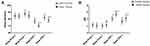Back to Journals » Nature and Science of Sleep » Volume 12
Effects of Transcutaneous Electrical Acupoint Stimulation on the Postoperative Sleep Quality and Pain of Patients After Video-Assisted Thoracoscopic Surgery: A Prospective, Randomized Controlled Trial
Authors Song B, Chang Y, Li Y, Zhu J
Received 9 July 2020
Accepted for publication 6 October 2020
Published 27 October 2020 Volume 2020:12 Pages 809—819
DOI https://doi.org/10.2147/NSS.S270739
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Steven A Shea
Bijia Song,1,2,* Yuanyuan Chang,1,* Yang Li,1 Junchao Zhu1
1Department of Anesthesiology, Shengjing Hospital of China Medical University, Shenyang, Liaoning, People’s Republic of China; 2Department of Anesthesiology, Friendship Hospital of Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Junchao Zhu
Department of Anesthesiology, Shengjing Hospital of China Medical University, Shenyang, Liaoning, People’s Republic of China
Email [email protected]
Objective: Postoperative sleep disturbances have serious adverse effects on postoperative outcomes. Our paper aimed to observe the effect of using transcutaneous electrical acupoint stimulation (TEAS) on sleep quality and complications after surgery in patients undergoing selective video-assisted thoracoscopic surgery.
Patients and Methods: Eighty-five patients were divided into the TEAS group or the control group randomly. Thirty minutes of TEAS treatment was performed on TEAS group at the following time points: the first night before surgery, at the end of surgery, and before sleeping on the second and third nights after surgery. The Portable Sleep Monitor (PSM) was performed to determine the sleep quality of the two nights before the operation (Sleep preop 2 and Sleep preop 1) and the first and third night after surgery (Sleep POD 1 and Sleep POD 3). The visual analog scale (VAS) was performed to evaluate pain scores after surgery and the Athens Insomnia Scale (AIS) was used for evaluating subjective sleep quality.
Results: Participants in the TEAS group had a lower AIS score and higher sleep efficiency at each time point except Sleep preop 2. Participants in the TEAS group showed significantly higher proportion of each sleep stage during Sleep-preop 1, Sleep POD 1, and Sleep POD 3. Patients in the TEAS group had significantly lower VAS scores at 2, 4, and 6 h during the first 24 h after surgery. The incidence of nausea and vomiting and dizziness in the control group was statistically higher than in the TEAS group.
Conclusion: Patients usually have sleep disturbances after video-assisted thoracoscopic surgery, such as decreased distribution of each sleep stage, lower sleep efficiency, and higher AIS score. Undergoing TEAS treatment perioperatively can improve sleep quality, and effectively promote the postoperative analgesic effect and alleviate postoperative complications.
Keywords: acupoints, general anesthesia, pain, sleep quality, transcutaneous electrical acupoint stimulation
Introduction
Despite improvements in surgical and anesthetic techniques, sleep disruption remains a challenging problem in surgical settings. Postoperative sleep disturbances (POSD) are defined as changes in the sleep structure and quality of patients during the early stages after surgery, which are manifested as significantly shortened rapid eye movement (REM) sleep, prolonged awake time, and sleep fragmentation.1 Long-term POSD may increase the risk of postoperative delirium or cognitive dysfunction and delay recovery, thereby worsening the patient’s physical condition.2,3 Although pharmacological interventions, such as short-acting non-benzodiazepine4 and multi-modal analgesia,5 have been reported to improve sleep quality after surgery, the potential risk of addiction and truncation still limits the clinical use of those drugs.
Transcutaneous electrical acupoint stimulation (TEAS) is a new acupuncture therapy developed by combining transcutaneous electrical nerve stimulation, more commonly used in Europe and America, and traditional Chinese acupuncture. TEAS treats disease through inputting a pulse current of different frequencies, intensities, and waveforms via electrode paste adhering to the skin. Previous studies have proven that TEAS can be successfully applied for various aspects such as relieving pain and nausea and vomiting after surgery,6–8 and improving sleep quality after surgery.9
The aim of our study was to investigate the effect of TEAS on postoperative pain and sleep quality among patients who have undergone video-assisted thoracoscopic surgery under general anesthesia. We hypothesized that TEAS intervention would effectively improve postoperative pain and sleep quality in these patients.
