Back to Journals » Infection and Drug Resistance » Volume 14
Effects of Tigecycline Combined with Azithromycin Against Biofilms of Multidrug-Resistant Stenotrophomonas maltophilia Isolates from a Patient in China
Authors Yue C, Shen W, Hu L , Liu Y , Zheng YH, Ye Y, Zhang Y, Li JB
Received 21 December 2020
Accepted for publication 4 February 2021
Published 26 February 2021 Volume 2021:14 Pages 775—786
DOI https://doi.org/10.2147/IDR.S298274
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
ChengCheng Yue,1,* WeiHua Shen,2,* LiFen Hu,1,3,4 YanYan Liu,1,3,4 YaHong Zheng,1 Ying Ye,1,3,4 Yuhao Zhang,5 JiaBin Li1,3,4,6
1Department of Infectious Diseases, The First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China; 2Department of Special Clinic, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, People’s Republic of China; 3Anhui Center for Surveillance of Bacterial Resistance, Hefei, Anhui, People’s Republic of China; 4Institute of Bacterial Resistance, Anhui Medical University, Hefei, Anhui, People’s Republic of China; 5Anhui Medical University, Hefei, Anhui, People’s Republic of China; 6Department of Infectious Diseases, The Chaohu Affiliated Hospital of Anhui Medical University, Hefei, Anhui, People’s Republic of China
*These authors contributed equally to this work
Correspondence: JiaBin Li; LiFen Hu
Department of Infectious Diseases, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, People’s Republic of China
Tel +86-551-62922713
; +86-551-62922258
Fax +86-551-62922281
Email [email protected]; [email protected]
Purpose: Our aim was to investigate in vitro biofilm formation by S. maltophilia and the effects of antibacterial agents used to prevent biofilm formation.
Methods: Two trimethoprim/sulfamethoxazole-resistant S. maltophilia strains were isolated from the pleural effusion of a patient with cancer. The minimum inhibitory concentrations (MICs) of amikacin, azithromycin, cefoperazone/sulbactam, and tigecycline were determined. The checkerboard method was used to determine the fractional inhibitory concentration indices (FICIs). A crystal violet biofilm assay and confocal laser scanning microscopy (CLSM) were used to observe biofilm formation. In vitro effects of azithromycin combined with tigecycline on biofilms of S. maltophilia strains were tested.
Results: The two S. maltophilia isolates were confirmed to produce strong biofilms. Crystal violet biofilm assay and CLSM analysis of S. maltophilia biofilm were in the initial adhesive stage after 2 h incubation. Biofilm was in the exponential phase of growth at 12 h and reached maximal growth at 36– 48 h. Compared with tigecycline or azithromycin alone, the combination of tigecycline and azithromycin increased the inhibiting effect S. maltophilia biofilm biomass after incubation for 12 h. Compared with the control group, in almost all strains treated with tigecycline and azithromycin, the biofilm was significantly suppressed significance (P< 0.001). We found that 2x MIC azithromycin combined with 1x MIC tigecycline had the best inhibiting effect against the biofilm, the biofilm inhibition rates of three strains were all over 60%, the biofilm thickness was inhibited from 36.00 ± 4.00 μm to 8.00 μm, from 40.00 μm to 6.67± 2.31 μm, and from 32.00 μm to 13.33 ± 2.31 μm in SMA1, SMA2 and ATCC17666, respectively.
Conclusion: Azithromycin combined with tigecycline inhibited biofilm formation by S. maltophilia. Our study provides an experimental basis for a possible optimal treatment strategy for S. maltophilia biofilm-related infections.
Keywords: Stenotrophomonas maltophilia, azithromycin, tigecycline, biofilm, trimethoprim/sulfamethoxazole
Introduction
Stenotrophomonas maltophilia (SMA) is recognized as a superbug that threatens human health by virtue of its natural and acquired resistances to a variety of antimicrobial agents.1,2 SMA causes healthcare-associated infections, and the infection rate has shown a substantial upward trend.3,4
SMA infection often occurs in elderly patients, in patients with tumors, in patients with cystic fibrosis, in immunocompromised patients, in patients with prolonged hospitalization, and as a result of misuse of antimicrobial agents, especially carbapenems1,5,6 Persistent infections caused by SMA create substantial challenges for clinicians. Multiple drug resistance contributes to persistent infections and subsequent mortality. Biofilm formation has been proven to enhance bacterial resistance. Nevertheless, the relationship between biofilm formation and intractable SMA infections remains unclear.
Biofilm formation is becoming a predominant feature in nosocomial infections. Because biofilms are increasingly resistant to antibiotics, making monotherapy ineffective, combination therapy seem to be important for their eradication.7 Macrolides in combination with other antibiotics have shown conflicting results with respect to antibiofilm properties on S. maltophilia. Azithromycin in combination with fluoroquinolones, particularly moxifloxacin, significantly reduced the biofilm-inhibiting effect on S. maltophilia biofilms.8 One possible mechanism is that azithromycin may interfere with protein synthesis and consequently with the bactericidal activity of fluoroquinolones.8 By contrast, erythromycin acts synergistically with levofloxacin, cefoperazone/sulbactam, and piperacillin, enhancing susceptibility against biofilms. These findings suggest that specific combined macrolide could be an effective treatment for S. maltophilia infection.9 Several studies have indicated that a combination of fluoroquinolones and azithromycin has a synergetic effect on Pseudomonas aeruginosa biofilms.10 Bacterial biofilm structure and composition confer inherent resistance to antimicrobial agents. The biofilm matrix serves as an obstacle to antimicrobial agents, which can be bound to matrix components or be consumed by them.11 Nevertheless, this trait varies by matrix composition, biofilm age, and specific antimicrobial agent.12,13 The aim of antibiofilm strategies was to prevent initial bacterial adhesion, inhibit biofilm maturation inhibition, and eradicate biofilm production. Premature biofilms are more susceptible to antimicrobial agents than are mature biofilms.14
In the present study, we measured the influence of biofilm production on refractory infection, and the in vitro effects of antimicrobial agents against biofilms produced by this organism.
