Back to Journals » Cancer Management and Research » Volume 12
Effects of Topical Hangeshashinto (TJ-14) on Chemotherapy-Induced Oral Mucositis
Authors Ozawa N, Onda T , Hayashi K, Honda H , Shibahara T
Received 13 November 2019
Accepted for publication 31 January 2020
Published 12 February 2020 Volume 2020:12 Pages 1069—1078
DOI https://doi.org/10.2147/CMAR.S238306
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xueqiong Zhu
Natsuo Ozawa,1 Takeshi Onda,1 Kamichika Hayashi,1 Hirona Honda,1 Takahiko Shibahara1,2
1Department of Oral and Maxillofacial Surgery, Tokyo Dental College, Chiba 261-8502, Japan; 2Oral Cancer Center, Tokyo Dental College, Chiba 272-8513, Japan
Correspondence: Takeshi Onda
Department of Oral and Maxillofacial Surgery, Tokyo Dental College, 1-2-2 Masago, Mihama-ku, Chiba 261-8502, Japan
Tel +81-43-270-3978
Fax +81-43-270-3979
Email [email protected]
Purpose: Hangeshashinto (TJ-14), a Kampo medicine comprising seven types of herbs, has been used in Japan to alleviate the side effects associated with anticancer drug treatments. However, the pharmacological effects of this medicine currently remain unclear. The present study aimed to demonstrate the efficacy of TJ-14 against anticancer drug-induced stomatitis, the pain associated with which may have a negative impact on mastication and swallowing.
Methods: Mucositis was induced in Sprague-Dawley rats by cancer chemotherapy. Changes in body weight, stomatitis grades, histopathological scores, and oral bacterial counts were examined among TJ-14-treated, saline-treated, and Control (no treatment) rats. In vitro studies, including cell proliferation and wound healing assays, using epidermal keratinocyte and fibroblast cell lines were conducted.
Results: The local application of TJ-14 exerted strong antibacterial effects and attenuated oral chemotherapy-induced stomatitis in rats. TJ-14 also increased the viability and invasion of epidermal keratinocytes and fibroblasts.
Conclusion: The present results demonstrated the potential of TJ-14 to attenuate chemotherapy-induced stomatitis.
Keywords: cancer chemotherapy, mucositis, Hangeshashinto, TJ-14, Kampo medicine, traditional Japanese medicine, oral cancer
Introduction
Significant advances have been achieved in the development of new anticancer agents and combination radiotherapy protocols to treat cancer;1,2 however, treatments for the side effects associated with these therapies remain limited.3 Previous studies reported that the incidence rates of oral mucositis, a common side effect of anticancer therapy, were 25–55%, 70–90%, and almost 100% in patients treated with anticancer agents for solid cancer, high-dose anticancer agents for hematopoietic stem cell transplantation, and anticancer agents and radiotherapy for head and neck cancer, respectively.1,4,5 Pain associated with stomatitis has a negative impact on mastication and swallowing, and, thus, nutrition, which reduces the quality of life of patients and increases their susceptibility to infections.6 Previous studies reported that stomatitis is a dose-limiting factor of cancer treatment,7 increases infection-related mortality,8 and may necessitate the withdrawal of or changes to treatment.9
Chemotherapy-induced stomatitis has been attributed to both primary and secondary causes, with the former being caused by mucosal inflammation as a result of reactive oxygen species generated by anticancer agents destroying cells in the oral mucosa, and the latter to large numbers of oral bacteria adhering to the ulcerated surface of the mucosa and causing local infections. These local infections, when combined with the metabolic damage and increased susceptibility to infections caused by anticancer agents, may become intractable or serious.10 Although various animal models have been employed to investigate stomatitis,11 the etiology of chemotherapy-induced stomatitis has not yet been elucidated in detail and may be more complex than originally considered.12