Patients and Methods
The study was approved by the Human Research Ethics Committee of Shengjing Hospital, Shenyang, Liaoning, China (Institutional Review Board registration number 2019PS646K) and complied with the purpose of the Declaration of Helsinki. Written informed consent was obtained from all patients participating in the study. The study was registered on Clinicaltrials.gov (NCT04124679) before the patients were enrolled.
Participants
Participants who received elective video-assisted thoracoscopic surgery at Shengjing Hospital of China Medical University were enrolled in this study. The inclusion criteria were as follows: (1) patients with American Society of Anesthesiologists grades I‒II; (2) patients with a body mass index of 18‒25 kg/m2; and (3) patients aged 40‒65 years. The following participants were excluded: (1) patients with central nervous system and mental diseases; (2) patients with preoperative sleep disturbances; (3) patients with a history of sedative, analgesic, or antidepression drug use; (4) patients with sleep apnea or moderate and severe obstructive sleep apnea-hypopnea syndrome; (5) patients with sinus bradycardia or atrioventricular block grade II or greater; and (6) patients with severe visual or hearing impairment or who were unable to communicate.
Sample Size
Based on our preliminary study, 30 patients were selected, including 15 in the TEAS group and 15 in the control group. According to the primary outcome of the Athens Insomnia Scale (AIS) score between the two groups and the calculation of the sample size by the formula (n1 = n2 = 2 × [(1.96 + 0.842) × δ/σ]2), 0.54 was chosen as the estimated variability between the two groups, and 0.8 was chosen as the standard deviation. Thus, 34 patients were recruited for each group in order to achieve a two-sided Type I error (α) of 0.05 and power of 80%. Due to drop out, a total of 85 patients were enrolled in this study.
Standardized Anesthesia
For all patients, surgeries were completed before 4:00 PM. Intramuscular midazolam (0.05 mg/kg) as a premedication was given to patients 60 min before transfer to the operating room. The induction of general anesthesia were propofol (2.0 mg/kg), sufentanil (0.3 μg/kg), and cisatracurium (0.15 mg/kg). Three minutes later, orotracheal intubation was performed with a double-lumen tube under video laryngoscopic guidance. The airway pressure was maintained at <30 cm H20 under one-lung ventilation. The pressure of end-tidal carbon dioxide was maintained between 35 and 45 mmHg. Intraoperative anesthetics administration was as follows: continuous propofol infusion at 4‒8 mg/kg/h and a separate 0.15‒0.20 μg/kg/min remifentanil infusion for maintaining sedation and analgesia; and inhalation of sevoflurane (0.6‒2%) to keep a minimum alveolar concentration of ≥ 0.7. Ramosetron (0.3 mg) was given prophylactically, and ketorolac tromethamine 45 mg was given at 30 min before the end of surgery to alleviate postoperative pain. Before chest wall closure, 0.1% ropivacaine solution (7 mL each) was delivered under thoracoscopic guidance at the proximal side of the fourth, fifth, and sixth intercostal nerves.
After the surgery, the patients were transferred to the postanesthesia care unit until complete recovery of consciousness and then transferred to a single occupant room. A patient-controlled analgesia (PCA) system was attached after surgery (4 mg butorphanol and 2 g propacetamol in 100 mL saline, every pump press resulting in a 2 mL infusion, with a 15-min lockout interval).
Study Protocol and Measurements
Eighty-five patients were divided to the TEAS group or control group randomly in a 1:1 ratio using a computer-generated randomization number sequence. Seal the group assignments in sequentially numbered opaque envelopes. Random assignment only informs the acupuncturist of the Department of Traditional Chinese Medicine of Shengjing Hospital. However, the acupuncturist was only responsible for placing the electrodes and turning the stimulator on and off. To be sure, there was no communication about the study between the acupuncturist and other researchers and study patients. Patients, attending anesthesiologists, surgeons and data collectors (the physicians in the sleep laboratory who were only responsible for evaluating sleep variables) were all blinded to the group assignment.
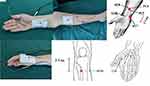
In the TEAS group, 30 min of TEAS treatment was performed bilaterally by an experienced acupuncturist. TEAS was performed at the HT7 (Shenmen) and Neiguan (PC6) acupoints on the night before surgery, by means of a stimulator (Hwato Electronic Acupuncture Treatment Instrument, model no.: SDZ-II; Suzhou Medical Appliances Co. Ltd, Suzhou, China) (Figure 2). Additionally, 30 min of TEAS treatment was also performed bilaterally at the Neiguan (PC6), HT7 (Shenmen), ST36 (Zusanli), and LI4 (Hegu) acupoints at the end of surgery, and before sleeping on the second and third nights after surgery (Figure 2). The TEAS device was calibrated before each treatment and was set at 2/10 HZ, which has been documented as the most effective frequency.10 The optimal intensity range is 6 to 15 mA, which can be adjusted according to the individual’s maximum tolerance to maintain minor muscle twitches.
