Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 15
Effects of Tofogliflozin and Anagliptin Alone or in Combination on Glucose Metabolism and Atherosclerosis-Related Markers in Patients with Type 2 Diabetes Mellitus
Authors Nomura S , Shouzu A, Taniura T, Okuda Y , Omoto S, Suzuki M, Ito T , Toyoda N
Received 14 March 2023
Accepted for publication 19 May 2023
Published 25 May 2023 Volume 2023:15 Pages 41—55
DOI https://doi.org/10.2147/CPAA.S409786
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Arthur E. Frankel
Shosaku Nomura,1 Akira Shouzu,2 Takehito Taniura,3 Yoshinori Okuda,4 Seitaro Omoto,5 Masahiko Suzuki,6 Tomoki Ito,7 Nagaoki Toyoda8
1Center of Thrombosis and Hemostasis, Kansai Medical University Medical Center, Moriguchi, Japan; 2Division of Diabetes, Saiseikai Izuo Hospital, Osaka, Japan; 3Division of Internal Medicine, Daiwa Hospital, Osaka, Japan; 4Division of Internal Medicine, Meisai Kinen Hospital, Osaka, Japan; 5Division of Internal Medicine, Yukeikai Hospital, Neyagawa, Japan; 6Division of Internal Medicine, Katano Hospital, Katano, Japan; 7First Department of Internal Medicine, Kansai Medical University, Hirakata, Japan; 8Second Department of Internal Medicine, Kansai Medical University, Hirakata, Japan
Correspondence: Shosaku Nomura, Center of Thrombosis and Hemostasis, Kansai Medical University Medical Center, 10-15 Fumizono-cho, Moriguchi, Osaka, 570-8507, Japan, Tel + 81 6 6992 1001, Fax + 81 6 6992 1066, Email [email protected]
Purpose: In people with type 2 diabetes mellitus (T2DM), both glucose metabolism abnormalities and atherosclerosis risk are significant concerns. This study aims to investigate the effects of the sodium-glucose cotransporter 2 inhibitor tofogliflozin (TOFO) and the dipeptidyl peptidase-4 inhibitor anagliptin (ANA) on markers of glucose metabolism and atherosclerosis when administered individually or in combination.
Methods: Fifty T2DM patients were divided into two groups (receiving either TOFO or ANA monotherapy) and observed for 12 weeks (observation points: 0 and 12 weeks). The TOFO and ANA groups were then further treated with ANA and TOFO, respectively, and the patients were observed for an additional 36 weeks (observation points: 24 and 48 weeks). Therapeutic effects and various biomarkers were compared between the two groups at the observation points.
Results: Combination therapy led to significant improvements in HbA1c levels and atherosclerosis markers. Additionally, the TOFO pretreatment group exhibited significant reductions in sLOX-1 and IL-6 levels.
Conclusion: The increase in sLOX-1 and IL-6 levels, which indicates the response of scavenger receptors to oxidized low-density lipoproteins in people with T2DM, is mitigated following TOFO and ANA combination therapy. TOFO alone or in combination with ANA may be beneficial for preventing atherosclerosis development in people with T2DM, in addition to its effect on improving HbA1c levels.
Keywords: tofogliflozin, anagliptin, type 2 diabetes mellitus, atherosclerosis, soluble LOX-1
Introduction
Atherosclerosis is characterized by the accumulation of plaque within arteries, leading to their narrowing.1–3 While often asymptomatic in its early stages, severe cases can result in ischemic heart disease, stroke, arteriosclerosis obliterans, and kidney damage, depending on the specific arterial locations affected.3,4 Atherosclerosis typically develops at a young age, and its impact tends to intensify with age.1–4 The precise etiology of atherosclerosis remains elusive, but several risk factors have been identified, such as diet, family history, lipid abnormalities, hypertension, obesity, and smoking.3,4 The simultaneous presence of lifestyle-related diseases, such as diabetes, hypertension, and dyslipidemia, can cause asymptomatic progression of arteriosclerosis, consequently elevating the risk of angina pectoris, myocardial infarction, and stroke.2–4 Among these conditions, type 2 diabetes mellitus (T2DM) is a critical contributor to atherothrombosis and embolism, making the development of effective treatment strategies for this disease crucial.5
The prevalence of T2DM is increasing in Japan, making its proper management a critical and timely concern.6 In addition to insulin therapy, several oral antidiabetic drugs (OADs) are employed to manage T2DM.6 Sodium-glucose cotransporter 2 (SGLT2) inhibitors, a relatively novel class of OADs, exhibit an insulin-independent mechanism of action and provide a low-risk treatment option for hyperglycemia.6,7 SGLT2 inhibitors primarily function by inhibiting glucose reabsorption in the renal proximal tubules, which leads to reduced circulating blood glucose.6,8 Not only do these inhibitors decrease hemoglobin A1c (HbA1c) levels, but they also demonstrate positive effects on renal protection and the cardiovascular system.9–11 SGLT2 inhibitors may prove beneficial when used in conjunction with other blood glucose-lowering agents.6,12 In particular, substantial HbA1c reduction may be achieved when combined with dipeptidyl peptidase-4 (DPP-4) inhibitors.13,14 Tofogliflozin (TOFO), a specific SGLT2 inhibitor, was approved for T2DM treatment in Japan in 2014.15 The HbA1c-lowering effect and safety of TOFO have been established through various clinical studies conducted in Japan.16–19 However, other potential therapeutic effects of TOFO, such as its impact on atherosclerosis development, have been rarely explored.
