in EAM rats for the EAM group; the over-synthesis of collagen caused excessive deposition and altered collagen I/III ratios damaging the left ventricular diastolic function, finally improving LVW and LVMI. The over-degradation of collagen is often accompanied by increased activity of the matrix metalloproteinase system. Excessive degradation of the ECM destroyed the heart collagen network and finally expanded the heart chamber which further affected the left ventricular systolic function. So, both MTX and TP can reduce myocardial interstitium fibrosis and delay left ventricular remodeling progress by improving indicators such as CVF, PVCA, type I collagen, type III collagen, and the ratio of collagen I/III. Moreover, literature indicates that animal weight can be used as a measure of systemic toxicity. During the treatment period, treatment group weights increased significantly which indicates that the systemic toxicity of MTX and TP seems slight.
Shioji et al observed an obvious expression of NF-κB in the myocardium using immunohistochemistry and its expression was related to the degree of myocardial lesion.20 In our experiment, we also detected the activity of NF-κB in the myocardium. We found that in the control group, NF-κB activity was very low while activated NF-κB and phosphate NF-κB were high in the EAM, MTX-L, MTX-H, TP-L, and TP-H groups. In addition, compared with the EAM group, activated NF-κB was reduced and phosphate NF-κB was reduced even more for the MTX-L, MTX-H, TP-L, and TP-H groups. Activated NF-κB refers to DNA binding activity and phosphate NF-κB refers to the transcriptional activity. NF-κB is the core of cellular signal transduction in inflammation. Activated NF-κB can regulate the expression of inflammatory-related molecules at the transcriptional level, and the inhibition of NF-κB can restrain inflammatory mediator synthesis and the waterfall cascade reaction, thus blocking pathological effects. Therefore, we considered that the factors for TP and MTX to reduce cytokine synthesis are inhibited by NF-κB activation and phosphorylation. This affects the classical activation pathway of NF-κB, thus causing nonspecific immune regulation on inflammatory cytokines.
The mechanism by which TP and MTX are effective in joint swelling and ulcer healing in EAM rats may be similar to the mechanism of improving RA for the two drugs. Furthermore, we compared MTX-L, MTX-H, TP-L, and TP-H groups. However, this comparison showed that although MTX and TP could improve many indicators in EAM rats, the concentration change did not affect inflammatory cytokine synthesis, LVW, LVMI, heart function, and collagen synthesis. This phenomenon may be related to the dose of the two drugs. More detailed differences in the effect and mechanism of effect need to be researched for these two drugs from an immunoregulatory aspect. Comparisons between different concentrations of MTX and TP may help in this regard.
Conclusion
In summary, EAM can be immunized with CM in Lewis rats. Both TP and MTX can reduce myocardial fibrosis, delay the left ventricular remodeling progress, mildly improve heart function, and nonspecifically regulate inflammatory cytokines in peripheral serum without a significant difference. The exact mechanism of nonspecific immunoregulation of inflammatory cytokines is still unclear, but the inhibition of NF-κB may partly contribute to it. This work proved that chemical (MTX) and natural (TP) nanodrugs, both as nonspecific immune regulatory drugs, could achieve a significant therapeutic effect toward EAM. More work concerning the internal mechanisms of these two drugs to cure EAM is still needed. Moreover, the results of this experiment are clinically relevant and provide a more rational theoretical basis for delaying or preventing the transformation from myocarditis to DCM.
Acknowledgment
This work was partly supported by the National Natural Science Foundation of China (81470381 and 81770262), and Jiangsu Key R&D Project in Social Development (BE2015663).
Disclosure
The authors report no conflicts of interest in this work.