Patients in the control group received electrical stimulation at a nonacupoint, which was located 4-cm interior to the bilateral Neiguan (PC6), HT7 (Shenmen), ST36 (Zusanli), and LI4 (Hegu) acupoints, similar to patients in the TEAS group. To ensure that patients remained blinded, the low-frequency stimuli were set to the same frequency (2/10 HZ), resulting in the patients believing that they were undergoing real TEAS therapy for 30 min. The control group also underwent this sham treatment on the first night before surgery, after surgery, and before sleeping on the second and third nights after surgery, similar to the TEAS group.
Data Collection
The Portable Sleep Monitor (PSM, PSM100A; Chengdu Sealand Technology Co., Ltd., Chengdu, China) is based on a patented, China Food and Drug Administration (CFDA)-cleared algorithm (CFDA approval: 20,162,210,145), which uses direct accessibility process to determine the quality of sleep. It requires a single-line electrocardiogram or photoplethysmography and accelerometer to produce an output that can be manipulated or interpreted without professional skills or training.11 The PSM was used on the following three nights from 23 PM to 6 AM: the second night and first night before surgery (Sleep preop 2 and Sleep-preop 1), the first and third night after surgery (Sleep POD1 and Sleep POD 3). The application of the PSM is shown in Figure 3.
The sleep process can be divided into waking period, non-rapid eye movement (NREM) and REM sleep. NREM includes Stages 1–4. Combining Stage 3 and Stage 4 represents “stable sleep”, at this stage the brain almost completely generates delta waves. When a person falls asleep, Stage 1 occurs for a short time. This is a very light stage of sleep because the person is drifting in and out of sleep. Stage 2 is defined as a slowing of brain waves and occasional sudden faster brain waves. The combination of Stage 1 and Stage 2 represents “light sleep” or “unstable sleep”.12
The following sleep variables were evaluated by physicians in the sleep laboratory, who were blinded to patient information: sleep efficiency (sleep time/recording time), subjective sleep quality score [AIS]), and percentages of REM sleep, stable sleep, and unstable sleep.
AIS is a self-assessment psychological questionnaire used to quantify sleep difficulties, based on the International Classification of Diseases-10th edition criteria.13 It comprises eight items: sleep induction, waking during the night, final awakening, total sleep duration, sleep quality, well-being, functioning capacity, and sleepiness during the day, and the total AIS scores range from 0 to 24 points, with ≥6 points indicating a diagnosis of insomnia.14,15
The postoperative pain score was evaluated by a visual analog scale (VAS) score,16 where 0 means no pain and 10 means severe pain. VAS scores were measured at 2, 4, 6, and 24 hours after surgery. Record the total number of PCA pump presses and appropriately treat the adverse reactions within 24 hours after surgery, such as hypotension, bradycardia, nausea and vomiting, and dizziness.
Statistical Analyses
PSS 23.0 and GraphPad Prism 6.0 software were used for the statistical analyses of study data. Quantitative data are presented as means ± standard deviations. Use chi-square test to analyze qualitative data. The Independent sample t-test and Wilcoxon rank- sum test were used between the TEAS Group and the Control Group to analyze AIS score, sleep efficiency and each sleep distribution.
All analyses were based on the intention-to-treat (ITT) population that was defined as participants who underwent at least one session of TEAS treatment or sham stimulation treatment and completed at least one measurement of AIS. Additional analyses were performed on the basis of the per-protocol (PP) population that was defined as participants who underwent 30 min of TEAS treatment or sham stimulation treatment and completed outcome measures at each timepoint. P < 0.05 was considered to be statistically significant.
