Back to Journals » Clinical Interventions in Aging » Volume 14
Effects of the head lift exercise and neuromuscular electrical stimulation on swallowing muscles activity in healthy older adults: a randomized pilot study
Authors Poorjavad M, Talebian Moghadam S, Ansari NN
Received 17 March 2019
Accepted for publication 18 May 2019
Published 21 June 2019 Volume 2019:14 Pages 1131—1140
DOI https://doi.org/10.2147/CIA.S209055
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Marziyeh Poorjavad,1 Saeed Talebian Moghadam,2 Noureddin Nakhostin Ansari2
1Department of Speech Therapy, School of Rehabilitation, Isfahan University of Medical Sciences, Isfahan, Iran; 2Department of Physical Therapy, School of Rehabilitation, Tehran University of Medical Sciences, Tehran, Iran
Purpose: Swallowing physiology exhibits several changes in advanced ages. The present study aimed to investigate and compare effects of a period of the head lift exercise (HLE) and neuromuscular electrical stimulation (NMES) on swallowing muscles activity in healthy elderly.
Patients and methods: A total of 23 older adults were randomized to either the HLE or NMES group for ten therapy sessions. They received pre- and post-therapy surface electromyography (sEMG) during water swallowing.
Results: For the HLE group, duration of suprahyoid muscles activity was significantly reduced at post-intervention compared to pre-intervention (p=0.036). Moreover after treatments, duration and latency between onset and peak amplitude of suprahyoid muscles activity was significantly shorter in the HLE group compare to the NMES group (respectively, p=0.007 and p=0.003).
Conclusion: Our findings suggest that the HLE, against the NMES, may be effective in reducing some aging effects on the suprahyoid muscles activity, especially in elders who demonstrate prolonged duration and latency between onset and peak of the suprahyoid muscles activity during swallowing.
Keywords: deglutition, aging, electromyography, exercise, electric stimulation therapy
Introduction
The process of physiological aging is associated with a decline in structure and function.1,2 Age-related changes cause incoordination of muscle activity3,4 and impair functional performance.5 Swallowing physiology has also been reported to exhibit several changes in advanced ages, including a longer delay in triggering the pharyngeal swallow,6–8 slower pharyngeal contraction wave,6 reduced maximum vertical and anterior hyoid movement during swallows,6,9 and less width of cricopharyngeal opening.6,10 Furthermore, the electromyographic (EMG) studies have shown an increase in duration of swallowing muscles activity,11–13 and in swallowing variability11,14 with increasing age. A decline in amplitude of EMG activity of the submental muscle group during volitional swallows has also been reported in the advanced ages.11 These physiologic changes in deglutition along with age-related diseases put the older individuals at higher risk of developing dysphagia.6 Epidemiologic studies have shown that the prevalence of oropharyngeal dysphagia15 and also aspiration pneumonia16 increases with advancing age. Roy et al17 reported dysphagia in 37.6% of elderly who live independently. Moreover, clinical signs of dysphagia have been reported in 55% of community dwelling older adults admitted to the hospital with a diagnosis of pneumonia. The prevalence of malnutrition and mortality at 30 days and 1-year follow-up have also been shown to be higher in the admitted patients who demonstrated signs of dysphagia.15
Decreased hyolaryngeal excursion, due to weak muscles,18,19 can decrease swallowing safety by increasing the risk of aspiration and have been commonly reported among patients with dysphagia and also asymptomatic older adults.6,10 The suprahyoid muscles, including the digastrics, geniohyoid, mylohyoid, and stylohyoid muscles, have been shown to elevate the hyolaryngeal complex in a superior and anterior pattern during swallowing.20 This movement facilitates opening the upper esophageal sphincter (UES) during swallowing.21 Considering this well recognized contribution of submental muscle activity to elevation of hyolaryngeal complex and opening of the UES,20 the clinical techniques have focused on strengthening the submental muscles.10,21–23 One of the most famous techniques in this field is the head lift exercise (HLE), commonly known as the Shaker exercise, which was introduced to strengthen the suprahyoid muscles in dysphagic patients.10,19,24
Previous research has shown that after 6 weeks of the HLE, asymptomatic older adults1,4,10 and also patients with dysphagia19,25 demonstrate a significant increase in anterior laryngeal1,4,10,19 and hyoid bone1,4 excursion and in UES opening.1,4,10,19,25 Post-swallow aspiration has also been reported to be significantly decreased following completion of the exercise regimen.19,25 With these promising findings, the HLE was recommended to prevent or diminish the swallowing changes in the advanced ages.4 Researches1,19and clinical experiences,26 however, show that completing the HLE period can be difficult for patients and even for healthy older adults. Neck soreness, dizziness and fatigue are introduced as reasons for discontinuation of the regimen.1 Moreover, back pain and cervical spine conditions may limit performance of the exercise. Easterling et al1 reported that about one fourth of the healthy elderly withdrew from participation in the exercise program, generally in the first 2 weeks. They also demonstrated that only 50% and 70% of their participants attained the isokinetic and isometric goals of the HLE exercise, respectively.1 Therefore, some researchers22,23,26,27–29 were encouraged to develop easy- to-perform exercises which can result in positive effects comparable to those of the HLE.