Materials and Methods
Clinical Observation of the Patient
On 22 May 2017, a 64-year-old man was admitted to the First Affiliated Hospital of Anhui Medical University with a 1-month history of recurrent fever. The patient presented with high fever, accompanied by chest tightness. He had a history of surgery for esophageal cancer one month prior. Chest CT revealed right pleural effusion. Pleural tap revealed a white blood cell of 2986 cell/mm3. K. pneumoniae was isolated after admission; two weeks later, SMA1 was obtained from the pleural effusion. Twenty days later, SMA2 was obtained from pleural effusion as well. The patient was diagnosed with an infected pleural effusion.
- K. pneumoniae was resistant to cefepime, aztreonam, and cefoperazone-sulbactam. The patient was treated with IV imipenem (0.5 g, q8h, 17 days) from 22 May to 8 June. After imipenem treatment for 16 days, WBC in pleural effusion decreased to 1951 cell/mm3. However, the patient maintained a low-grade fever. SMA1 was isolated in the pleural effusion. The anti-bacterial therapy was changed to IV tigecycline (50 mg, q12h) for 14 days. However, the fever rebounded to 39°C on 16 June, and SMA2 was isolated from the pleural effusion. At this time, the thoracic drainage tube was removed. The anti-bacterial therapy was changed to IV tigecycline (2 g, q8h) combined with azithromycin (0.5 g, qd) for 17 days. Finally, the patient recovered. This study was conducted in accordance with the Declaration of Helsinki. We confirmed that written informed consent has been provided by the patient to publish the case details. This study was approved by the local Ethics Committee of Anhui Medical University.
S. maltophilia Isolates
SMA1 and SMA2 were isolated from the patient pleural fluid on 5 June and 19 June, respectively. The isolates were confirmed using the VITEK GNI system (bioMerieux Vitek Inc., Hazelwood, MO, USA) and Clin-ToF-II.
Antimicrobial Susceptibility Testing and Detection of Resistance Mechanisms
Antimicrobial susceptibility testing was determined using the microdilution method, according to the Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, 23rd informational supplement.15 The following antimicrobial agents were tested against S. maltophilia strains: trimethoprim/sulfamethoxazole (SXT), minocycline (MIN), levofloxacin (LVX), ticarcillin–clavulanate (TIC/CIA), chloramphenicol (CHL), ceftazidime (CAZ), amikacin (AMK), azithromycin (AZM), tigecycline (TGC), cefoperazone/sulbactam, aztreonam (ATM), imipenem (IPM), and meropenem (MEP). All antibiotics are from Sigma-Aldrich China, Inc. All experiments were repeated three times. The breakpoint of tigecycline for S. maltophilia was based on the standard of the US Food and Drug Administration. S. maltophilia 17,666, Pseudomonas aeruginosa ATCC 27,853, and Escherichia coli ATCC 25,922 were used as controls for antimicrobial susceptibility testing.
Polymerase chain reaction was used to detect β-lactamase genes (L1 and blaL2), sulfamethoxazole resistance genes (sul1, sul2, and sul3), trimethoprim resistance genes (dfrA1, dfrA5, dfrA12, dfrA13, and dfrA17), quinolone resistance genes (qnrX, qnrY, and qnrZ gene), and class 1, 2, and 3 integrons. All experiments were repeated three times.
Checkerboard Assay
The checkerboard broth microdilution assay was performed in 96-well microtiter plates using 2-fold dilutions of two antibiotics diluted in cation-adjusted Mueller–Hinton broth (CAMHB). AZM ranging from 1/64 × MIC to 2× MIC was dispensed in every row. TGC ranging between 1/64× MIC and 2× MIC was added in each column. An equal volume of standardized bacterial suspension of 1 × 106 CFU/mL was added and then all plates were incubated at 37°C in an aerobic atmosphere for 24 h. Fractional inhibitory concentration index (FICI) values were calculated as FICI = FIC A (MIC antibiotic A in combination)/(MIC antibiotic A) + FIC B (MIC antibiotic B in combination)/(MIC antibiotic B). To categorize the drug combination that consistently generated the lowest FICI after repeating the experiment in duplicate on two further occasions, the results were grouped as follows: FICIs of ≤0.5 were interpreted as synergistic; FICIs of >0.5 but ≤1 were considered additive; FICIs of >1 but ≤4 were considered indifferent; and FICIs >4 were interpreted as antagonistic.16 The measurements were performed on three different samples of each.