Few evidence-based studies have been conducted on the relationship between stomatitis and cancer treatments, and effective, reliable methods for the treatment of this condition have not been established. In clinical practice, there is no standard treatment for chemotherapy-induced oral mucositis, so currently symptomatic treatments are actually employed. The prophylactic measures and treatments for this disease reported so far include oral care, nutritional therapy, topical steroid ointment, cryotherapy, low-level laser therapy and azulene sulfonate sodium hydrate, as well as the mouth rinse with allopurinol, camostat mesylate, and rebamipide for removing active oxygen species and protecting mucous membranes. However, their effects are limited, which often makes the treatment difficult.13 Mouth rinse with lidocaine hydrochloride, as well as acetaminophen, NSAIDs, and opioids are also used for pain management, but they are not definitive cares. In recent years, the effectiveness of palifermin, a keratinocyte growth factor-1, for this disease has been reported and raising expectation. However, it has not been approved in Japan. In addition, its use means the administration of growth factors to the patients with malignant tumors, of which the safety has not been established yet.13 Kampo medicine (traditional Japanese medicine) has been used to treat the side effects associated with anticancer drug treatments in Japan.14,15 Hangeshashinto (TJ-14) has been empirically reported to attenuate the side effects associated with cancer chemotherapies.13,15,16 It comprises seven herbal extracts (Coptis rhizome, ginseng, glycyrrhiza, jujube, Pinellia tuber, processed ginger, and Scutellaria root) and has been approved for the treatment of acute and chronic gastrointestinal catarrh, fermentative diarrhea, and acute gastroenteritis and oral mucositis by the Ministry of Health, Labour and Welfare of Japan.17 TJ-14 reportedly promotes the healing of stomatitis induced by lung,18 colon,19 stomach,20 and head and neck cancer15 treatments, and suppresses its exacerbation. A double-blind, randomized, Phase II clinical trial for the chemotherapies for colorectal19 and gastric20 cancers has reported that TJ-14 reduced the severity of these diseases and shortened the periods for healing compared with placebo controls. However, the mechanism for the improvement of stomatitis by TJ-14 and its pharmacological effects still remain unclear. Recent studies reported the antioxidant,21 anti–inflammatory,22 bactericidal,23 analgesic17 and healing-promoting24 effects of TJ-14.
In the present study, we used an animal model of mucositis induced by cancer chemotherapy to investigate the effects of TJ-14 on stomatitis. In vitro studies were also conducted to clarify the effects of TJ-14 on epidermal keratinocytes and fibroblasts.
Materials and Methods
Hangeshashinto (TJ-14)
TJ-14 (serial number: J08372) was obtained from Tumura & Co. (Tokyo, Japan) and diluted to 100 mg/mL in distilled water.
Animals
All procedures using live animals conformed to the ethical guidelines established by the Japanese Council on Animal Care and were approved by the Animal Care Committee of Tokyo Dental College (permit number 282403). Fourteen-week-old male Sprague-Dawley rats (n = 21) were obtained from Sankyo Laboratory (Tokyo, Japan). Animals were housed in a room maintained under standardized light (12:12-h light/dark cycle), temperature (23 ± 2°C), and humidity (55% ± 5%) conditions and food pellets and drinking water were available ad libitum.1
Animal Model for Mucositis Induced by Cancer Chemotherapy
The protocol used to induce oral mucositis was modified from a previously reported method.1,25 5-FU (Wako Pure Chemical Industries, Osaka, Japan) was administered intraperitoneally to all rats at 60 mg/kg/day on Days 1–5. Anesthesia was induced on Day 6 by the inhalation of 4% sevoflurane (Maruishi Pharmaceutical, Osaka, Japan), followed by an intraperitoneal injection of sodium pentobarbital (30 mg/kg body weight, Somnopentyl; Kyoritsu Seiyaku, Tokyo, Japan). Fifty microliters of 100% acetic acid (Wako Pure Chemical Industries) was then applied to the lingual dorsum with a Plaut brush® (Oral Care, Tokyo, Japan) and rubbed in to induce stomatitis. Animals were then divided into three groups (n = 7 each): control (no treatment); saline-treated; and TJ-14-treated. After the development of stomatitis, mouths were rinsed four times daily (every 6 h) with physiological saline in the saline group and with TJ-14 in the TJ-14 group using a Doltz EW1211® water flosser (Panasonic Healthcare, Tokyo, Japan) at a water pressure of 4.0 kgf/cm2. Body weights were measured on Days 6, 9, 11, and 16. Stomatitis grades and bacterial counts were assessed before rinsing 3, 5, and 10 days after acetic acid irritation (Days 9, 11, and 16, respectively).