In addition to voluntary muscle contractions, neuromuscular electrical stimulation (NMES) can also induce muscle strengthening30 and improve muscle function in the elderly.31 The use of NMES to improve swallowing function has received increasing attention in the last few decades. It has been shown to be safe and well tolerated by most patients.32,33 Several NMES protocols have been studied to enhance the swallowing function in patients with swallowing disorders.32–34,35–37 Some studies have also explored how biomechanical aspects of swallowing are affected by NMES in healthy participants.18,38,39
The HLE and most NMES protocols were designed to strengthen the suprahyoid muscles.4,10,19,35 However, against NMES which is well tolerated by most individuals,32,33 performing the HLE has been found to be challenging specially for the elderly.1,19,26 To explore an effective and easy to perform alternative to the HLE, therefore, the present study aimed to investigate and compare the effects of a period of suprahyoid muscles’ NMES and a similar period of the HLE on swallowing muscles activity in healthy elderly. We hypothesized that the same period of NMES training and the HLE exercise would result in comparable gains in function of swallowing muscles. If the equal gains are observed following the NMES protocol, it will be recommended to those older individuals who find the HLE physically challenging. Moreover although gains in some biomechanical aspects of swallowing function were documented following the HLE4,10,19,25 and NMES training,18,33,35,37 their effects on the neuromuscular parameters of the swallow are less studied.18,26,40,41 Knowing the exact effects of both the HLE and NMES on swallowing neuromuscular characteristics would provide insights into the future directions of management and prevention of swallowing disorders.
Materials and methods
Participants
A total of 108 community dwelling older adults who attended a health care center to receive routine elderly medical care were interviewed in order to assess for eligibility to participate in this pilot clinical study. The volunteers were included if they desired to involve in such a health program and didn’t have neck pain; neck skin wound; malignancy; oral anatomical abnormalities; and any history of neurological disorders, head and neck cancer, and difficulties in swallowing. Moreover, because dysphagia is more prevalent among aging adults, they were also clinically assessed using the Oral Pharyngeal and Clinical Swallowing Examination42,43 to ensure that their swallowing function is normal. A volunteer was not recruited to participate in the study if he/she reported a history of swallowing problems or other diseases affecting swallowing function, and also if the performed examination indicated swallowing difficulties. Finally, 23 individuals were assigned to the HLE (n=12) or NMES group (n=11) based on a simple randomization method. A reluctance to participate in such a preventive health program (47%) and chronic neck pain (22%) were the most prevalent causes which limit our sample size. The study was approved by the medical research ethics committee of Tehran University of Medical sciences (IR.TUMS.REC.1394.2180) and all participants provided written informed consent. This study was conducted in accordance with the Declaration of Helsinki.