Biofilm Formation Assay
Crystal Violet Biofilm Assay
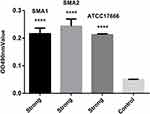
To observe the biofilm formation and established biofilms, crystal-violet staining experiments were performed as previously described with some changes.17–19 Briefly, overnight cultures of S. maltophilia were standardized to a 0.5 MacFarland standard (equivalent to 1.5 × 108 CFU/mL), and then diluted (1:100) with fresh Luria Bertani broth. Aliquots (200 μ L) of standardized inocula were added to the wells of sterile flat-bottom 96-well polystyrene tissue culture plates (Thermo Fisher Scientific, USA) and incubated at 37°C over a series of time-points (2, 4, 6, 8, 10, 12, 24, 36, and 48 h) in a closed and humidified plastic container. The medium was then discarded and non-adherent cells were removed by washing three times in sterilized ultrapure water. The cells were stained with 0.01% (w/v) crystal violet for 15 min. The excess stain was then removed by washing with water and the stained biofilms were dried for 30 min at ambient room temperature and extracted with 33% (v/v) glacial acetic acid. The amount of biofilm produced was quantified by measuring the optical density at 490 nm (OD490) using a plate reader (VarioskanFlash; Thermo Fisher Scientific, USA). Non-inoculated media were used as a control. The low cut-off point for biofilm production was chosen according to the criteria described by Di Bonaventura et al, the cut-off point was defined as three standard deviations above the mean optical density of the control (ODc) wells.17 Based on this cut-off, strains were classified into the following categories: no biofilm producer (OD ≤ ODc), weak biofilm producer (ODc < OD ≤ 2 × ODc), moderate biofilm producer (2 × ODc < OD ≤ 4 × ODc), and strong biofilm producer (4 × ODc < OD).20,21 Each isolate was assayed three times and the results were presented as the average of the three assays.8,22
Confocal Laser Scanning Microscopy (CLSM) of Biofilm Formation
Overnight bacterial culture of SMA1, SMA2 and ATCC17666 were standardized to a 0.5 MacFarland standard (equivalent to 1.5 × 108 CFU/mL) and then diluted (1:100) with fresh luria bertani broth. Then 400 μL of the dilution were inoculated into tissue culture 24-well glass-bottom imaging plates and incubated at 37°C over a series of time-points (2, 6, 12, 24, 36 and 48 h) in a closed and humidified plastic container.23 After washing out non-adherent cells with phosphate-buffered saline (PBS), biofilms were fixed with 3.7% (v/v) formaldehyde for 1 h, washed with sterile PBS, and then extracellular nucleic acids stained with fluorescent dyes 0.01% acridine orange (AO, Sigma) for 15 min at room temperature (RT). After staining, wells were washed with 1 mL of PBS to remove unbound dye and dried for 15 min. All procedures were conducted in the dark. The excitation laser wavelength for AO was 488 nm, the emission wavelength is 512~654 nm. Confocal laser microscopy was used to observe the growth of biofilms, as well as structure and thickness at various times.24 The biofilms were examined using a Zeiss LSM 510 META microscope (Zeiss, Germany).20 CLSM was used to measure biofilm thickness ImageJ program.25 Images were constructed using ZEN software (Carl Zeiss, Thornwood, NY, USA), as described previously.26 The measurements were performed on three different samples of each.27
Effect of Combined Tigecycline and Azithromycin on S. maltophilia Biofilms Using CLSM
CLSM was performed to visualize biomass and cells of biofilm affected by tigecycline and azithromycin using published procedures, with some modifications.28 Briefly, overnight bacterial culture of S. maltophilia was adjusted and 200 μL dilution was inoculated into tissue culture-treated 24-well glass bottom imaging plates (Eppendorf AG, Hamburg, Germany, Cat. no. 0030741021). Sterile cell slides are placed at the bottom of the 24-well plates. After 12 h incubation, suspension medium was discarded and each well was washed three times with 1 mL of sterile PBS. Various concentrations of TGC (0.5x, 1x, and 2x of the MIC) were diluted by prepared fresh CAMHB in the wells of the plate. Various concentrations of azithromycin (0.5x, 1x, and 2x of the MIC) were added to wells on the same plate. Fresh CAMHB without antibiotics was added to the control wells. The plates were incubated at 37°C for 12 h. After washing out non-adherent cells with PBS, biofilms were fixed with 3.7% (v/v) formaldehyde for 1 h, washed with sterile PBS, and then extracellular nucleic acids stained with 0.01% AO for 15 min at RT. After staining, wells were washed with 1 mL of PBS to remove unbound dye and dried for 15 min. All procedures were conducted in the dark. The excitation laser wavelength for AO was 488 nm, the emission wavelength was 512~654nm. Confocal laser microscopy was used to observe the growth of biofilms in different drug treatment groups, including structure and thickness. The biofilms were examined under Zeiss LSM 510 META microscope (Zeiss, Germany). CLSM was used to measure biofilm thickness using the ImageJ program. Images were constructed using ZEN software (Carl Zeiss, Thornwood, NY, USA), as described previously.26 The measurements were performed on three different samples of each.
In vitro Effects of Azithromycin, Tigecycline, Amikacin, Cefoperazone/Sulbactam Alone on S. maltophilia Biofilms
This test was performed on the S. maltophilia strains (SMA1, SMA2, and ATCC17666). After 12 h incubation periods at 37 °C, the supernatants from each well were gently discarded. Each well was then washed three times with sterile saline water without destroying the attached biofilm. Various concentrations of azithromycin, tigecycline, amikacin, and cefoperazone/sulbactam alone (1x, 2x, and 4x of the MIC) were prepared following dilution in 100 μL of fresh CAMHB in the wells of the micro-titer plate. Fresh CAMHB without antibiotics was added to the control wells. The plates were incubated at 37 °C for 12 h and the OD490 nm was measured. All measurements were performed three times.