References
Nagata Y, Yamagishi M, Konno T, et al. Heat failure phenotypes induced by knockdown of DAPIT in zebrafish: a new insight into mechanism of dilated cardiomyopathy. Sci Rep. 2017;7(1):17417. | ||
Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation. 2001;104(9):1076–1082. | ||
Tahir H, Daruwalla V, Hayat S. Myocarditis leading to severe dilated cardiomyopathy in a patient with dengue fever. Case Rep Cardiol. 2015;2015(2):1–4. | ||
Cohn JN. Structural basis for heart failure. ventricular remodeling and its pharmacological inhibition. Circulation. 1995;91(10):2504–2507. | ||
Mason JW, O’Connell JB, Herskowitz A, et al. A clinical trial of immunosuppressive therapy for myocarditis. The myocarditis treatment Trial Investigators. N Engl J Med. 1995;333(5):269–275. | ||
Lauer B, Schannwell M, Kühl U, Strauer BE, Schultheiss HP. Antimyosin autoantibodies are associated with deterioration of systolic and diastolic left ventricular function in patients with chronic myocarditis. J Am Coll Cardiol. 2000;35(1):11–18. | ||
Sonderegger I, Röhn TA, Kurrer MO, et al. Neutralization of IL-17 by active vaccination inhibits IL-23-dependent autoimmune myocarditis. Eur J Immunol. 2006;36(11):2849–2856. | ||
Gong X, Feng H, Zhang S, et al. Increased expression of CCR5 in experimental autoimmune myocarditis and reduced severity induced by anti-CCR5 monoclonal antibody. J Mol Cell Cardiol. 2007;42(4):781–791. | ||
Okura Y, Yamamoto T, Goto S, et al. Characterization of cytokine and iNOS mRNA expression in situ during the course of experimental autoimmune myocarditis in rats. J Mol Cell Cardiol. 1997;29(2):491–502. | ||
Gong F, Hu Y, Chen L, Gu W. The therapeutic effect of intravenous immunoglobulins and vitamin C on the progression of experimental autoimmune myocarditis in the mouse. Med Sci Monit. 2007;13(11):BR240–BR246. | ||
Takada H, Kishimoto C, Hiraoka Y, Kurokawa M, Shiraki K, Sasayama S. Captopril suppresses interstitial fibrin deposition in Coxsackievirus B3 myocarditis. Am J Physiol. 1997;272(1 Pt 2):H211–H219. | ||
Brown PM, Pratt AG, Isaacs JD. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat Rev Rheumatol. 2016;12(12):731–742. | ||
Riksen NP, Smits P, Rongen GA. The nonspecific anti-inflammatory therapy with methotrexate for patients with chronic heart failure. Am Heart J. 2006;151(5):e5. | ||
Lin KX, Wang CZ, Qian GS. [Effect of Tripterygium wilfordii on Th1, Th2 cytokines production in asthma patients]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2001;21(1):22–24. Chinese. | ||
Li J, Schwimmbeck PL, Tschope C, et al. Collagen degradation in a murine myocarditis model: relevance of matrix metalloproteinase in association with inflammatory induction. Cardiovasc Res. 2002;56(2):235–247. | ||
Rezkalla S, Kloner RA, Khatib G, Smith FE, Khatib R. Effect of metoprolol in acute coxsackievirus B3 murine myocarditis. J Am Coll Cardiol. 1988;12(2):412–414. | ||
Horii T, Tambara K, Nishimura K, Suma H, Komeda M. Residual fibrosis affects a long-term result of left ventricular volume reduction surgery for dilated cardiomyopathy in a rat experimental study. Eur J Cardiothorac Surg. 2004;26(6):1174–1179. | ||
Seitz M, Loetscher P, Dewald B, et al. Methotrexate action in rheumatoid arthritis: stimulation of cytokine inhibitor and inhibition of chemokine production by peripheral blood mononuclear cells. Br J Rheumatol. 1995;34(7):602–609. | ||
Ho JC, Tipoe G, Zheng L, et al. In vitro study of regulation of IL-6 production in bronchiectasis. Respir Med. 2004;98(4):334–341. | ||
Shioji K, Kishimoto C, et al. Upreagulation of thioredoxin expression in gaint cell myocarditis in rats. FEBS Lett. 2000;472(1):109–113. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.