This prospective randomized open study (exploratory study) sought to examine the effects of TOFO and anagliptin (ANA), a DPP-4 inhibitor, on glucose metabolism and atherosclerosis-related markers in people with T2DM when administered either independently or in combination.
Materials and Methods
Patients and Study Design
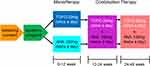
We conducted a multicenter, randomized, prospective study to assess the efficacy of TOFO and ANA monotherapies (0–12 weeks of study) and their combination therapy (13–48 weeks of study) in people with T2DM. The combination therapy persisted for 36 weeks. This study adhered to the principles outlined in the Declaration of Helsinki and followed the ethical guidelines for clinical research as recommended by the Ministry of Health, Labour and Welfare. The study was registered with the Japan Registry of Clinical Trials (jRCTs031180440).
Eligible patients with T2DM met the following criteria:
- Once-daily use of any DPP-4 inhibitor.
- HbA1c levels between 7.0% and 9.0% at the time of consent.
- A body mass index (BMI) of 18.0 kg/m2 or higher at the time of consent
- Males or females aged 20–95 years.
- Provision of written informed consent.
Participants were allocated to one of two groups; Group A (TOFO group) or Group B (ANA group). Both groups were followed up for 12 weeks, with observation points at 0 and 12 weeks. Subsequently, Group A and Group B received additional ANA and TOFO treatments, respectively, for an additional 36 weeks (48 weeks in total). The concomitant use of diabetes medications that were used prior to the study was allowed, but in principle, no drugs were added, discontinued, or doses changed. Discontinuation criteria for glycemic control in the elderly were established from the viewpoint of preventing severe hypoglycemia. In cases of concomitant sulfonylurea, glinide, or insulin use, the treatment was halted when HbA1c levels reached < 6.3% (65–75 years) or < 6.8% (75 years and older). Study physicians closely monitored compliance with the medications during patient visits, documenting the results in the medical records. Non-compliant patients were excluded from the final statistical analysis.
The primary endpoint of this study focused on alterations in HbA1c levels at 12, 24, and 48-week intervals. Secondary endpoints encompassed the following parameters: (1) changes in weight, BMI, and abdominal circumference; (2) changes in blood pressure; (3) fluctuations in glucose metabolism, as evidenced by fasting blood glucose (FBG) levels; (4) changes in lipid profiles, including total cholesterol (TC), low-density lipoprotein (LDL)-C, high-density lipoprotein (HDL)-C, and triglyceride (TG) levels; (5) changes in liver function markers, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyl transpeptidase (γ-GTP) levels; (6) changes in renal function indicators, including serum creatinine levels, estimated glomerular filtration rate (eGFR), and uric acid levels; and (7) changes in platelet-, inflammatory-, and atherosclerosis-associated markers, comprising platelet-derived microparticles (PDMP), high mobility group box protein 1 (HMGB1), adiponectin, plasminogen activator inhibitor (PAI)-1, monocyte chemoattractant protein (MCP)-1, soluble vascular cell adhesion molecule (sVCAM)-1, soluble lectin-like oxidized LDL receptor (sLOX)-1, and interleukin (IL)-6.
The concurrent use of SGLT2 inhibitors, DPP-4 inhibitors (including fixed-dose combinations), and GLP-1 receptor agonists other than the study medications was disallowed in both groups. During the monotherapy phase (0–12 weeks of the study), the TOFO-precedence group and ANA-precedence group were precluded from using ANA and TOFO, respectively. While there were no restrictions concerning the concomitant use of restricted medications, the study protocol generally discouraged the introduction, discontinuation, or alteration of dosage for any new drugs throughout the research period.
Procedures
This multicenter study involved six facilities, including Kansai medical University, Saiseikai Izuo Hospital, Daiwa Hospital, Mesisei Kinen Hospital, Yukeikai Hospital, and Katano Hospital. Allocation of research participants to study groups was managed through an allocation system using fax communication. Case report forms (CRFs) were employed in a paper-based format to gather background information and clinical data. Participants were chosen based established inclusion and exclusion criteria. Once the participants were selected, the investigator presented the study details using a consent document and obtained written consent. Subsequently, the investigator sent a form containing necessary information (ie, gender, HbA1c levels) for allocation via fax to the research office. The research office executed dynamic allocation using a computer and transmitted the results to the investigator by fax.
Drug administration commenced in accordance with the allocation outcome (TOFO or ANA group). Adverse events were monitored as needed for safety evaluation, independent of the observation points in the study. The investigator adhered to the research protocol without deviation or alteration, unless approval was granted by the principal investigator and the ethics review committee. If protocol deviation or change was necessary to prevent imminent danger to participants or for other unavoidable medical reasons, the specifies were documented in the medical record and CRF. Observations continued to the greatest extent possible, even in cases of protocol deviation.