Effect of Tigecycline Combined with Azithromycin on S. maltophilia Biofilms
This test was performed on the S. maltophilia strains (SMA1, SMA2, and ATCC17666). After 12 h incubation periods at 37 °C, the supernatants from each well were gently discarded. Each well was then washed three times with sterile PBS without destroying the attached biofilm. Various concentrations of TGC (0.5x, 1x, and 2x of the MIC) were prepared following dilution in 100 μL of fresh CAMHB in the wells of the micro-titer plate. Various concentrations of azithromycin (0.5x, 1x, and 2x of the MIC) were added to wells on the same plate. Fresh CAMHB without antibiotics was added to the control wells. The plates were incubated at 37 °C for 12 h and the OD490 nm was measured. The inhibition results were calculated using Eq (1) and were expressed as a percentage: % inhibition = 100 − (ODsample/ODcontrol) x 100 (1).29 All measurements were performed three times at least.
Statistical Analysis
All tests were performed in triplicate and repeated three times. Multiple comparisons of responses to antibiotics and inhibition of biofilm formation were performed using one-way analysis of variance. A P-value <0.05 was considered statistically significant. Statistical analysis was conducted using GraphPad Prism 6.
Results
Antimicrobial Susceptibility and Resistance Genes
Antimicrobial susceptibility testing showed that two strains displayed resistance to SXT, LVX, TIC/CIA, AZM, IPM, MEP, CAZ, and ATM, and were sensitive to SCF, MIN, CHL, and TGC. As shown in (Figure S1), the two isolates contained sul1, qnrX, qnrY and qnrZ genes, and class 1 and 3 integrase of integrons.
In vitro Synergy Testing Using the Checkerboard Method
As shown in Table 1, the combination of azithromycin– tigecycline resulted in synergy for three strains, with FICIs ranging from 0.1875 to 0.375. The combination of cefoperazone/sulbactam–azithromycin resulted in additivity for three strains, with FICIs ranging from 0.625 to 1.0. The combination of amikacin–tigecycline resulted in no interaction for the three strains, with FICIs ranging from 1.5 to 3.0. The combination of amikacin–cefoperazone/sulbactam resulted in no interaction for three strains, with FICIs ranging from 1.25 to 2.25. The combination of tigecycline–cefoperazone/sulbactam resulted in no interaction for three strains, with FICIs ranging from 1.25 to 4.0.
 |
Table 1 Checkerboards of the Various Antibiotics Used in This Study, with Their FICIs in S. maltophilia Strains |
In vitro Biofilm Formation by S. maltophilia
Crystal Violet Biofilm Assay
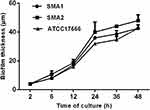
As shown in Figure 1, SMA1, SMA2 and ATCC17666 were in the initial adhesive stage after 2 h of incubation, and in the exponential phase of growth after 12 h of incubation. Maximum growth was achieved after about 48 h of incubation (plateau phase). As shown in Figure 2, the ability of the three strains to produce biofilm was strong.
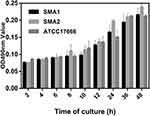 |
Figure 1 Average ODs at different time points (hours) of S. maltophilia strains. Results are expressed as means ± SDs. |
CLSM Analysis of Biofilm Formation
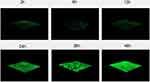
CLSM analysis of biofilm formation in clinical isolates strains (SMA1, SMA2) and standard strain ATCC17666. CLSM analysis showed that the thicknesses of ATCC17666 biofilms were 3.99 ± 0.01 μm at 2 h, 8.00 μm at 6 h, 16.00 μm at 12 h, 36.00 μm at 24 h, 34.67 ± 2.31 μm at 36 h, and 42.67 ± 2.31 μm at 48 h. Thicknesses of the SMA1 biofilms were 4.00 μm at 2 h, 8.00 μm at 6 h, 17.33 ± 2.31 μm at 12 h, 36.00 μm at 24 h, 38.67 ± 2.31 μm at 36 h, and 42.67 ± 2.31 μm at 48 h. Thicknesses of the SMA2 biofilms were 4.00 μm at 2 h, 10.67 ± 2.31 μm at 6 h, 18.67 ± 2.31 μm at 12 h, 40.00 ± 6.93 μm at 24 h, 44.00 μm at 36 h, and 48.00 ± 4.00 μm at 48 h (Figure 3). CLSM analysis showed bacterial biofilm beginning to form after 2 h incubation. The biological membrane structure is close, and the thickness increased over 12–24 h incubation. The three-dimensional space network structure gradually formed between 36–48 h incubation (Figure 4). The growth rates of the strains biofilms were similar between 0–12 h incubation, however between 12 h and 48 h incubation, the growth rates of SMA1 and SMA2 became faster than that of ATCC17666.