Stomatitis Grading
A modified version of the National Cancer Institute-Common Terminology Criteria for Adverse Events26 was used to grade chemotherapy-induced oral mucositis in rats on Days 9, 11, and 16 (before mouth rinsing) as follows:1 Grade 0, normal mucosa; Grade 1, redness of the mucosa with punctate ulcers or a pseudomembrane; Grade 2, confluent ulceration or a pseudomembrane with no bleeding following a slight stimulation; Grade 3, confluent ulceration or a pseudomembrane with bleeding following a slight stimulation; and Grade 4, tissue necrosis or spontaneous bleeding (Figure 1A).
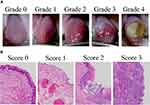 |
Figure 1 Rat chemotherapy-induced stomatitis grading and scoring criteria. Notes: (A) Representative examples from Grades 0 to 4 are shown; (B) Representative examples from Scores 0 to 3 are shown. |
Histopathological Analysis of Oral Mucositis
Tongue samples were collected from rats for a histopathological analysis 3, 5, and 10 days after acetic acid irritation (Days 9, 11, and 16, respectively). Specimens were fixed in 10% neutral-buffered formalin, dehydrated, and embedded in paraffin. Five-micrometer-thick sections were obtained for hematoxylin and eosin staining and examined under a light microscope (×40). Histological parameters were assessed in a single-blind manner and graded as follows:27 Score 0, a normal epithelium and connective tissue with no vasodilatation, cellular infiltration, hemorrhagic areas, ulceration, or abscesses; Score 1, mild vasodilatation, re-epithelization areas and inflammatory infiltration with large numbers of mononuclear cells, and no hemorrhagic areas, edema, ulceration, or abscesses; Score 2, moderate vasodilatation, areas of hydropic epithelial degeneration, inflammatory infiltration with large numbers of neutrophils, the presence of hemorrhagic areas, edema, and eventual ulceration, and no abscesses; and Score 3, severe vasodilatation, inflammatory infiltration with large numbers of neutrophils, the presence of hemorrhagic areas, edema, and extensive ulceration, and abscesses (Figure 1B).
Oral Bacterial Counts
Oral bacterial counts were assessed before rinsing 3, 5, and 10 days after acetic acid irritation (Days 9, 11, and 16, respectively). Bacterial counts were measured in the oral cavity using a bacteria detection apparatus (DU-AA01; Panasonic Healthcare), as described previously.28 Bacterial counts was assessed using the dielectrophoretic impedance measurement technique.1,29
Statistical Analysis
The distribution of weight (g) on Day 6 and bacterial counts (×105 cfu/mL) on Day 9 were tested for normality using the Shapiro–Wilk test. Weight and stomatitis grading were determined and the significance of differences among the three groups was examined using the Kruskal–Wallis test. Comparisons between two groups were performed using the Mann–Whitney U-test followed by the Steel-Dwass test. Oral bacteria were counted, and differences among all three groups were investigated using an analysis of variance. Comparisons between two groups were made using the t-test, and p values were adjusted using Tukey’s method. SAS version 9.4 statistical software (SAS Institute, Cary, NC) was used for statistical analyses, with p < 0.05 (two-tailed) considered to be significant.1
Anti-Inflammatory Effects of TJ-14 in the Experimental Inflammatory Rat Model
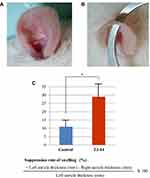
Phorbol 12-myristate 13-acetate (PMA; 5 mg; Sigma, St. Louis, MO) was dissolved in 250 μL of dimethyl sulfoxide (DMSO) in accordance with a previous study.30 A total of 1.59 mL of acetone was added to 10 μL of this solution to obtain a PMA solution (125 μg/mL), 10 μL of which was applied to the right and left auricles of healthy rats. TJ-14 was then applied to both sides of the right auricle once every hour, and the swelling inhibition rate was calculated after 6 h. Ice water was applied to both sides of the right auricle in the controls. The swelling inhibition rate was calculated using the following formula: suppression rate of swelling (%) = [left auricle thickness (mm) - right auricle thickness (mm)]/left auricle thickness (mm) ×100 (%).
Cells
The PSVK1 epidermal keratinocyte cell line and KD fibroblast cell line, obtained from the Japanese Collection of Research Bioresources Cell Bank, Osaka, Japan, were maintained in 150 × 20-mm tissue culture dishes (Nunc, Roskilde, Denmark) at 37°C (humidified atmosphere, 5% CO2/95% air) and cultured in Dulbecco’s modified Eagle’s medium (Sigma) with 10% fetal bovine serum (Sigma) and 50 units/mL penicillin and streptomycin.