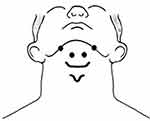 | Figure 1 Placement of electrodes used for NMES application.Abbreviation: NMES, neuromuscular electrical stimulation. |
Description of the therapies
Both treatment groups participated in ten therapy sessions over 2 weeks; five consecutive days per week. The HLE were prescribed for a 6-week period in the literature.1,4,10,19 We, however, found that most of the volunteers were not motivated to participate in such a long period of preventive exercise program. Moreover, Easterling et al1 showed that about one fourth of healthy older volunteers withdrew from the HLE program, generally in the first 2 weeks. They suggested further studies to determine the necessary minimum duration of the HLE to achieve an improvement in swallowing function.1 We therefore chose a 2-week period, as a modified exercise protocol.
The HLE protocol has been well described in previous studies.10,19,25 Briefly, the isometric portion of the HLE consists of three sustained 60-second head lifts in the supine position with a 60-second rest period between each trial. This portion is followed by 30 consecutive repetitions of head-lifts without holding (isokinetic part of the HLE). The exercise protocol was performed 3 times in each daily session. Easterling et al1 showed that during the first 2 weeks of the HLE program, only 30% and 52% of healthy older adults attained the isometric and isokinetic goals of the exercise, respectively. Therefore we expected that our volunteers might not be able to successfully achieve the goals during 2 weeks. So each participant was instructed about the HLE goals and encouraged to perform the exercise completely. However if he/she was not able to perform the isometric part of the exercise completely, the numbers of seconds for each sustained head lift were recorded in a logbook which had been provided to write down details of exercise performance. They were also allowed to take a brief rest (a few seconds) between the repetitions during the isokinetic part, whenever they needed. Therefore, the participants of this study performed a modified and more flexible form of the HLE protocol.
The subjects in the NMES group received surface electrical stimulation applied to the suprahyoid muscles at rest for 10 daily sessions over 2 weeks. Electrode placement was according to Kim and Han.38 Two paired electrodes (self-adhesive SKINTACT Ag/AgCl electrodes, with a diameter of about 10 mm, Leonhard Lang Ltd, Stroud, Gloucestershire, UK) were used to stimulate the muscles bilaterally. The hyoid bone was palpated and the active electrodes were attached to midpoints between the chin and the edges of the hyoid bone. The reference electrodes were placed at midpoints between the chin and the bilateral mandibular angles35,38,44,45 (Figure 1). Electrical stimulation was supplied using a dual-channel electrotherapy device (FarMed, Tehran, Iran). Currents with high frequency have been shown to produce stronger contractions and facilitate muscular strength.46 Therefore, a pulse rate of 100 HZ with duration of 1 ms (with a duty cycle of 10) was used. In order to increase the participants’ tolerance level, stimulus duration was set at 7 seconds and 8-second resting intervals were given after each stimulus. The intensity of stimulation was adjusted according to the participant's tolerance. The maximal tolerable intensity, as described by Kim and Han,38 was applied for 15-minutes without any additional exercise. Each participant received three 15-minutes sets of stimulation a day with 5-minute rest intervals given between stimulation sets to avoid muscle fatigue. The stimulus intensity was adjusted for each channel independently.
All therapy sessions in both groups were conducted and supervised by the same speech pathologist. Prior to beginning each therapy session, the participants were asked about the relevant experienced complaints during the last day.
Pre- and post-intervention evaluations
A few days before beginning of sessions and immediately after completing the therapies, surface EMG of the swallowing muscles was performed during water swallow. Activity of supra- and infrahyoid muscle groups during swallowing were simultaneously investigated. The EMG signals were recorded using two pairs of surface silver/silver chloride electrodes. Center to center distance of the electrodes was 20 mm47 and the signals were passed through a differential amplifier (sampling frequency =1000 Hz, input impedance>10,000,000 ohms, CMRR>96 dB, bandwidth=20–450Hz) (Biometrics Ltd NOS.SX230, Ynysddu, Wales, UK).
The first pair of electrodes was placed beneath the chin midway between the mandible and the hyoid bone, 1 cm from midline to the right.47 The second pair was placed approximately 2 cm lateral to the thyroid cartilage on the left side of the neck47,48 (Figure 2). The reference electrode was attached on the left wrist. Medical adhesive tape was used to increase the electrode-to-skin contact.