In vitro Effects of Azithromycin, Tigecycline, Amikacin, and Cefoperazone/Sulbactam on S. maltophilia Preformed Biofilm
As shown in Figure 5, low concentrations of antibiotics had a weak inhibitory effect on biofilms and inhibited biofilm formation in the three S. maltophilia strains in a dose-dependent manner. Amikacin had a far greater inhibitory effect on the biofilm of the premature biofilms than did the other antibiotics. Compared with the control group, the inhibitory effect of amikacin on the biofilms of the three strains was statistically significant (P <0.01). Both tigecycline and cefoperazone/sulbactam had inhibitory effects on the biofilms of SMA1 and SMA2 (biofilms formed after 12 h incubation). Compared with the control group, the inhibitory effects of tigecycline and cefoperazone/sulbactam on the biofilms of the two clinical isolates strains were statistically significant (P <0.01); however, the inhibitory effect of tigecycline and cefoperazone/sulbactam on the biofilm of the ATCC17666 was not statistically. As shown in Figure 5, the inhibitory effects of azithromycin alone against preformed biofilms in SMA1, SMA2, and ATCC17666 occurring at various concentrations were poor. This may be because all three strains were resistant to azithromycin. Compared with the control group, the inhibitory effect of azithromycin S. maltophilia (SMA1, SMA2, ATCC17666) strains that have formed biofilms has almost not reached statistical significance.
Effect of the Combination of Tigecycline and Azithromycin on Preformed Biofilm of S. maltophilia Using Crystal Violet Biofilm Assay
Based on the synergistic effect of azithromycin and tigecycline, the effects of various concentrations of azithromycin combined with tigecycline to inhibit the biofilms were tested.
Compared with tigecycline or azithromycin alone, the combination of tigecycline and azithromycin increased the inhibitory effect on S. maltophilia preformed biofilms. We tested several combinations of various drug concentrations, and all of the combinations increased the associated inhibition (Figure 6). Although this had the effect of synergistically inhibiting biofilm, it did not reach statistical significance at low concentrations; however, compared with the control group, in almost all groups treated with antibiotics, biofilm formation was significantly suppressed (P <0.001). The best inhibitory effect was observed when 2x MIC azithromycin was combined with 1x MIC tigecycline (Figure 6) and when 2x MIC azithromycin was combined with 1x MIC tigecycline. The biofilm inhibition rates were 64% for SMA1, 76% for SMA2, and 67% for ATCC17666. The biofilm inhibition rates of three strains were all over 60%, suggesting good results.
Effect of the Combination of Azithromycin and Tigecycline on S. maltophilia Preformed Biofilm Thickness and Structure Using CLSM Analysis
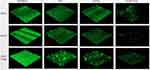
To further study the effects of antibiotics on biofilm formation by S. maltophilia, a combination of azithromycin and tigecycline was tested on the three S. maltophilia strains. Interestingly, compared with S. maltophilia treated by azithromycin or tigecycline alone, the combination of azithromycin and tigecycline increased the inhibitory effect on S. maltophilia preformed biofilm. As shown in Figure 7, compared with the control group, the biofilms of almost all antibiotic treatment groups were significantly inhibited. Compared with tigecycline alone, the best inhibitory effect was observed with 2x MIC azithromycin combined with 1x MIC tigecycline (P<0.01). When 2x MIC azithromycin combined with 1x MIC tigecycline was used, the biofilm thickness of SMA1 was inhibited from 36.00 ± 4.00 μm to 8.00 μm; that of SMA2 was inhibited from 40.00 μm to 6.67 ± 2.31 μm; and that of ATCC17666 was inhibited from 32.00 μm to 13.33 ± 2.31 μm.
As shown in the CLSM images, compared with S. maltophilia treated with azithromycin or tigecycline alone, the combination of azithromycin and tigecycline increased the inhibitory effect on S. maltophilia preformed biofilms. As shown in Figure 7, 2x MIC azithromycin combined with 1x MIC tigecycline showed the best inhibitory effect, consistent with the results of the 96-well biofilm assays (Figure 6). Micrographs of no-antibiotic treatment groups showed spatial biomass distribution of the formed biofilms and relatively thick coating of the biofilms with well-organized architecture characterized by large clumps over a glass surface. However, after treatment with 1x MIC tigecycline and 2x MIC azithromycin alone, or 1x MIC tigecycline combined with 2x MIC azithromycin (Figure 8), the established biofilms were disrupted and scattered as microcolonies, and the biofilm cells remained in the form of flakes, evacuated, indicating that cells within the formed biofilms were probably challenged, killed, and detached from the established biofilms. Compared with S. maltophilia biofilm treated with 1x MIC tigecycline and 2x MIC azithromycin alone, biofilms of S. maltophilia were almost disrupted by 1x MIC tigecycline combined with 2x MIC azithromycin.
CLSM was used to visualize the biofilms formed on the glass bottom plates. Compared with S. maltophilia biofilm treated with tigecycline and azithromycin alone, microscopic examination showed that tigecycline combined with azithromycin largely eradicated the biofilms formed on the glass bottom surface. This suggests that there were few biofilm cells.