Cell Proliferation Assay
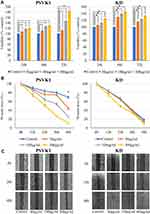
PSVK1 and KD cells were plated onto 96-well plates (density, 5 ×103 cells/well) in sextuplicate and incubated at 37°C in a humidified 5% CO2 atmosphere. After an overnight attachment period, the cells were treated with TJ-14 (30, 100, or 300 μg/mL) or 0.01% DMSO (as a control). The number of viable cells was counted at 24, 48, and 72 h using the RealTime-Glo MT Cell Viability Assay (Promega Corporation, Wisconsin, USA) and a GloMax 96 Microplate Luminometer (Promega Corporation). All assays were performed in five technical replicates and each assay was repeated three times.
Wound Healing Assay
PSVK1 and KD cells were seeded at 3.0 ×105 cells/insert on culture inserts (80206; ibidi GmbH, Munich, Germany) in triplicate and then exposed to TJ-14 (30, 100, 300 μg/mL) or 0.01% DMSO. Wound healing was assessed as described in our previous study.31 The closure of the gap that was created during the cell culture was viewed under a microscope (200× magnification) and immediately photographed after the same culture medium had been added at the indicated time points. The area of the gap was measured at each time point using the MTrackJ plugin (http://www.imagescience.org/meijering/software/mtrackj/manual/) in ImageJ software (version 1.50; NIH, Bethesda, MA).
Results
Data Distribution
The Shapiro–Wilk test was significant (p=0.0004) for body weight on Day 6, but not for bacterial counts on Day 9 (p=0.0068).
Body Weight Changes
Body weight decreased after the development of stomatitis in all rats in the three groups (Figure 2A). Significant differences were observed in body weight on Day 16 between the Control and TJ-14 groups and the saline and TJ-14 groups (p < 0.05). Within-group comparisons revealed significant differences in body weight between Day 6 and Days 9 and 11 in the three groups (p < 0.05). Significant differences were also noted between Days 6 and 16 in the Control and saline groups.
Stomatitis Grading
Stomatitis grades were the lowest on Days 11 and 16 in the TJ-14 group (Figure 2B). Grades did not improve during the experimental period in the Control group. In the saline group, no significant change in grade was observed on Day 11, whereas improvements were noted on Day 16. In the TJ-14 group, stomatitis grades improved on Days 11 and 16, exhibiting overall improvements with time. Significant differences were observed on Days 11 and 16 between the Control and TJ-14 groups and the saline and TJ-14 groups (Figure 2B). Intragroup comparisons revealed significant differences between Days 9 and 16 in the TJ-14 group.
Histopathological Scoring
Histopathological scores were the lowest on Days 11 and 16 in the TJ-14 group (Figure 2C), but improved on Days 11 and 16, exhibiting overall improvements with time. Significant differences were noted on Days 11 and 16 between the Control and TJ-14 groups and the saline and TJ-14 groups (Figure 2C). Intragroup comparisons revealed significant differences between Days 9, 11 and 16 in the TJ-14 group.
Oral Bacterial Count Measurements
Oral bacterial counts slightly decreased over time in all three groups (Figure 2D), with the largest decrease being observed in the TJ-14 group, followed by the saline group. The steepest reduction in bacterial counts occurred in the TJ-14 group. Significant differences were observed between all groups, except for the Control and saline groups on Days 9 and 11 (Figure 2D). Significant differences were also noted in all intragroup comparisons.
Suppression of Experimental Inflammation by TJ-14 in vivo
The Control group (cold water application) showed a 10% suppression in swelling (Figure 3A and C), whereas a 30% suppression was noted in the TJ-14 group (p < 0.01; Figure 3B and C).
TJ-14 Promotes the Viability and Invasion of Keratinocytes and Fibroblasts in vitro
As shown in Figure 4A, TJ-14 significantly increased the viability of PSVK1 and KD cells after 48 h of exposure (p < 0.05). Wound closure was observed within 48 h in TJ-14-treated PSVK1 and KD cells; in contrast, the process was significantly slower in Control cells (Figure 4B and C).