Results
We initially assessed 109 patients for eligibility to participate in this study. Among them, 15 patients did not meet the inclusion criteria, and 9 patients refuse to participate (Figure 1). Finally, we included 85 patients in the ITT population and 78 in the PP population (three patients were allergic to TEAS, two patients were transferred to the intensive care unit after surgery, and two patients removal of the drainage tube was delayed).
 |
Figure 1 Flow diagram showing the patients that were included and excluded in this study. |
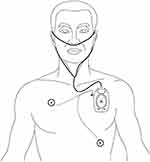 |
Figure 3 The application of using the Portable Sleep Monitor. |
Demographic Characteristics of the Two Groups
There were no statistically significant differences between the two groups in terms of patient gender (P = 0.595), age (P = 0.152), total dose of sufentanil consumption (P = 0.322), duration of surgery (P = 0.125), intraoperative fluid input volume (P = 0.424), bleeding volume (P = 0.515), urine volume (P = 0.689), and type of surgeries (P = 0.808) (Table 1).
 |
Table 1 Comparison of Demographic Characteristics Between the Two Groups |
Primary Outcome: Comparison of Perioperative Sleep Quality Between the Two Groups
There was no significant difference in the AIS score and sleep efficiency of patients in the TEAS or control groups at Sleep-preop 2 (P = 0.205 and P = 0.771, respectively). At Sleep preop 1, Sleep POD 1 and Sleep POD 3, the AIS scores of the TEAS group were lower than those of the control group. Patients in the TEAS group had a lower AIS score than those in the control group at Sleep-preop 1, Sleep POD 1, and Sleep POD 3 (P = 0.036, P < 0.001, and P < 0.001, respectively). Moreover, patients in the TEAS group had a significantly higher sleep efficiency than those in the control group at Sleep-preop 1, Sleep POD 1, and Sleep POD 3 (P < 0.001). The sleep efficiency presented lower and AIS scores presented higher among the two groups during Sleep POD 1 and Sleep POD 3 than Sleep preop 1 (all P < 0.001) (Figure 4A and B; Table 2). There were also significant differences in AIS score (P = 0.025, P < 0.001, and P < 0.001) and sleep efficiency (all P < 0.001) when compared with the previous time point, for patients in the TEAS group. No statistically significant differences were found in AIS score and sleep efficiency at Sleep-preop 1 as compared with Sleep-preop 2 for patients in the control Group (P = 0.160 and P = 0.235, respectively) (Figure 4A and B; Table 2). The distribution of sleep stages is shown in Figure 5 and Table 2. The proportions of REM, stable sleep and unstable sleep during Sleep POD 1 and Sleep POD 3 among the two groups were lower than those during Sleep preop 1 (P < 0.001). Patients in the TEAS group showed a significantly higher ratio of each sleep stage than those in the control group at Sleep-preop 1 (P = 0.013, P < 0.001, and P = 0.018, respectively), Sleep POD 1 (P < 0.001), and Sleep POD 3 (P < 0.001).
 |
Table 2 The Comparison of Sleep Efficiency, AIS Score and Each Sleep Stage at Different Timepoints Between the Two Groups |
Secondary Outcome: Comparison of Postoperative Pain and Adverse Effects Between the Two Groups
Patients in the TEAS group had significantly lower VAS scores than those in the control group at 2, 4, and 6 h after the surgery (P = 0.001, P < 0.001, and P = 0.007, respectively, Table 3). The pump press numbers of PCA in the control group was greater than that in the TEAS group (P < 0.001, Table 3). The incidence of nausea and vomiting, and of dizziness was also significantly higher in the control group than in the TEAS group (P = 0.039 and P = 0.012, respectively, Table 3).
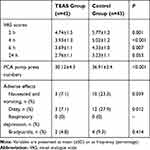 |
Table 3 Degree of Postoperative Pain and Incidence of Adverse Effects Between the TEAS Group and the Control Group |
Discussion
Our results confirmed that patients undergoing video-assisted thoracoscopic surgery were prone to sleep disorders such as reduced distribution of each sleep stage, reduced sleep efficiency, and higher AIS scores. However, patients who underwent TEAS before and after the surgery had better perioperative sleep efficiency and subjective sleep quality, and TEAS promoted the postoperative analgesic effect and alleviated postoperative complications, as compared with patients in the control group.