Discussion
S. maltophilia is an opportunistic pathogen that is widely distributed in nature. The pathogen is the third most common infectious non-fermentative following P. aeruginosa and Acinetobacter baumannii.3,4 Biofilms lead to greater resistance to antimicrobial drugs than non-biofilm forming bacteria and are therefore more difficult to treat.30,31 The origin of various chronic infections is usually the formation of a biofilm by the infecting organism. Therefore, one of the important considerations for effective treatment of most chronic infections is to target the biofilm stage of the organism.32,33
In the present study, a patient with esophageal cancer had an infected pleural effusion after surgery. After treatment of thoracic drainage tube drainage and imipenem for two weeks, SXT-resistant S. maltophilia was isolated from the pleural effusion. SXT-resistant S. maltophilia was still isolated after an interval of 20 days. Our result revealed the use of imipenem for two weeks selected the S. maltophilia as the dominant pathogenic bacteria. The two S. maltophilia isolates were confirmed to produce strong biofilms, which may have contributed to the refractory infection. The use of thoracic drainage tube drainage may contribute to biofilm formation. Interestingly, SMA2 had a higher ability to produce biofilm. There are many infectious bacteria, including S. maltophilia, P. aeruginosa and Staphylococcus aureus, which have the ability to form biofilms.34 The ability of S. maltophilia to form biofilm is an important feature of its virulence.19 In our research, crystal violet biofilm assay and CLSM analysis of S. maltophilia biofilm were in the initial adhesive stage after 2 h incubation. Biofilm was in the exponential phase of growth at 12 h, and reached maximal growth at 36–48 h. Crystal violet biofilm assay and CLSM analysis showed these adherent cells are embedded within a self-produced matrix of an extracellular polymeric substance, and finally forming a complex three-dimensional architecture, which is consistent with the two previous investigations.19,20,25,35 The antibiotics used to treat S. maltophilia infections are usually selected based on the results of conventional antimicrobial susceptibility of plankton. However, it is known that organisms such as S. maltophilia actually grow in the form of biofilms that are more resistant to bacteria than bacteria floating on airway epithelial cells.8,36,37
Previous research showed levofloxacin prevented biofilm formation of S. maltophilia isolates,38 however, according to our study, a refractory infection in pleural effusion was caused by S. maltophilia with high biofilm production, characterized by resistance to SXT, LVX, and ceftazidime.11 The patient was treated using TGC at first, however, the effect was not good, and biofilm production in the isolates may have contributed to the poor efficacy. The patient was recovered by the combination of azithromycin and tigecycline at last.
Macrolides in combination with other antibiotics have shown conflicting results regarding antibiofilm properties against S. maltophilia. Azithromycin in combination with fluoroquinolones significantly reduced the biofilm-inhibiting effect on S. maltophilia performed biofilms, especially combined with moxifloxacin.8 One possible mechanism is that azithromycin may interfere with the protein synthesis and consequently with the bactericidal activity of fluoroquinolones.8 In this research, we found that azithromycin–tigecycline showed synergistic effects against biofilm growth in the clinical S. maltophilia isolates, which contradicts to the previous research.8 By contrast, erythromycin acts synergistically with levofloxacin, cefoperazone/sulbactam, and piperacillin, enhancing susceptibility in biofilms. For this reason, specific macrolides could be effective adjuncts for treatment of S. maltophilia infections.9 Several studies have indicated that a combination of fluoroquinolones and azithromycin has synergetic effects against P. aeruginosa biofilms.7,10 The results of these studies are consistent with our findings in SMA1, SMA2, and ATCC17666.
In our study, the clinical S. maltophilia isolates were resistant to SXT; however, the combination of azithromycin–tigecycline showed synergistic effect on the inhibition of clinical S. maltophilia isolates in vitro. Furthermore, both 0.5x MIC azithromycin and 0.5x MIC tigecycline had inhibitory effects on biofilms in the two clinical S. maltophilia isolates. The inhibitory effect was more substantial with the increase of drug concentration. Based on the synergistic effect of azithromycin and tigecycline, the effects of various concentrations of azithromycin combined with tigecycline to inhibit the biofilms were tested. For azithromycin, 0.5x MIC combined with 1x MIC tigecycline inhibited the biofilm to half the thickness. Inhibition of biofilms is critical to controlling recurrent infection. CLSM analysis showed that 2x MIC azithromycin combined with 1x MIC tigecycline almost inhibited biofilm growth in two clinical S. maltophilia strains. It penetrate the biofilm, eventually killing the bacteria penetrating the biofilm, and eventually killing the bacteria.39
When mature biofilms are completely formed by biofilm-forming pathogens, conventional antibiotics usually fail to eradicate the infection because the multilayer architecture of the biofilm acts as a diffusion barrier. This can provide protection to biofilm-grown cells against antimicrobial agents or antibiotics. Therefore, high biofilm disrupting concentrations are required, and strategies focusing on destroying the formed biofilms are of particular importance against infection.40 Compared with S. maltophilia biofilm treated with tigecycline and azithromycin alone, azithromycin combined with tigecycline increased the inhibitory effect on S. maltophilia biofilms more efficiently. Our results suggest that the combination of tigecycline and azithromycin induced synergistic disruption of the complex architecture of the biofilms, as measured both by microtiter plate assay and by CLSM analysis. We believe that antibiotics are likely to only slow down the progress of biofilm formation by eliminating unprotected planktonic bacteria and reducing even stopping the metabolic activity of bacteria on the biofilm surface, and our results agree with most studies.41–43
Conclusion
Biofilm formation of S. maltophilia contributes to persistent infection. We found that azithromycin combined with tigecycline increased the inhibiting effect on S. maltophilia biofilms compared with tigecycline and azithromycin alone. The use of antibiotics can better inhibit the growth of bacterial at the early stage of biofilm formation, accordingly reducing the incidence of resistance. Our research provides an experimental basis for possible optimal treatment strategy for S. maltophilia biofilm-related infections; however, additional evidence is required to confirm these findings in future therapeutic studies.