Discussion
Vasoconstriction, blood clot formation, fibrin formation, inflammatory cell infiltration, cell proliferation, neovascularization, and epithelial regeneration all contribute to wound healing in the oral mucosa. Local factors that influence wound healing include an insufficient oxygen supply, local infection, and the presence of foreign bodies, while systemic factors include age, sex, circulatory impairments, an immunocompromised status, the nutritional status, systemic disease, and the use of concomitant medications, such as steroids and anticancer agents.1,32 Chemotherapy-induced stomatitis is characterized by the adhesion of large numbers of oral bacteria to the ulcerated surface of the oral mucosa, which causes local infections. Oral mucosal cells are damaged by the free radicals generated by anticancer drugs, which induces a strong inflammatory reaction and the formation of erosions and ulcers. In addition, the oral mucosal epithelium is vulnerable to mechanical stimulation, which easily erodes the surface. Wound healing is delayed due to metabolic disorders caused by anticancer drugs, and protection against infection is compromised by immunosuppression.10 Therefore, local infections prolong healing in patients with stomatitis and increase their susceptibility to further infection. Local infection and delayed healing in chemotherapy-induced stomatitis act synergistically to promote critical colonization.33 Critical colonization may be prevented by decreasing bacterial counts, which may be achieved via chemical removal using pharmaceutical agents or physical removal by rinsing or similar approaches. Critical colonization is attributed to large numbers of resident bacteria in the mouth. Furthermore, ulcerated surfaces are covered by necrotic material during stomatitis, which promotes the proliferation of bacteria. Collectively, these factors reduce the efficacy of pharmaceutical agents.29,34 In the present study, bacterial counts were significantly lower in the saline and TJ-14 groups than in the Control group, and may be attributed to the physical removal of mucous and necrotic substances by water flossing.
The antibacterial effects of TJ-14 have been categorized into two types: those caused by the constituents of TJ-14 itself and those by the antimicrobial peptides produced by the body. The former involves homogentisic acid, baicalein, baicalin, berberine, coptisine, ginsenoside Rb1, and 6-shogaol, which have been reported to exert antibacterial effects against Gram-negative bacteria. A previous study reported that TJ-14 was ineffective against Gram-positive resident bacteria, and is less effective against the resident bacterial environment in the oral cavity.23 On the other hand, antibacterial peptide production involves 3,4-dihydroxybenzaldehyde, baicalin, and ginsenoside Rb1, which act on oral mucosal epithelial cells and are considered to protect them against bacterial infection by enhancing the production of the antimicrobial peptide calprotectin.35 The present results revealed markedly lower numbers of bacteria in the TJ-14 group, suggesting antibacterial activity in the mucosa of anticancer drug-induced stomatitis.
No significant differences were observed in body weight changes or food intake on Days 9 and 11 between the three groups. A decreased food intake was noted after the onset of stomatitis along with a corresponding reduction in body weight, and these changes were attributed to the pain associated with stomatitis. On Day 16, body weights were higher in the TJ-14 group than in the Control and saline groups, and may have been due to an increased food intake because pain during eating was alleviated by the healing of stomatitis.
The growth of granulation tissues is an important step in the oral mucosal repair throughout the healing process of stomatitis. Fibroblasts play the most important role also in the formation of granulation tissues. During the repair process, fibroblasts migrate, proliferate and secrete lots of collagen fibers and matrix components to form granulation tissues along with new capillaries. They also compensate for tissue defects and create the conditions for epidermal keratinocytes to cover them.36 Significant increases in the cell proliferation rate and cell migration in a concentration-dependent manner were observed in epidermal keratinocytes and fibroblasts in the TJ-14 group. Furthermore, macroscopic and histopathological findings indicated more rapid healing in the TJ-14 group than in the Control and saline groups. The repair of the oral mucosa was considered to be promoted by the cell migration-promoting effects of TJ-14.
The present results did not support the analgesic and free-radical-scavenging effects of TJ-14. However, the anti–inflammatory, wound healing, and antibacterial properties of TJ-14 play important roles in stomatitis, suggesting its potential to promote healing in stomatitis.
Among the seven components in TJ-14, those contributing to its antibacterial, healing-promoting, and anti–inflammatory effects have not yet been identified. Furthermore, the synergistic effects of each herbal medicine remain unclear. Therefore, future studies are needed to investigate these properties in more detail. Additionally, we think it necessary to compare the effectiveness of TJ-14 for this disease with those of other currently available treatment methods in the future.