Determination of Various Biomarkers
Peripheral venous fasting blood samples were obtained from the patients using 21-gauge needles to minimize platelet activation and collected into vacutainers containing EDTA-ACD (NIPRO Co. Ltd., Osaka, Japan). The tubes were gently inverted once or twice from mixing and then maintained at room temperature for no more than 2–3 hours. Following centrifugation at 8000g for 5 minutes, 200 μL of supernatant was carefully collected from the 2 mL samples to avoid platelet contamination. These samples were preserved at −40°C until analysis. ELISA kits for PDMP measurements were procured from JIMRO Co. Ltd. (Tokyo, Japan). Serum levels of sVCAM-1, MCP-1, PAI-1, and IL-6 were measured using monoclonal antibody-based ELISA kits obtained from Invitrogen International Inc. (Camarillo, California, USA). The plasma adiponectin level was determined using the Adiponectin ELISA kit from Otsuka Pharmaceuticals Co. Ltd (Tokyo, Japan). Serum sLOX-1 levels were evaluated using the sLOX-1 ELISA Kit (R&D Systems, USA), and HMGB1 levels were measured employing the HMGB1 ELISA kit (Shino-test Corp., Kanagawa, Japan). Each assay utilized recombinant products and standard solutions provided by the commercial kits as positive controls. The manufacturer’s instructions were followed for all kits. The intra- and inter-assay coefficients of variation were 4% and 5%, respectively.
Statistical Analyses
We employed full analysis set (FAS) and per protocol set (PPS) approaches to evaluate the primary and secondary endpoints, maintaining a 5% significance level for both analysis. Pearson’s chi-square test was used to examine Fisher’s direct probability for between-group comparisons. Paired-sample t-tests were conducted for quantitative parametric data analysis. To identify within-group changes (prior to following 12 and 24 weeks of intervention), paired-sample t-tests were employed for quantitative parametric data, while the Wilcoxon test was used for non-parametric quantitative data. A medical statistician developed a separate statistical analysis plan, outlining the specific statistical methods and data handling procedures. Prior to data preparation, three analysis populations were defined for each case after assessing deviations from the research protocol, the extent of deviations, and the timing of participant dropout: (1) FAS: This population included participants who received the study drug. Individuals with serious violations of the study design, such as failure to obtain consent or enrollment outside the designated period, were excluded. (2) PPS: This target population conformed to the research protocol. From the FAS, participants with serious violations concerning the study drug or concomitant therapy were excluded, including violations of eligibility criteria, exclusion criteria, prohibited concomitant drug usage, and significant deviations from medication compliance rates. (3) Safety analysis population: This population consisted of participants who were enrolled in the study and received either the assigned treatment or all of the treatments.
Course of the Trial Period
This investigation is structured into two distinct phases: the case enrollment phase and the research phase. The initial approval from the IRB at Kansai Medical University was obtained on July 24, 2017 (No. 2017009). As part of the review process by the Medical Ethics Committee of Kansai Medical University, registration with the University Hospital Medical information Network Clinical Trials Registry (UMIN-CRT)) was Mandated. Consequently, we registered the study with UMIN-CRT (UMIN000028634). The enrollment of the first cases commenced on March 1, 2018. During this enrollment phase, supplementary IRB applications were submitted due to the incorporation of additional participating sites and investigators. Following the reporting of these modifications to the IRB, the study received approval from the Hattori Clinic Clinical Review Board (CRB) on February 8, 2019 (CRB3180027). The study was also registered with the Japan Registry of Clinical Trials (jRCTs031180440) by the Ministry of Health, Labour and Welfare. The UMIN-CRT number (UMIN000028634) is referenced in the jRCT documentation under the section pertaining to registration with other clinical research registries. The inaugural case study was initiated on February 13, 2019. The observation period for the final patient concluded (last visit) on February 28, 2022. The CRB approved the study termination procedure on February 3, 2023. The termination report was received approved from the jRCT in Japan, and the study will be officially documented as terminated on May 31, 2023.
Results
Patient Baseline Characteristics
The study design is illustrated in Figure 1. A total of 50 patients were randomly assigned, and followed protocol evaluation, 22 patients in the TOFO group and 24 patients in the ANA group were included in the statistical analysis. Figure 2 presents a summary of the study medication and observation period. Throughout the study, no patients were found to have deviated from the prescribed medication regimen. Table 1 displays the baseline characteristics of the 46 patients who eventually received the combination therapy, prior to the initiation of monotherapy. No significant differences were observed between the TOFO and ANA groups, except for MCP-1 and sVCAM-1 levels. The ANA group exhibited significantly higher MCP-1 levels, while the TOFO group had significantly higher sVCAM-1 levels. At baseline (week 0), the mean age, HbA1c level, and BMI were 63.1 ± 11.0 years, 8.19 ± 0.66%, and 28.7 ± 4.52 kg/m2, respectively. The mean HbA1c levels and BMI prior to initiating combination therapy (12 weeks) were 7.33 ± 0.72% and 27.8 ± 4.39 kg/m2, respectively. Discontinuation criteria for glycemic control in the elderly were established from the viewpoint of preventing severe hypoglycemia. The current study recommended the following protocol for the concomitant use of antidiabetic agents; in cases involving concurrent sulfonylurea, glinide, or insulin use, treatment was to be discontinued when HbA1c levels reached < 6.3% (for patients aged 65–75 years) or < 6.8% (for patients aged 75 years and older). Nonetheless, no patients meeting these criteria were observed. Concomitant use of antidiabetic agents in this study was 16 cases in the TOFO group (sulfonylurea: 8, glinide 5, and insulin use 3) and 17 cases in the ANA group (sulfonylurea: 7, glinide 6, and insulin use 4) (Table 1). Since these concomitant cases did not show any change or discontinuation of use during the study period, they were judged to be acceptable for inclusion in the study. Therefore, they were considered acceptable for all statistical analyses. A high proportion of the included patients received concomitant antidyslipidemic drugs (TOFO: 68.2%, ANA: 62.5%). The concomitant medications administered included pitavastatin (2 mg/day), atorvastatin (10 mg/day), and pravastatin (10 mg/day). No bias in the use of concomitant medications was detected between the two groups.