Acknowledgments
We thank all the authors and people who helped us to accomplish this work. This study was supported by the National Natural Science Foundation of China (No 81101313 and No 81973983), the National Science and Technology Major Project (No. 2017ZX10204401), the Borrowing and Transferring Subsidy Project in 2019, Hefei (J2019Y04), the Human Resources and Social Security of Anhui province (2018H194).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chang Y, Lin C, Chen Y, Hsueh P. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front Microbiol. 2015;6:893. doi:10.3389/fmicb.2015.00893
2. Jeon YD, Jeong WY, Kim MH, et al. Risk factors for mortality in patients with Stenotrophomonas maltophilia bacteremia. Medicine. 2016;95(31):e4375. doi:10.1097/MD.0000000000004375
3. Hu L, Xu X, Li H, et al. Surveillance of antimicrobial susceptibility patterns among Stenotrophomonas maltophilia isolated in China during the 10-year period of 2005–2014. J Chemother. 2018;30(1):25–30. doi:10.1080/1120009X.2017.1378834
4. Kang YR, Cha YK, Kim JS, et al. Imaging findings ofStenotrophomonas maltophilia pneumonia: emphasis on CT findings between immunocompromised and immunocompetent patients. Acta Radiol. 2020;61(7):903–909. doi:10.1177/0284185119885117
5. Adegoke AA, Stenström TA, Okoh AI. Stenotrophomonas maltophilia as an emerging ubiquitous pathogen: looking beyond contemporary antibiotic therapy. Front Microbiol. 2017;8. doi:10.3389/fmicb.2017.02276
6. Cabaret O, Bonnal C, Canoui-Poitrine F, et al. Concomitant presence of aspergillus fumigatus and Stenotrophomonas maltophilia in the respiratory tract: a new risk for patients with liver disease? J Med Microbiol. 2016;65(5):414–419. doi:10.1099/jmm.0.000233
7. Saini H, Chhibber S, Harjai K. Azithromycin and ciprofloxacin: a possible synergistic combination against Pseudomonas aeruginosa biofilm-associated urinary tract infections. Int J Antimicrob Agents. 2015;45(4):359–367. doi:10.1016/j.ijantimicag.2014.11.008
8. Wang A, Wang Q, Kudinha T, Xiao S, Zhuo C. Effects of fluoroquinolones and azithromycin on biofilm formation of Stenotrophomonas maltophilia. Sci Rep. 2016;6(1).
9. Sun E, Liang G, Wang L, et al. Antimicrobial susceptibility of hospital acquired Stenotrophomonas maltophilia isolate biofilms. Braz J Infect Dis. 2016;20(4):365–373. doi:10.1016/j.bjid.2016.04.002
10. Nichols DP, Caceres S, Caverly L, et al. Effects of azithromycin in Pseudomonas aeruginosa burn wound infection. J Surg Res. 2013;183(2):767–776. doi:10.1016/j.jss.2013.02.003
11. Roisin L, Melloul E, Woerther P, et al. Modulated response of aspergillus fumigatus and Stenotrophomonas maltophilia to antimicrobial agents in polymicrobial biofilm. Front Cell Infect Microbiol. 2020;10.
12. Mah T. Biofilm-specific antibiotic resistance. Future Microbiol. 2012;7(9):1061–1072. doi:10.2217/fmb.12.76
13. Olsen I. Biofilm-specific antibiotic tolerance and resistance. Eur J Clin Microbiol. 2015;34(5):877–886. doi:10.1007/s10096-015-2323-z
14. Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15(2):167–193. doi:10.1128/CMR.15.2.167-193.2002
15. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Third Informational Supplement, M100-S23. Wayne, PA: Clinical and Laboratory Standards Institute; 2013.
16. Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52(1):1. doi:10.1093/jac/dkg301
17. Di Bonaventura G, Spedicato I, D’Antonio D, Robuffo I, Piccolomini R. Biofilm formation by Stenotrophomonas maltophilia: modulation by quinolones, trimethoprim-sulfamethoxazole, and ceftazidime. Antimicrob Agents Chemother. 2004;48(1):151–160. doi:10.1128/AAC.48.1.151-160.2004
18. O’Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30(2):295–304. doi:10.1046/j.1365-2958.1998.01062.x
19. Passerini DRB, Calenda M, Vay C, Franco M. Biofilm formation by Stenotrophomonas maltophilia isolates from device-associated nosocomial infections. Rev Argent Microbiol. 2007;39(4):204–212.