Surgical trauma, general anesthetics, operation time, and intraoperative bleeding volume are important surgical factors that may cause POSD in patients, such as decreased REM sleep and slow-wave sleep, changes in sleep structure, and sleep fragmentation.17,18 This may be because general anesthetics may interfere with the normal sleep-wake cycle, disrupt melatonin metabolism, and affect circadian clock genes which are expressed in the suprachiasmatic nucleus.19,20 Moreover excessive stress caused by surgical trauma might disrupt the neuroendocrine system, hormone and cytokine levels, and immune function, and may cause inflammatory reactions.21 In our study, we found that patients in both groups presented a lower sleep efficiency and a higher AIS score characterized by a decreased proportion of each sleep stage during Sleep POD 1 and Sleep POD 3, as compared with Sleep-preop 1, which was similar to the finding of Lin et al, who reported that sleep disturbances are frequently observed during the postoperative period in patients who have undergone open thoracotomy or minimally invasive surgery.22
Acupuncture has been utilized in Chinese health care for at least 2500 years. It involves the insertion of thin needles at specific acupoints to balance the body and restore its physiological function, without affecting the natural sleep‒wake cycle.23 TEAS combines transcutaneous electrical nerve stimulation with traditional acupuncture to provide specific low-frequency pulse currents to the body through the skin. Compared with traditional acupuncture or electroacupuncture, it has many advantages, including reduced pain and injury, reduced incidence of infection and higher patient tolerance. In our study, we chose PC6 (Neiguan) and HT7 (Shenmen) as acupoints to improve sleep quality; moreover, the dense-disperse frequency of 2/10 Hz was chosen based on previous literature.24–26 We found that during the perioperative period, compared with the control group, the TEAS group had higher sleep efficiency and lower AIS scores. Although patients in both groups presented with a lower postoperative proportion of each sleep stage during the postoperative period than during Sleep-preop 1 and Sleep-preop 2, patients in the TEAS group still showed a higher proportion of these sleep phases than those in the control group.
The possible mechanisms underlying the effects of TEAS in the treatment of decreased postoperative sleep quality may be as follows: First, TEAS can regulate the levels of serotonin (5-HT), norepinephrine, cortisol, melatonin, and other endogenous factors to improve sleep quality indirectly. Rat studies have shown that electroacupuncture can improve sleep quality by restoring normal neurotransmitters (eg, 5-HT and 5-hydroxyindoleacetic acid), interleukin-1β, and tumor necrosis factor levels, and decreasing norepinephrine and dopamine levels in the brain of an insomnia rat model.27,28 Second, Heng and Jia showed that acupuncture may stably affect human brain electrical activity through inhibiting the hippocampus β wave and activating δ wave, thereby effectively reducing nerve stress function and exerting sedative and hypnotic effects.24
In addition to the decline in postoperative sleep quality caused by surgical trauma, stress, and general anesthetics, postoperative complications after major surgery may also reduce postoperative sleep quality. Acupoint electrical stimulation may effectively improve postoperative sleep quality by reducing postoperative complications. Patients undergoing video-assisted thoracic surgery are generally expected to experience less postoperative pain because of the less-invasive surgery; however, Rizk et al reported that patients usually experienced similar pain intensity and a similar prevalence of chronic post-surgical pain as patients undergoing thoracotomy.29 Wang et al also found that patients who underwent video-assisted thoracic surgery had higher pain levels on the first night after surgery.30
Acupoint electrical stimulation has been proven to have obvious analgesic and sedative effects, which can enhance immune function and reduce postoperative complications, such as pain, and nausea and vomiting, which could improve postoperative sleep quality and promote the recovery of patients.31,32 According to previous studies about the analgesic effect of stimulation at ST-36 (Zusanli) and LI-4 (Hegu) acupoints,33,34 we used these two acupoints in combination with PC6 (Neiguan) and HT7 (Shenmen) acupoints after surgery in our study. Thirty minutes of TEAS treatment was also performed bilaterally by an experienced acupuncturist at these four acupoints on the first three nights after surgery. We found that patients in the TEAS group had significantly lower VAS scores than those in the control group at 2, 4, 6, and 24 h after surgery. Patients in the control group used more PCA doses (more PCA pump presses) than those in the TEAS group. The possible mechanisms, according to animal studies, may be as follows: First, electroacupuncture-upregulated endocannabinoids may directly inhibit pain because cannabinoid receptor 2 activation inhibits sensory nerve activities in a rat pain model.35 Second, electroacupuncture at PC6 (Neiguan) and LI4 (Hegu) can change the phosphorylation level of the N-methyl-D-aspartate receptor (NMDA) subunit 2B of the NMDA receptor in the C1‒C3 segment of the spinal cord, and can up-regulate the expression of 5-hydroxytryptamine 2A receptor mRNA and protein, which could effectively increase the pain threshold and relieve pain.36,37
Moreover, in terms of other postoperative complications, we found that the incidence of nausea and vomiting, and of dizziness were significantly lower in the TEAS group than in the control group. This may be because, first, electroacupuncture at PC6 (Neiguan) and ST-36 (Zusanli) could cause free flow of qi, regulate the digestive system, and help relax the gastrointestinal track.38,39 Second, electroacupuncture may induce the release of peripheral opioids to relieve pain and reduce the use of opioids, which may improve postoperative nausea and vomiting.40 Third, electroacupuncture may accelerate motility of the gastrointestinal track and mitigate delayed gastric emptying via corticotrophin-releasing factor type-2 receptors, which mediate outflow of the parasympathetic efferent pathway.38,39,41
There are some limitations in this study. First, we only collected data on sleep quality in the short-term perioperative period after TEAS. The effect of TEAS on long-term sleep quality after surgery needs further study. Second, although we tried to reduce the confounding factors of postoperative sleep quality such as light, noise or interference due to nursing care in the study, there may be other inevitable factors that may affect postoperative sleep quality. Third, it is necessary to study the effect of TEAS on postoperative sleep quality in large-scale multi-center studies and other types of surgery under general anesthesia in the future.