 |
Table 1 Comparison of Two Groups at the Start of Initial Treatment |
 |
Figure 1 Study design. |
Changes in Glycemic Parameters, BMI, and HMGB1 Levels
After 12 weeks of monotherapy with either TOFO or ANA, HbA1c levels and FBG values were assessed, as well as following combination therapy at 24 and 48 weeks of the study. HbA1c levels exhibited a reduction of −0.34 ± 0.72% (p = 0.056) and −0.13 ± 0.73% (p = 0.0412) after 12 weeks of TOFO and ANA monotherapy, respectively. Upon combination therapy at the 24-week time point, HbA1c level experienced a decline of −1.08 ± 0.68% relative to the baseline value (p < 0.001) (Table 2). The progression of HbA1c level changes at 12, 24, and 48 weeks of the study can be observed in Figure 3A. A significant decrease in FBG levels (−19.3 ± 25.4 mg/dL, p = 0.036) was noted after 12 weeks of TOFO monotherapy, though not with ANA (Table 2). FBG level alterations at 12, 24, and 48 weeks of the study are depicted in Figure 3B.
 |
Table 2 Comparison with Baseline Values for Monotherapy and Combination Therapy |
Relative to the baseline, BMI experienced a significant decline (−0.8 ± 0.91 kg/m2) in the TOFO group (p = 0.038) after 12 weeks of treatment, while no significant change was observed in the ANA group (Table 2). BMI level alterations at 12, 24, and 48 weeks of the study are presented in Figure 3C. A comparable trend was observed in HMGB1 level changes in both the TOFO and ANA groups (Table 2 and Figure 3D).
Changes in Endothelial Cell-Related Parameters and PDMP and Adiponectin Levels
The impact of 12-week monotherapy with TOFO or ANA, as well as combination therapy (24 and 48 weeks of study), on endothelial cell-related parameters was assessed by examining sVCAM-1 and PAI-1 concentrations. Following 12-weeks of TOFO and ANA monotherapy, sVCAM-1 levels exhibited a decrease of −76.3 ± 88.5 ng/mL (p = 0.053) and −125.9 ± 224.9 ng/mL (p = 0.029), respectively. Additionally, a substantial reduction in sVCAM-1 levels was observed with combination therapy (p < 0.001) (Table 2 and Figure 4A). Nonetheless, 12 weeks of TOFO or ANA monotherapy did not result in a significant decrease in PAI-1 and PDMP concentrations, whereas combination therapy contributed to a notable reduction in both PAI-1 and PDMP concentrations (Table 2, Figure 4B and 5C). Moreover, 12 weeks of TOFO or ANA monotherapy did not induce any significant alterations in adiponectin concentrations, whereas combination therapy led to a considerable elevation in adiponectin concentrations (Table 2 and Figure 4D).
Changes in Diastolic Blood Pressure and γ-GTP, sLOX-1, IL-6 Levels
Following a 12-week regimen of TOFO monotherapy, substantial reductions were observed in sLOX-1 levels (−89.5 ± 100.2 ng/mL, p = 0.007), IL-6 levels (−0.82 ± 4.4 pg/mL, p = 0.041), diastolic blood pressure (−3.0 ± 2.7 mmHg, p = 0.048), and γ-GTP levels (−7.5 ± 12.1 IU/mL, p = 0.043) (Table 2 and Figure 5A–D). In contrast, no significant alterations in diastolic blood pressure or sLOX-1, IL-6, and γ-GTP levels were detected after a 12-week course of ANA monotherapy (Table 2 and Figure 5A–D). The administration of combined TOFO and ANA therapy, encompassing 24 and 48 weeks of the study, resulted in marked decreases in diastolic blood pressure and sLOX-1, IL-6, and γ-GTP levels (Table 2 and Figure 5A–D).
Changes in Lipid Profile and Renal, Liver, and Additional Parameters
NO significant changes were observed in total cholesterol, HDL cholesterol, triglycerides, and LDL cholesterol levels following both monotherapy and combination therapy (Table 2). Similarly, renal (creatinine levels and eGFR), liver (AST and ALT levels), and other evaluated parameters (systolic blood pressure and uric acid levels) remained unchanged after administration of TOFO or ANA monotherapy and combination therapy (Table 2). Notably, 12-week TOFO monotherapy resulted in a significant reduction in body weight (−2.2 ± 3.4 kg, p = 0.023) and waist circumference (−0.8 ± 0.91 cm, p = 0.038) (Table 2). Moreover, at the 24-week assessment point, combination therapy significantly decreased both body weight (−2.4 ± 0.98 kg, p < 0.001) and waist circumference (−2.6 ± 1.8 cm, p < 0.001) (Table 2).