The present results suggest that the local application of TJ-14 is a higher-quality cancer treatment. The role of TJ-14 is expected to increase in importance as newly developed cancer drugs exert their expected effects. Nevertheless, the present results suggest that TJ-14 is useful as an agent in cancer support therapy.
Disclosure
The authors reports no conflicts of interest in this work.
References
1. Hayashi K, Onda T, Honda H, et al. Effects of ozone nano-bubble water on mucositis induced by cancer chemotherapy. Biochem Biophys Rep. 2019;20:100697. doi:10.1016/j.bbrep.2019.100697
2. Chabner BA, Longo DL. Cancer Chemotherapy and Biotherapy: Principles and Practice.
3. Oral Complications of Chemotherapy and Head/Neck Radiation (PDQ®)–Health Professional Version [homepage on the Internet]. Bethesda: National Cancer Institute website; 2018. Available from: http://www.cancer.gov/cancertopics/pdq/supportivecare/oralcomplications/HealthProfessional.
4. Peterson DE. New strategies for management of oral mucositis in cancer patients. J Support Oncol. 2006;4(2 Suppl 1):9–13.
5. Naidu MU, Ramana GV, Rani PU, Mohan IK, Suman A, Roy P. Chemotherapy-induced and/or radiation therapy-induced oral mucositis–complicating the treatment of cancer. Neoplasia. 2004;6(5):423–431. doi:10.1593/neo.04169
6. Rubenstein EB, Peterson DE, Schubert M, et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer. 2004;100(9 Suppl):2026–2046. doi:10.1002/cncr.20163
7. Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, Rubenstein EB. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer. 2003;98(7):1531–1539. doi:10.1002/(ISSN)1097-0142
8. Sonis ST, Oster G, Fuchs H, et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol. 2001;19(8):2201–2205. doi:10.1200/JCO.2001.19.8.2201
9. Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66(3):253–262. doi:10.1016/S0167-8140(02)00404-8
10. Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent Clin North Am. 2008;52(1):61–77. doi:10.1016/j.cden.2007.10.002
11. Viet CT, Corby PM, Akinwande A, Schmidt BL. Review of preclinical studies on treatment of mucositis and associated pain. J Dent Res. 2014;93(9):868–875. doi:10.1177/0022034514540174
12. Sonis ST, Elting LS, Keefe D, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100(9 Suppl):1995–2025.
13. Miyano K, Ueno T, Yatsuoka W, Uezono Y. Treatment for cancer patients with oral mucositis: assessment based on the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer in International Society of Oral Oncology (MASCC/ISOO) in 2013 and Proposal of Possible Novel Treatment with a Japanese Herbal Medicine. Curr Pharm Des. 2016;22(15):2270–2278. doi:10.2174/1381612822666160219120842
14. Sharma R, Tobin P, Clarke SJ. Management of chemotherapy-induced nausea, vomiting, oral mucositis, and diarrhea. Lancet Oncol. 2005;6(2):93–102. doi:10.1016/S1470-2045(05)01735-3
15. Kono T, Takeda H, Shimada M, Kase Y, Uezono Y. Novel therapeutics for adverse effects of antitumor therapy: the promise of multicomponent, traditional Japanese herbal remedies. J Carcinog Mutagen. 2014;S8:007. doi:10.4172/2157-2518.S8-007
16. Hatakeyama H, Takahashi H, Oridate N, et al. Hangeshashinto improves the completion rate of chemoradiotherapy and the nutritional status in patients with head and neck cancer. ORL J Otorhinolaryngol Relat Spec. 2015;77(2):100–108. doi:10.1159/000381026
17. Hitomi S, Ono K, Yamaguchi K, et al. The traditional Japanese medicine Hangeshashinto alleviates oral ulcer-induced pain in rat model. Arch Oral Biol. 2016;66:30–37. doi:10.1016/j.archoralbio.2016.02.002
18. Mori K, Kondo T, Kamiyama Y, Kano Y, Tominaga K. Preventive effect of Kampo medicine (Hangeshashin-to) against irinotecan-induced diarrhea in advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. 2003;51(5):403–406. doi:10.1007/s00280-003-0585-0
19. Matsuda C, Munemoto Y, Mishima H, et al. Double-blinded placebo-controlled, randomized phase II study of TJ-14 (Hangeshashinto) for infusional fluorinated-pyrimidine-based colorectal cancer chemotherapy-induced oral mucositis. Cancer Chemother Pharmacol. 2015;76(1):97–103. doi:10.1007/s00280-015-2767-y
20. Aoyama T, Nishikawa K, Takiguchi N, et al. Double-blind, placebo-controlled, randomized phase II study of TJ-14 (hangeshashinto) for gastric cancer chemotherapy-induced oral mucositis. Cancer Chemother Pharmacol. 2014;73(5):1047–1054. doi:10.1007/s00280-014-2440-x
21. Matsumoto C, Sekine-Suzuki E, Nyui M, et al. Analysis of the antioxidative function of the radioprotective Japanese traditional (Kampo) medicine, hangeshashinto, in an aqueous phase. J Radiat Res. 2015;56(4):669–677. doi:10.1093/jrr/rrv023
22. Kato T, Segami N, Sakagami H. Anti-inflammatory activity of Hangeshashinto in IL-1β-stimulated gingival and periodontal ligament fibroblasts. In Vivo. 2016;30(3):257–263.