Conclusion
In conclusion, patients undergoing video-assisted thoracoscopic surgery experienced marked postoperative sleep disturbances, characterized by higher AIS scores, lower sleep efficiency, and decreased proportions of each sleep phase. Our findings show that TEAS can effectively improve postoperative sleep efficiency and subjective sleep scores, relieve postoperative complications, such as postoperative pain, and nausea and vomiting, which may further help to improve postoperative sleep quality and promote the recovery of patients.
Data Sharing Statement
The individual deidentified participant data in our study could be shared with readers. Readers can obtain the data by emailing the corresponding author ([email protected]). We did not include specific data and documents from previous reports in our study. All the data in our study are available for 10 years.
Acknowledgments
The authors would like to thank Raymond C. Koehler, MD, Ph.D., from the Departments of Anesthesiology and Critical Care Medicine, Johns Hopkins, University, Baltimore, MD, USA, and Dr. Weifeng Song, MD, Ph.D., from the Department of Anesthesiology and Perioperative Medicine, School of Medicine, the University of Alabama at Birmingham, Birmingham, AL, USA, for their discussion and advice on this study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. Bijia Song and Yuanyuan Chang contributed equally to the work.
Funding
The present study was funded by the Joint Plan of Key R&D of Liaoning Provincial Science and Technology Department (2020JH2/10300123) and the Support Plan for Innovative Talents in Liaoning Higher Education Institution (grant no. 201834).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Knill RL, Moote CA, Skinner MI, Rose EA. Anesthesia with abdominal surgery leads to intense REM sleep during the first postoperative week. Anesthesiology. 1990;73(1):52–61. doi:10.1097/00000542-199007000-00009
2. Su X, Wang DX. Improve postoperative sleep: what can we do? Curr Opin Anaesthesiol. 2018;31(1):83–88. doi:10.1097/ACO.000000000.0000538
3. Kjolhede P, Langstrom P, Nilsson P, Wodlin NB, Nilsson L. The impact ofqualityofsleep onrecoveryfromfast-trackabdominal hysterectomy. J Clin Sleep Med. 2012;8(4):395–402. doi:10.5664/jcsm.2032.
4. Krenk L, Jennum P, Kehlet H. Postoperative sleep disturbances after zolpidem treatment in fast-track hip and knee replacement. J Clin Sleep Med. 2014;10(3):321–326. doi:10.5664/jcsm.354018.
5. Dolan R, Huh J, Tiwari N, Sproat T, Camilleri-Brennan J. Aprospective analysis of sleep deprivation and disturbance in surgical patients. Ann Med Surg. 2016;6:1–5. doi:10.1016/j.amsu.2015.12.046.