Adverse Events
One patient on TOFO experienced frequent urination and urinary incontinence, while drug-induced liver injury was reported in one patient on ANA. None of these adverse events were determined to be causally related to the study drug, and all adverse events were classified as non-serious.
Discussion
The current prospective and randomized trial found that both TOFO monotherapy and combination therapy with TOFO and ANA demonstrate notable therapeutic efficacy in people with T2DM. Additionally, TOFO exhibits significant anti-atherosclerotic effects and improves sLOX-1 levels. To the best of our knowledge, this is the first study to report an improvement in sLOX-1 levels following TOFO treatment.
Blood pressure reduction, lipid profile improvement, and weight loss have been associated with the use of SGLT2 inhibitors in previous studies.6–8 Our findings are in line with these observations, as we discovered that TOFO monotherapy can effectively induce weight loss and decrease BMI, abdominal circumference, and diastolic blood pressure. Previous clinical studies have also reported weight reduction effects of TOFO.18,20 Suzuki et al21 demonstrated that these effects are linked to a metabolic shift from carbohydrate oxidation to fatty acid oxidation and caloric losses due to increased urinary glucose excretion. Kamei et al22 further highlighted that a significant reduction in body water content may be a crucial factor contributing to weight loss in T2DM patients treated with TOFO. In a direct comparison between TOFO and glimepiride among T2DM patients, Kizawa et al23 found that TOFO can substantially decrease both BMI and abdominal circumference. Therefore, results of current study are consistent with these prior reports.
SGLT2 inhibitors have been demonstrated to improve abnormalities related to lipid metabolism.6,7 This beneficial effect of SGLT2 inhibitors is particularly relevant in the context T2DM-associated nonalcoholic fatty liver disease (NAFLD).24 In the current study, we observed a significant reduction in γGTP levels with TOFO monotherapy, although no notable changes were evident in markers of lipid metabolism. Notably, the combination of TOFO and ANA significantly decreased both ALT and AST levels. Matsuba et al25 previously reported improvements in ALT, AST, and γGTP levels in T2DM patients treated with a DPP-4 inhibitor in conjunction with TOFO. They attributed these effects to a TOFO-induced reduction in body fat mass, ultimately leading to enhanced insulin sensitivity. Interestingly, a recent clinical trial investigating TOFO in NAFLD people with T2DM also documented significant improvements in ALT, AST, and γGTP levels, as well as body weight.26,27 Our findings are consistent with these prior reports.
Although SGLT2 inhibitors target the kidneys, they do not overburden renal function, and when combined with other antidiabetic medications, these inhibitors are anticipated to exhibit increased efficacy.28,29 In the current study, neither TOFO monotherapy nor its combination with ANA impacted renal functions. Nunoi et al30 demonstrated that TOFO ameliorates albuminuria and confers protective effects on renal tubules. These renal effects of TOFO suggest its potential role in mitigating the progression of renal disease, a common complication in people with T2DM.31
In this study, the combination therapy of TOFO and ANA demonstrated significant improvements in both PDMP and adiponectin levels. PDMPs are known to facilitate coagulation system activation and play a crucial role in thrombus formation processes.32–36 Furthermore, PDMPs also act on leukocytes and vascular endothelial cells, inducing the expression of cell adhesion molecules in both cell types, which increases adhesion between them and contributes to the development of early atherosclerosis lesions.37 Adiponectin, on the other hand, reduces visceral fat, mitigates obesity, and exhibits remarkable anti-atherosclerotic effects.38–40 Consequently, the TOFO and ANA combination therapy may be a critical factor in inhibiting atherosclerosis development in people with T2DM. Iwamoto et al41 demonstrated that one of the underlying mechanisms of TOFO’s atherosclerosis inhibitory effect is its capacity to reduce apolipoprotein-E levels. Additionally, the UTOPIA study of clinical cases revealed that TOFO exhibits exceptional therapeutic effects on atherosclerosis.42,43 Therefore, the combination therapy with TOFO may represent an optimal treatment strategy for diabetes, encompassing measures against atherosclerosis.
The most notable finding of this study is the considerable enhancement in sLOX-1 levels following TOFO monotherapy and the combined treatment of TOFO and ANA. sLOX-1 has gained prominence as a biomarker for atherosclerosis44–46 and has also been identified as a markers for diabetic complications.47 The current study postulates that TOFO ameliorates sLOX-1 levels by diminishing oxidative stress and reactive oxygen species (ROS) production (Figure 6). The presence of ROS in patients with T2DM may be strongly correlated with complications and disease progression.48 ROS may facilitate the conversion of LDL to oxidized LDL in people with T2DM.49 A substantial accumulation of oxidized LDL in macrophages via scavenger receptors has been documented.2,3,50 Subsequently, macrophages transform into foam cells and contribute to plaque formation, leading to atherosclerosis development and plaque rupture.2,50 Previous reports indicate that TOFO possesses inhibitory effects on ROS production,51 suggesting that TOFO may prevent the sequence of events depicted in Figure 6. The improvements in IL-6 levels induced by TOFO further supports this hypothesis. Consequently, TOFO holds significant potential for preventing the development of atherosclerosis in people with T2DM.