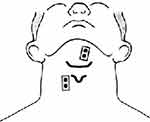 | Figure 2 Placement of sEMG electrodes on the supra- and infrahyoid muscles.Abbreviation: sEMG, surface electromyography. |
The participants sat comfortably on a chair and test instructions were reviewed. After the electrode placement, sEMG data were obtained during three swallow trials, each consisting of 13.5 mL of water. Each trial was performed to the examiner’s command. We chose the volume of 13.5 mL as the mean of normal bolus volume for older adults (ages 61–70 years) based on Vaiman et al.13 The same volume of water was used for all the trials.
The obtained raw signals were saved and coded for subsequent blind analysis. DataLog PC Software (Version 7.50) was used to obtain the rectified and smoothed EMG signals using the root mean square (RMS) method (with the time constant of 50 ms) (Figure 3).The onset and offset of each swallowing event was determined based on 10% of the peak amplitude as a threshold level. The “onset” of swallow was defined as a point where the EMG trace exceeded the threshold level and the “offset” was determined as the point at which the trace returned to below the threshold level. The mean amplitude, duration, and also latency between onset and peak amplitude within the swallow events49 were measured from each sEMG channel. The mean amplitude of each swallowing event was normalized to the mean amplitude of a 500 milliseconds period of the baseline before the swallow. All sEMG data were recorded and analyzed by the same rater. For each participant, sEMG measures were averaged from three recorded trials.
Statistical analysis
Statistical analyses were performed using SPSS statistical software (version 19.0; SPSS Inc., IBM Corporation, Armonk, NY, USA) and statistical significance was set at p≤0.05. The normality of data distribution for all variables was demonstrated by the Shapiro-Wilk’s test and then two-way mixed analyses of variance (ANOVA) were performed on the variables. The mean age of participants was compared between the groups using an independent-samples t-test.
Results
Of the 23 enrolled participants, two subjects in the HLE group withdrew from the therapy (one for an obligatory trip, and the other because of neck pain that she believed limited her daily activities). One subject in the NMES group completed therapy and assessments but he is excluded due to the low quality of sEMG signals of both supra- and infrahyoid muscles. Overall, 20 participants (ten HLE, ten electrotherapy), therefore, completed therapy sessions and received both pre- and post-therapy electromyographic studies (Figure 4). The mean age of the participants was 67.35±6.10 years (range: 60–81 years), and 90% were women. The mean age was not significantly different between the two groups (69.1±6.89 in the HLE group vs 65.6±5.19 in the NMES group, p=0.22).
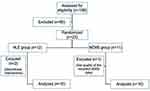 | Figure 4 Subject dropout.Abbreviations: HLE, head lift exercise; NMES, neuromuscular electrical stimulation. |
During the 2-week period of the HLE, five participants experienced neck muscle soreness and one experienced dizziness. Headache and mild to severe stomachache were also reported, each by two subjects. One participant reported mild pain or discomfort in the chest and one experienced back pain. Participants in the NMES group also reported some complaints. A mild irritation of the skin, globus sensation, and mild mandibular and anterior teeth pain were reported, each by one participant.
For all sEMG measures of the supra- and infrahyoid muscles, the data was normally distributed (p>0.05) and there were homogeneity of variances (p>0.05) and covariances (p>0.001), as assessed by Levene’s test of homogeneity of variances and Box’s M test, respectively. Table 1 demonstrated the sEMG measures of the suprahyoid muscles before and after treatments in both groups. There was no statistically significant interaction between the treatment and time on mean amplitude of the suprahyoid muscles activity, F(1, 17) =0.020, p=0.88. The main effect of time did not show a statistically significant difference in mean of mean amplitude of the suprahyoid muscles activity at the different time points, F(1, 17) =0.529, p=0.477. The main effect of group also showed that there was not a significant difference in mean of this measure between intervention groups F(1, 17) =0.707, p=0.412. Regarding duration of and latency between onset and peak amplitude of the suprahyoid muscles activity, there were statistically significant interactions between the treatment and time, respectively F(1, 17) =4.698, p=0.045; and F(1, 17) =5.376, p=0.033. At the baseline, there were not significant differences in duration of and latency between onset and peak amplitude of the suprahyoid muscles activity between groups, respectively F(1, 17) =0.342, p=0.57; and F(1, 17) =0.132, p=0.72. After treatment, significant differences in duration of and latency between onset and peak amplitude of the suprahyoid muscles activity between groups were observed, respectively F(1, 17) =9.214, p=0.007; and F(1, 17) =11.627, p=0.003. For the HLE group, duration of the suprahyoid muscles activity was significantly reduced at post-intervention compared to pre-intervention, F(1, 8) =6.326, p=0.036. But for the NMES group, duration of the suprahyoid muscles activity was not significantly different between pre- and post-intervention time points, F(1, 9) =0.973, p=0.35. There were not statistically significant effects of time on latency between onset and peak amplitude of the suprahyoid muscles activity for both groups, F(1, 8) =3.33, p=0.106 for the HLE group; and F(1, 9) =2.42, p=0.155 for the NMES group.