6. Asmussen S, Maybauer DM, Chen JD, et al. Effects of acupuncture in anesthesia for craniotomy: a meta-analysis. J Neurosurg Anesthesiol. 2017;29(3):219–227. doi:10.1097/ANA.0000000000000290
7. Sahmeddini MA, Farbood A, Ghafaripuor S. Electro-acupuncture for pain relief after nasal septoplasty: a randomized controlled study. J Altern Complement Med. 2010;16(1):53–57. doi:10.1089/acm.2009.0288
8. Xu M, Zhou SJ, Jiang CC, et al. The effects of P6 electrical acustimulation on postoperative nausea and vomiting in patients after infratentorial craniotomy. J Neurosurg Anesthesiol. 2012;24(4):312–316. doi:10.1097/ANA.0b013e31825eb5ef
9. Chiou YF, Yeh ML, Wang YJ. Transcutaneous electrical nerve stimulation on acupuncture points improves myofascial pain, moods, and sleep quality. Rehabil Nurs. 2020;45(4):225–233. doi:10.1097/RNJ.0000000000000198
10. Yao Y, Zhao Q, Gong C, et al. Transcutaneous electrical acupoint stimulation improves the postoperative quality of recovery and analgesia after gynecological laparoscopic surgery: a randomized controlled trial. Evid Based Complement Alternat Med. 2015;2015:324–360. doi:10.1155/2015/324360
11. Miller JN, Kupzyk KA, Zimmerman L, et al. Comparisons of measures used to screen for obstructive sleep apnea in patients referred to a sleep clinic. Sleep Med. 2018;51:15–21. doi:10.1016/j.sleep.2018.06.007
12. Hassan AR, Bhuiyan MIH. Dual tree complex wavelet transform for sleep state identification from single channel electroencephalogram. IEEE Int Conference Telecommunications Photonics. 2016;
13. Okajima I, Nakajima S, Kobayashi M, Inoue Y. Development and validation of the Japanese version of the athens insomnia scale. Psychiatry Clin Neurosci. 2013;67(6):420–425. doi:10.1111/pcn.12073
14. Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens insomnia scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48(6):555–560. doi:10.1016/s0022-3999(00)00095-7
15. Soldatos CR, Dikeos DG, Paparrigopoulos TJ. The diagnostic validity of the athens insomnia scale. J Psychosom Res. 2003;55:263–267. doi:10.1016/S0022-3999(02)00604-9
16. El Sherif FA, Othman AH, Abd El-Rahman AM, Taha O. Effect of adding intrathecal morphine to a multimodal analgesic regimen for postoperative pain management after laparoscopic bariatric surgery: a prospective, double-blind, randomized controlled trial. Br J Pain. 2016;10(4):209–216. doi:10.1177/2049463716668904
17. Gögenur I, Wildschiøtz G, Rosenberg J. Circadian distribution of sleep phases after major abdominal surgery. Br J Anaesth. 2008;100(1):45–49. doi:10.1093/bja/aem340
18. Krenk L, Jennum P, Kehlet H. Sleep disturbances after fast-track hip and knee arthroplasty. Br J Anaesth. 2012;109(5):769–775. doi:10.1093/bja/aes252
19. Tian JX, Yin C, Chu SS, Ma ZL, Gu XP. Murine clock gene express in the suprachiasmatic nuclei and peripheral blood mononuclear cells during the daily sleep-wake rhythm and after isoflurane anesthesia. Sleep Biolog Rhythm. 2015;13:357–365.
20. Buhr ED, Takahashi JS. Molecular components of the Mammalian circadian clock. Handb Exp Pharmacol. 2013;217:3–27. doi:10.1007/978-3-642-25950-0_1
21. Alazawi W, Pirmadjid N, Lahiri R, Bhattacharya S. Inflammatory and immune responses to surgery and their clinical impact. Ann Surg. 2016;264(1):73–80. doi:10.1097/SLA.0000000000001691
22. Lin S, Chen Y, Yang L, Zhou J. Pain, fatigue, disturbed sleep and distress comprised a symptom cluster that related to quality of life and functional status of lung cancer surgery patients. J Clin Nurs. 2013;22(9–10):1281–1290. doi:10.1111/jocn.12228
23. Yin X, Gou M, Xu J, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. 2017;37:193–200. doi:10.1016/j.sleep.2017.02.012
24. Heng Z, Jia L. Comparative study on effect of acupuncture at neiguan (pc6) and shenmen (ht7) acupoints on brain wave of sleep deprivation model rats. Hubei J TCM JUN. 2017;39(6):13–16.
25. Ni X, Xie Y, Wang Q, et al. Cardioprotective effect of transcutaneous electric acupoint stimulation in the pediatric cardiac patients: a randomized controlled clinical trial. Paediatr Anaesth. 2012;22(8):805–811. doi:10.1111/j.1460-9592.2012.03822.x
26. Kotani N, Hashimoto H, Sato Y, et al. Preoperative intradermal acupuncture reduces postoperative pain, nausea and vomiting, analgesic requirement, and sympathoadrenal responses. Anesthesiology. 2001;95(2):349–356. doi:10.1097/00000542-200108000-00015
27. Tang L, You F, Hu X, Li YF. Electroacupuncture improves insomnia by down-regulating peripheral benzodiazepine receptor expression in hippocampus, and up-regulating 5-HT, 5-HIAA, TNF-α and IL-1β contents in hypothalamus in insomnia rats. Zhen Ci Yan Jiu. 2019;44(8):560–565. doi:10.13702/j.1000-0607.180610.