23. Fukamachi H, Matsumoto C, Omiya Y, et al. Effects of Hangeshashinto on growth of oral microorganisms. Evid Based Complement Alternat Med. 2015;2015:512947. doi:10.1155/2015/512947
24. Kono T, Kaneko A, Matsumoto C, et al. Multitargeted effects of Hangeshashinto for treatment of chemotherapy-induced oral mucositis on inducible prostaglandin E2 production in human oral keratinocytes. Integr Cancer Ther. 2014;13(5):435–445. doi:10.1177/1534735413520035
25. Vilela-Goulart M, Teixeira RT, Rangel DC, Niccoli-Filho W, Gomes MF. Homogenous amniotic membrane as a biological dressing for oral mucositis in rats: histomorphometric analysis. Arch Oral Biol. 2008;53(12):1163–1171. doi:10.1016/j.archoralbio.2008.07.003
26. National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) [homepage on the Internet]. Bethesda: National Cancer Institute website; 2018. Available from: http://www.cancer.gov/.
27. Leitão RF, Ribeiro RA, Lira AM, et al. Glutamine and alanyl-glutamine accelerate the recovery from 5-fluorouracil-induced experimental oral mucositis in hamster. Cancer Chemother Pharmacol. 2008;61(2):215–222. doi:10.1007/s00280-007-0463-2
28. Hamada R, Suehiro J, Nakano M, Kikutani T, Konishi K. Development of rapid oral bacteria detection apparatus based on dielectrophoretic impedance measurement method. IET Nanobiotechnol. 2011;5(2):25–31. doi:10.1049/iet-nbt.2010.0011
29. Funahara M, Hayashida S, Sakamoto Y, et al. Efficacy of topical antibiotic administration on the inhibition of perioperative oral bacterial growth in oral cancer patients: a preliminary study. Int J Oral Maxillofac Surg. 2015;44(10):1225–1230. doi:10.1016/j.ijom.2015.06.002
30. De Young LM, Kheifets JB, Ballaron SJ, Young JM. Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions. 1989;26(3–4):335–341. doi:10.1007/BF01967298
31. Sekikawa S, Onda T, Miura N, et al. Underexpression of α-1-microglobulin/bikunin precursor predicts a poor prognosis in oral squamous cell carcinoma. Int J Oncol. 2018;53(6):2605–2614. doi:10.3892/ijo.2018.4581
32. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–229. doi:10.1177/0022034509359125
33. Healy B, Freedman A. Infections. BMJ. 2006;332(7545):838–841. doi:10.1136/bmj.332.7545.838
34. Hayashida S, Funahara M, Sekino M, et al. The effect of tooth brushing, irrigation, and topical tetracycline administration on the reduction of oral bacteria in mechanically ventilated patients: a preliminary study. BMC Oral Health. 2016;16(1):67. doi:10.1186/s12903-016-0224-x
35. Hiroshima Y, Bando M, Inagaki Y, et al. Effect of Hangeshashinto on calprotectin expression in human oral epithelial cells. Odontology. 2016;104(2):152–162. doi:10.1007/s10266-015-0196-3
36. Li LJ, Wang MZ, Yuan TJ, et al. The crude ethanol extract of Periplaneta americana L. stimulates wound healing in vitro & in vivo. Chin Med. 2019;14:33. doi:10.1186/s13020-019-0259-4
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.