20. Melloul E, Luiggi S, Anaïs L, et al. Characteristics of aspergillus fumigatus in association with stenotrophomonas maltophilia in an in vitro model of mixed biofilm. PLoS One. 2016;11(11):e166325. doi:10.1371/journal.pone.0166325
21. Zhuo C, Zhao QY, Xiao SN. The impact of spgM, rpfF, rmlA gene distribution on biofilm formation in Stenotrophomonas maltophilia. PLoS One. 2014;9(10):e108409. doi:10.1371/journal.pone.0108409
22. Stepanovic S, Cirkovic I, Ranin L, Svabic-Vlahovic M. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett Appl Microbiol. 2004;38(5):428–432. doi:10.1111/j.1472-765X.2004.01513.x
23. Kim H, Lee D, Eom Y. Anti-biofilm and anti-virulence efficacy of celastrol against Stenotrophomonas maltophilia. Int J Med Sci. 2018;15(6):617–627. doi:10.7150/ijms.23924
24. Runci F, Bonchi C, Frangipani E, Visaggio D, Visca P. Acinetobacter baumannii biofilm formation in human serum and disruption by gallium. Antimicrob Agents Chemother. 2017;61(1). doi:10.1128/AAC.01563-16
25. Melloul E, Roisin L, Durieux M, et al. Interactions of Aspergillus fumigatus and Stenotrophomonas maltophilia in an in vitro mixed biofilm model: does the strain matter? Front Microbiol. 2018;9. doi:10.3389/fmicb.2018.02850
26. Woo S, Lee S, Lee S, Lim K, Ha E, Eom Y. Activity of novel inhibitors of Staphylococcus aureus biofilms. Folia Microbiol. 2017;62(2):157–167. doi:10.1007/s12223-016-0485-4
27. Del Carpio-Perochena AE, Bramante CM, Duarte MAH, et al. Biofilm dissolution and cleaning ability of different irrigant solutions on intraorally infected dentin. J Endod. 2011;37(8):1134–1138. doi:10.1016/j.joen.2011.04.013
28. Nosyk O, Ter Haseborg E, Metzger U, Frimmel FH. A standardized pre-treatment method of biofilm flocs for fluorescence microscopic characterization. J Microbiol Methods. 2008;75(3):449–456. doi:10.1016/j.mimet.2008.07.024
29. Liu Y, Jiang Y, Zhu J, Huang J, Zhang H. Inhibition of bacterial adhesion and biofilm formation of sulfonated chitosan against Pseudomonas aeruginosa. Carbohydr Polym. 2019;206:412–419. doi:10.1016/j.carbpol.2018.11.015
30. Balcázar JL, Subirats J, Borrego CM. The role of biofilms as environmental reservoirs of antibiotic resistance. Front Microbiol. 2015;6. doi:10.3389/fmicb.2015.01216
31. Romero R, Schaudinn C, Kusanovic JP, et al. Detection of a microbial biofilm in intraamniotic infection. Am J Obstet Gynecol. 2008;198(1):131–135. doi:10.1016/j.ajog.2007.11.026
32. Wu K, Yau YCW, Matukas L, Waters V. Biofilm compared to conventional antimicrobial susceptibility of stenotrophomonas maltophilia isolates from cystic fibrosis patients. Antimicrob Agents Chemother. 2013;57(3):1546–1548. doi:10.1128/AAC.02215-12
33. Flores-Treviño S, Bocanegra-Ibarias P, Camacho-Ortiz A, Morfín-Otero R, Salazar-Sesatty HA, Garza-González E. Stenotrophomonas maltophilia biofilm: its role in infectious diseases. Expert Rev Anti Infect Ther. 2019;17(11):877–893. doi:10.1080/14787210.2019.1685875
34. Mah TC, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9(1):34–39. doi:10.1016/S0966-842X(00)01913-2
35. Huang T, Somers EB, Wong ACL. Differential biofilm formation and motility associated with lipopolysaccharide/exopolysaccharide-coupled biosynthetic genes in Stenotrophomonas maltophilia. J Bacteriol. 2006;188(8):3116–3120. doi:10.1128/JB.188.8.3116-3120.2006
36. Ciacci N, Boncompagni S, Valzano F, et al. In vitro synergism of colistin and N-acetylcysteine against Stenotrophomonas maltophilia. Antibiotics. 2019;8(3):101. doi:10.3390/antibiotics8030101
37. Brooke JS. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev. 2012;25(1):2–41.
38. Minardi D, Montanari MP, Tili E, et al. Effects of fluoroquinolones on bacterial adhesion and on preformed biofilm of strains isolated from urinary double J stents. J Chemother. 2008;20(2):195–201. doi:10.1179/joc.2008.20.2.195
39. Ackermann M. A functional perspective on phenotypic heterogeneity in microorganisms. Nat Rev Microbiol. 2015;13(8):497–508. doi:10.1038/nrmicro3491
40. Tu QP, Genevaux P, Pajunen M, et al. Isolation and characterization of biofilm formation-defective mutants of Staphylococcus aureus. Infect Immun. 2007;75(3):1079–1088. doi:10.1128/IAI.01143-06
41. Ishida H, Ishida Y, Kurosaka Y, Otani T, Sato K, Kobayashi H. In vitro and in vivo activities of levofloxacin against biofilm-producing Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42(7):1641–1645. doi:10.1128/AAC.42.7.1641
42. Aaron SD, Ferris W, Ramotar K, Vandemheen K, Chan F, Saginur R. Single and combination antibiotic susceptibilities of planktonic, adherent, and biofilm-grown pseudomonas aeruginosa isolates cultured from sputa of adults with cystic fibrosis. J Clin Microbiol. 2002;40(11):4172–4179. doi:10.1128/JCM.40.11.4172-4179.2002
43. Carsenti-Etesse H, Durant J, Entenza J, et al. Effects of subinhibitory concentrations of vancomycin and teicoplanin on adherence of staphylococci to tissue culture plates. Antimicrob Agents Chemother. 1993;37(4):921–923. doi:10.1128/AAC.37.4.921
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.