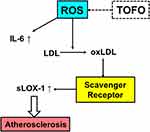 |
Figure 6 Mechanism. |
The present study is subject to several limitations that warrant consideration in future research. First, the small sample size precludes drawing definitive conclusions regarding the differences in effects between once- and twice-daily DPP-4 inhibitors. Indeed, no significant differences were observed between the two study groups at the 12-week observation point. Consequently, future studies employing larger sample sizes are needed to yield more robust results. Second, this study did not conduct a multivariate analysis of factors influencing the treatment effect on HbA1c or the disparity in sLOX-1 trends following combination therapy. Therefore, further investigation of these factors is necessary in subsequent studies with additional cases. Third, as the study exclusively enrolled Japanese participants, the generalizability of the findings to other populations is limited. Fourth, we were not able to examine the effect of concomitant medications, especially insulin, on the study results. We would like to increase the number of cases in the future and conduct further studies. Lastly, the study did not reveal significant pre- and post-treatment differences in lipid metabolism-related markers for either study group. While previous research has demonstrated that TOFO subsequently improves lipid and liver profiles in patients with NAFLD, the prevalence of NAFLD among patients in this study was unclear. Thus, further examination of this issue is warranted.
Conclusion
In conclusion, our findings demonstrate that the combined therapy of TOFO and ANA exhibits superior efficacy in ameliorating HbA1c levels and atherosclerotic biomarkers compared to monotherapeutic approaches with TOFO or ANA in people with T2DM. Moreover, a sequential treatment strategy involving initial TOFO administration followed by ANA therapy results in a substantial reduction of sLOX-1 and IL-6 levels. Consequently, the use of TOFO, either as a standalone treatment or in conjunction with ANA, holds promise for the prevention of atherosclerosis in addition to the improvement of glycemic control in people with T2DM.
Abbreviations
T2DM, type 2 diabetes mellitus; OADs, antidiabetic drugs; SGLT2, sodium-glucose cotransporter 2; HbA1c, hemoglobin A1c; DPP-4, dipeptidyl peptidase-4; TOFO, tofogliflozin; ANA, anagliptin; BMI, body mass index; FBG, fasting blood glucose; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglycerides; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γ-GTP, γ-glutamyl transpeptidase; eGFR, estimated glomerular filtration rate; PDMP, platelet-derived microparticles; HMGB1, high mobility group box protein 1; PAI-1, plasminogen activator inhibitor-1; MCP-1, monocyte chemoattractant protein-1; sVCAM-1, soluble vascular cell adhesion molecule-1; sLOX-1, soluble lectin-like oxidized LDL receptor-1; IL-6, interleukin-6; CRFs, case report forms; FAS, full analysis set; PPS, per protocol set; NAFLD, nonalcoholic fatty liver disease; ROS, reactive oxygen species.
Data Sharing Statement
The datasets utilized and analyzed in this research are accessible from the corresponding authors upon request in the future without any specific rationale.
Ethics Approval and Consent to Participate
IRB approval for this investigation was granted by the Medical Ethics Committee of Kansai Medical University (study number 2017009). CRB approval was provided by the Accredited Clinical Research Review Committee (Hattori Clinic Clinical Research Review Board) with study number CRB3180027. The research adhered to the principles of the Declaration of Helsinki and the ethical guidelines for clinical research established by the Ministry of Health, Labour and Welfare. The study was registered with the Japan Registry of Clinical Trials (jRCTs031180440) and the University Hospital Medical Information Network (UMIN-CRT) (UMIN000028634).
Acknowledgments
The authors express their gratitude to Dr. Shigeaki Ohtsuki for his assistance with the statistical methods employed for data analysis.
Author Contributions
All authors made significant contributions in all areas, including the conception, design, and execution of the study, as well as patients data acquisition, individual data analysis, and interpretation. The authors participated in drafting and revising the manuscript, provided final approval for the publication version, agreed to the chosen journal for submission, and accepted responsibility for all of their work.
Funding
No specific budget was allocated for the conduct of this study.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. doi:10.1056/NEJMra043430
2. Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6(7):508–519. doi:10.1038/nri1882
3. Libby P. The changing landscape of atherosclerosis. Nature. 2021;592(7855):524–533. doi:10.1038/s41586-021-03392-8
4. Baeyens N, Bandyopadhyay C, Coon BG, Yun S, Schwartz MA. Endothelial fluid shear stress sensing in vascular health and disease. J Clin Invest. 2016;126(3):821–828. doi:10.1172/JCI83083
5. Barbu E, Popescu MR, Popescu AC, Balanescu SM. Inflammation as a precursor of atherothrombosis, diabetes and early vascular aging. Int J Mol Sci. 2022;23(2):963. doi:10.3390/ijms23020963
6. Kaneko S. Novel approaches to pharmacological management of type 2 diabetes in Japan. Expert Opin Pharmacother. 2021;22(16):2235–2249. doi:10.1080/14656566.2021.1974401
7. Abdul-Ghani MA, Norton L, Defronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev. 2011;32(4):515–531. doi:10.1210/er.2010-0029
8. Imprialos KP, Sarafidis PA, Karagiannis AI. Sodium–glucose cotransporter-2 inhibitors and blood pressure decrease: a valuable effect of a novel antidiabetic class? J Hypertens. 2015;33(11):2185–2197. doi:10.1097/HJH.0000000000000719
9. Solini A, Giannini L, Seghieri M, et al. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16(1):138. doi:10.1186/s12933-017-0621-8