 | Table 1 The mean ± standard deviation of sEMG parameters of the suprahyoid muscle activity before and after treatment |
The sEMG measures of the infrahyoid muscles before and after treatments in both groups are presented in Table 2. There were no statistically significant interactions between the treatment and time on mean amplitude of, duration of, and latency between onset and peak amplitude of the infrahyoid muscles activity, respectively F(1, 17) =0.535, p=0.474; F(1, 17) =2.24, p=0.153; and F(1, 17) =0.910, p=0.353. The main effect of time did not show statistically significant differences in these measures at the different time points, respectively F(1, 17) =0.925, p=0.35; F(1, 17) =0.211, p=0.652; and F(1, 17) =0.632, p=0.438.The main effect of group also showed that there were no significant differences in the mean of these measures between intervention groups, respectively F(1, 17) =0.006, p=0.94; F(1, 17) =0.021, p=0.886; and F(1, 17) =0.850, p=0.369.
 | Table 2 The Mean±standard deviation of sEMG parameters of the infrahyoid muscle activity before and after treatment |
Discussion
The present study aimed to compare the efficacy of two dysphagia interventions developed to strengthen the suprahyoid muscles. A total of 23 healthy older adults were randomized to one of the two therapy groups: the HLE and the NMES for a 2-week period. The two groups were compared on the electromyographic features of the supra- and infrahyoid muscles during swallowing.
Pain and discomfort was reported by the majority of subjects who performed the HLE, whilst a few subjects in the NMES group reported a few mild complications. Furthermore, in the HLE group one person withdrew from the therapy due to severe neck pain. These findings are in agreement with studies1,26 reporting that completing the HLE period is difficult for patients and even for healthy older adults and warning that these difficulties may reduce the clinical feasibility of the HLE.
Our results indicated that none of the therapies changed the mean amplitude of the suprahyoid muscles activity during swallowing. Although the HLE was developed to strengthen the suprahyoid muscles during swallowing, Mishra et al26 also observed no effects over a 6-week period of the HLE on peak amplitude of the submental muscles activity during swallowing in healthy young adults. In the current study, we used 13.5 mL of water which has been described as the mean of normal bolus volume for the older adults (ages 61–70).13 The studied swallowing task, therefore, can be considered as a submaximal task for healthy older adults which does not need significantly more activation of the suprahyoid muscles.26
Physiological changes, such as reduced movement velocity,56 longer duration of the submental,11,12 infrahyoid and orbicularis oris activity during swallowing12 have been reported in advanced ages. The results of the current study indicate that a short period of the HLE can decrease duration of the suprahyoid muscles activity during swallowing in the healthy older adults. It means the subjects who performed the HLE could complete the swallow task with the reduced total activity time of the laryngeal elevator muscles. It shows a faster recruitment of the motor units in the suprahyoid muscles.11 Ding et al48 and also Ertekin et al50 showed a strong temporal relationship between laryngeal elevation and the submentals EMG activity during swallowing. Crary et al21 also found a significant relatively strong correlation51 between total duration of hyoid movement and the submentals EMG signal (r=0.539).21 Azola et al52 also demonstrated that duration of submental muscles activity during Mendelsohn Maneuver is moderately correlated with both duration of maximum hyoid elevation (r=0.423) and with duration of laryngeal vestibule closure (r=0.430). Furthermore, Alfonsi et al53 who observed a significantly prolonged duration of suprahyoid sEMG activity during swallowing in patients with multiple sclerosis (MS) compared to controls, showed a positive correlation between duration of suprahyoid sEMG activity and Penetration-Aspiration Scale scores (PAS).55 Given the prolongation of duration of suprahyoid sEMG activity in aging and also the reported relationship between the suprahyoid sEMG activity and the events of pharyngeal swallowing, it can probably be concluded that the HLE by decreasing total duration of the suprahyoid sEMG activity has diminished some aging effects on the swallowing physiology. In contrast to the HLE, the NMES could not reduce duration of the suprahyoid muscles activity during swallowing and a significant between-group difference in duration of the suprahyoid muscles activity during swallowing was therefore observed after the different therapies. This finding may be considered in line with the videofluoroscopic findings of Nam et al.35 They showed that repeated sessions of electrical stimulation on the suprahyoid muscles don’t increase the velocity of hyolaryngeal elevation.