28. Cheng C, Liu P, Wu Q, et al. Effects of electroacupuncture on anxiety and sympathetic-adrenal medulla system in rats with insomnia. Zhongguo Zhen Jiu. 2015;35(9):923–926.
29. Rizk NP, Ghanie A, Hsu M, et al. A prospective trial comparing pain and quality of life measures after anatomic lung resection using thoracoscopy or thoracotomy. Ann Thorac Surg. 2014;98(4):1160–1166. doi:10.1016/j.athoracsur.2014.05.028
30. Wang H, Li S, Liang N, Liu W, Liu H, Liu H. Postoperative pain experiences in Chinese adult patients after thoracotomy and video-assisted thoracic surgery. J Clin Nurs. 2017;26(17–18):2744–2754. doi:10.1111/jocn.13789
31. Teoh AYB, Chong CCN, Leung WW, et al. Electroacupuncture-reduced sedative and analgesic requirements for diagnostic EUS: a prospective, randomized, double-blinded, sham-controlled study. Gastrointest Endosc. 2018;87(2):476–485. doi:10.1016/j.gie.2017.07.029.
32. Wu MS, Chen KH, Chen IF, et al. The efficacy of acupuncture in post-operative pain management: a systematic review and meta-analysis. PLoS One. 2016;11(3):e0150367. doi:10.1371/journal.pone.0150367.
33. Praveena S, Bhojwani K, Abdullah N. Intraoperative electroacupuncture reduces postoperative pain, analgesic requirement and prevents postoperative nausea and vomiting in gynaecological surgery: a randomised controlled trial. Anesth Pain Med. 2016;6(6):e40106. doi:10.5812/aapm.40106
34. Xiao J, Yi W, Wu L. Effects of electroacupuncture on reducing labor pain and complications in the labor analgesia process of combined spinal-epidural analgesia with patient-controlled epidural analgesia. Arch Gynecol Obstet. 2019;299(1):123–128. doi:10.1007/s00404-018-4955-6
35. Anand P, Whiteside G, Fowler CJ, Hohmann AG. Targeting CB2 receptors and the endocannabinoid system for the treatment of pain. Brain Res Rev. 2009;60:255–266. doi:10.1016/j.brainresrev.2008.12.003
36. Qiao LN, Yang YS, Wang JY, et al. Effects of electroacupuncture at “Futu” (LI 18), etc. on expression of spinal 5-HT 1 AR mRNA, 5-HT 2 AR mRNA and protein in rats with neck incision pain. Zhen Ci Yan Jiu. 2011;36(6):391–396.
37. Yan LP, Liu YG, Wu XT, Li SD, Ma C. Effect of electroacupuncture intervention on N-methyl-D-aspartic acid receptor expression in spinal cord in rats with chronic constrictive injury of the sciatic nerve. Zhen Ci Yan Jiu. 2013;38(5):380–385.
38. Xie J, Chen LH, Ning ZY, et al. Effect of transcutaneous electrical acupoint stimulation combined with palonosetron on chemotherapy-induced nausea and vomiting: a single-blind, randomized, controlled trial. Chin J Cancer. 2017;36(1):6. doi:10.1186/s40880-016-0176-1
39. Alizadeh R, Esmaeili S, Shoar S, Bagheri-Hariri S, Shoar N. Acupuncture in preventing postoperative nausea and vomiting: efficacy of two acupuncture points versus a single one. J Acupunct Meridian Stud. 2014;7(2):71–75. doi:10.1016/j.jams.2013.04.005
40. Fukuda H, Suenaga K, Tsuchida D, et al. The selective mu opioid receptor antagonist, alvimopan, improves delayed GI transit of postoperative ileus in rats. Brain Res. 2006;1102(1):63–70. doi:10.1016/j.brainres.2006.02.092
41. Takahashi T. Mechanism of acupuncture on neuromodulation in the gut–a review. Neuromodulation. 2011;14(1):8–12. doi:10.1111/j.1525-1403.2010.00295.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.