10. Lim S, Eckel RH, Koh KK. Clinical implications of current cardiovascular outcome trials with sodium glucose cotransporter-2 (SGLT2) inhibitors. Atherosclerosis. 2018;272:33–40. doi:10.1016/j.atherosclerosis.2018.03
11. Giugliano D, Maiorino MI, Bellastella G, Chiodini P, Esposito K. Glycemic control, preexisting cardiovascular disease, and risk of major cardiovascular events in patients with type 2 diabetes mellitus: systematic review with meta-analysis of cardiovascular outcome trials and intensive glucose control trials. J Am Heart Assoc. 2019;8(12):e012356. doi:10.1161/JAHA.119.012356
12. Scheen AJ. SGLT2 inhibitors as add-on therapy to metformin for people with type 2 diabetes: a review of placebo-controlled trials in Asian versus non-Asian patients. Diabetes Met Obes. 2020;13:2765–2779. doi:10.2147/DMSO.S193528
13. Cai X, Han X, Luo Y, Ji L. Efficacy of dipeptidyl-peptidase-4 inhibitors and impact on β-cell function in Asian and Caucasian type 2 diabetes mellitus patients: a meta-analysis. J Diabetes. 2015;7(3):347–359. doi:10.1111/1753-0407.12196
14. Zhou Y, Geng Z, Wang X, Huang Y, Shen L, Wang Y. Meta-analysis on the efficacy and safety of SGLT2 inhibitors and incretin based agents combination therapy vs. SGLT2i alone or add-on to metformin in type 2 diabetes. Diabetes Metab Res Rev. 2020;36(2):e3223. doi:10.1002/dmrr.3223
15. Poole RM, Pressler JE. Tofogliflozin: first global approval. Drugs. 2014;74(8):939–944. doi:10.1007/s40265-014-0229-1
16. Kaku K, Watada H, Iwamoto Y, et al. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc Diabetol. 2014;13:65. doi:10.1186/1475-2840-13-65
17. Tanizawa Y, Kaku K, Araki E, et al. Long-term safety and efficacy of tofogliflozin, a selective inhibitor of sodium/glucose cotransporter 2, as monotherapy or in combination with other oral antidiabetic agents in Japanese patients with type 2 diabetes mellitus: multicenter, open-label, randomized controlled trials. Expert Opin Pharmacother. 2014;15(6):749–766. doi:10.1517/14656566.2014.887680
18. Ikeda S, Takano Y, Cynshi O, et al. A novel and selective sodium-glucose cotransporter-2 inhibitor, tofogliflozin, improves glycaemic control and lowers body weight in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015;17(10):984–993. doi:10.1111/dom.12538
19. Terauchi Y, Tamura M, Senda M, Gunji R, Kaku K. Long-term safety and efficacy of tofogliflozin as add-on to insulin in patients with type 2 diabetes: results from a 52-week, multicentre, randomized, double-blind, open-label extension, Phase 4 study in Japan (J-STEP/INS). Diabetes Obes Metab. 2018;20(5):1176–1185. doi:10.1111/dom.13213
20. Iwahashi Y, Hirose S, Nakajima S, Seo A, Takahashi T, Tamori Y. Evaluation of metabolic parameters and body composition in Japanese patients with type 2 diabetes mellitus who were administered tofogliflozin for 48 weeks. Diabetol Int. 2016;8(2):205–211. doi:10.1007/s13340-016-0295-6.
21. Suzuki M, Takeda M, Kito A, et al. Tofogliflozin, a sodium/glucose cotransporter 2 inhibitor, attenuates body weight gain and fat accumulation in diabetic and obese animal models. Nutr Diabetes. 2014;4(7):e125. doi:10.1038/nutd.2014.20
22. Kamei S, Iwamoto M, Kameyama M, et al. Effect of tofogliflozin on body composition and glycemic control in Japanese subjects with type 2 diabetes mellitus. J Diabetes Res. 2018;2018:6470137. doi:10.1155/2018/6470137
23. Kitazawa T, Seino H, Ohashi H, et al. Comparison of tofogliflozin versus glimepiride as the third oral agent added to metformin plus a dipeptidyl peptidase-4 inhibitor in Japanese patients with type 2 diabetes: a randomized, 24-week, open-label, controlled trial (STOP-OB). Diabetes Obes Metab. 2020;22(9):1659–1663. doi:10.1111/dom.14059
24. Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol. 2018;53(3):362–376. doi:10.1007/s00535-017-1415-1
25. Matsuba R, Matsuba I, Shimokawa M, Nagai Y, Tanaka Y. Tofogliflozin decreases body fat mass and improves peripheral insulin resistance. Diabetes Obes Metab. 2018;20(5):1311–1315. doi:10.1111/dom.13211
26. Takeshita Y, Honda M, Harada K, et al. Comparison of tofogliflozin and glimepiride effects on nonalcoholic fatty liver disease in participants with type 2 diabetes: a randomized, 48-week, open-label, active-controlled trial. Diabetes Care. 2022;45(9):2064–2075. doi:10.2337/dc21-2049
27. Yoneda M, Kobayashi T, Honda Y, et al. Combination of tofogliflozin and pioglitazone for NAFLD: extension to the ToPiND randomized controlled trial. Hepatol Commun. 2022;6(9):2273–2785. doi:10.1002/hep4.1993
28. Nespoux J, Vallon V. SGLT2 inhibition and kidney protection. Clin Sci. 2018;132(12):1329–1339. doi:10.1042/CS20171298
29. DeFronzo RA, Beeves WB, Awad AS. Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat Rev Nephrol. 2021;17(5):319–334. doi:10.1038/s41581-021-00393-8
30. Nunoi K, Sato Y, Kaku K, Yoshida A, Suganami H. Effects of sodium-glucose cotransporter 2 inhibitor, tofogliflozin, on the indices of renal tubular function in patients with type 2 diabetes. Endocrinol Diabetes Metab. 2018;1(2):e00015. doi:10.1002/edm2.15
31. Li Z, Murakoshi M, Ichikawa S, et al. The sodium-glucose cotransporter inhibitor tofogliflozin prevents diabetic kidney disease progression in type 2 diabetic mice. FEBS Open Bio. 2020;10(12):2761–2770. doi:10.1002/2211-5463.13014
32. Miyazaki Y, Nomura S, Miyake T, et al. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood. 1996;88(9):3456–3464. doi:10.1182/blood.V88.9.3456.bloodjournal8893456
33. Nomura S, Ozaki Y, Ikeda Y. Function and role of microparticles in various clinical settings. Thromb Res. 2008;123(1):8–23. doi:10.1016/j.thromres
34. Nomura S, Shouzu A, Taomoto K, et al. Assessment of an ELISA kit for platelet-derived microparticles by joint research at many institutes in Japan. J Atheroscler Thromb. 2009;16(6):878–887. doi:10.5551/jat.2642.
35. Nomura S. Microparticle and atherothrombotic diseases. J Atheroscler Thromb. 2016;23(1):1–9. doi:10.5551/jat.32326
36. Nomura S. Extracellular vesicles and blood diseases. Int J Hematol. 2017;105(4):392–405. doi:10.1007/s12185-017-2180-x
37. Nomura S, Tandon NN, Nakamura T, Cone J, Fukuhara S, Kambayashi J. High-shear-stress-induced activation of platelets and microparticles enhances expression of cell adhesion molecules in THP-1 and endothelial cells. Atherosclerosis. 2001;158(2):277–287. doi:10.1016/s00219150(01)004336
38. Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24(1):29–33. doi:10.1161/01.ATV.0000099786.99623.EF.
39. Takahara M, Katakami N, Kaneto H, Noguchi M, Shimomura I. Contribution of visceral fat accumulation and adiponectin to the clustering of metabolic abnormalities in a Japanese population. J Atheroscler Thromb. 2014;21(6):543–553.
40. Ouchi N. Adipocytokines in cardiovascular and metabolic diseases. J Atheroscler Thromb. 2016;23(6):645–654. doi:10.5551/jat.34918
41. Iwamoto M, Kubota T, Sakurai Y, et al. The sodium-glucose co-transporter 2 inhibitor tofogliflozin suppresses atherosclerosis through glucose lowering in ApoE-deficient mice with streptozotocin-induced diabetes. Pharmacol Res Perspect. 2022;10(4):e00971. doi:10.1002/prp2.971
42. Katakami N, Mita T, Yoshii H, et al. The influence of tofogliflozin on treatment-related quality of life in patients with type 2 diabetes mellitus. Diabetes Ther. 2021;12(9):2499–2515. doi:10.1007/s13300-021-01125-8
43. Katakami N, Mita T, Maeda N, Sato Y, Watada H, Shimomura I. Evaluation of the effect of tofogliflozin on the tissue characteristics of the carotid wall-a sub-analysis of UTOPIA trial. Cardiovasc Diabetol. 2022;21(9):19. doi:10.1186/s12933-022-01451-6
44. Sawamura T, Kume N, Aoyama T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386(6620):73–77. doi:10.1038/386073a0
45. Yokota C, Sawamura T, Watanabe M, et al. High levels of soluble lectin-like oxidized low-density lipoprotein receptor-1 in acute stroke: an age- and sex-matched cross-sectional study. J Atheroscler Thromb. 2016;23(10):1222–1226. doi:10.5551/jat.32466
46. Sawamura T, Fujita Y, Horiuchi S, Kakino A. LOX-1 in ischemic stroke. J Atheroscler Thromb. 2017;24(6):566–568. doi:10.5551/jat.ED071.2009
47. Fukui M, Tanaka M, Senmaru T, et al. LOX-1 is a novel marker for peripheral artery disease in patients with type 2 diabetes. Metabolism. 2013;62(7):935–938. doi:10.1016/j.metabol.2013.01.018
48. Rehman K, Akash MSH. Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: how are they interlinked? J Cell Biochem. 2017;118(11):3577–3585. doi:10.1002/jcb.26097
49. Yuan T, Yang T, Chen H, et al. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019;20:247–260. doi:10.1016/j.redox.2018.09.025
50. Yu XH, Fu YC, Zhang DW, Yin K, Tang CK. Foam cells in atherosclerosis. Clin Chim Acta. 2013;424:245–252. doi:10.1016/j.cca.2013.06.006
51. Ishibashi Y, Matsui T, Yamagishi S. Tofogliflozin, a highly selective inhibitor of SGLT2 blocks proinflammatory and proapoptotic effects of glucose overload on proximal tubular cells partly by suppressing oxidative stress generation. Horm Metab Res. 2016;48(3):191–195. doi:10.1055/s-0035-1555791
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.