Although neither of the therapies significantly changed the latency between onset and peak amplitude of the suprahyoid muscles activity, this measure after the NMES was significantly longer than after the HLE. A shorter latency between onset and peak amplitude of the suprahyoid muscles activity in subjects doing the HLE compared to those receiving the NMES may indicate that the muscles could reach the maximum level of needed activation in a shorter time.
Swallowing includes coordinated and simultaneous function of different muscle groups.57 Although the treatments studied here were designed to strengthen the hyolaryngeal elevator muscles, we hypothesized the change in the hyolaryngeal elevators function will affect the hyolaryngeal depressors and so the overall swallowing function. However no significant changes in the sEMG parameters of the infrahyoid muscles activity during swallowing were observed after treatments. One possible explanation for this finding could lie in the short duration of treatments in this study. A 2-week period of strengthening techniques on the suprahyoid muscles may not have been able to change the physiology so much that it affects myoelectrical activity of the other related muscles. Therefore further studies with longer duration of treatments are needed to investigate the infrahyoid muscles’ response to techniques designed for strengthening the hyolaryngeal elevator muscles.
Our study has a number of methodological limitations. Although more than 100 older adults were assessed for eligibility for this study, only a small sample size finally participated and completed the treatments and evaluations. We need therefore to be cautious in interpreting data. The reluctance of elderly subjects to participate in such a preventive health program was the most common reason that led to the small sample size.54 In addition, our participants performed only a short period of the exercise and most of them were not able to attain the performance goals of the HLE during this period, especially in the isometric portion. However, it’s worth noting that even this short and more flexible protocol of the HLE seems to be able to reduce some age-related changes in swallowing physiology in healthy older adults. Nonetheless, given that the detraining effects have been shown to occur more rapidly than the training effects,58 further research is needed to evaluate the long-term maintenance of the observed changes following cessation of the exercise. Also considering the short treatment duration in this study, investigating effects of longer periods of performing the studied therapies on the myoelectrical activity of swallowing muscles also merits further research. The results may be different after a longer duration of treatments. In addition, as mentioned before, electromyographic measures of hyolaryngeal muscles were of interest in the current study. However, if biomechanical aspects of swallowing had also been evaluated via videofluoroscopy, we would have reached more comprehensive conclusion regarding comparative effects of the HLE and NMES in the elderly. Also given lack of a control group in the current study and evaluating the electromyographic activity during only one bolus consistency and volume, the findings should be interpreted with caution.
Conclusion
Although the short and flexible protocol of the head lift exercise did not affect the mean amplitude of the muscles activity, it did result in a significant decrease in the duration of the suprahyoid muscles activity during water swallowing in healthy older adults. Significant shorter duration and shorter latency between onset and peak amplitude of the suprahyoid muscles activity were also observed in subjects performing the HLE compared to those receiving the NMES. These findings suggest that the HLE may be more effective than the NMES in reducing some aging effects on the suprahyoid muscles activity, especially in elders who demonstrate prolonged duration and latency between onset and peak of the suprahyoid muscles activity during swallowing. However future studies with larger sample sizes, which evaluate different aspects of swallowing function in different bolus volumes and consistencies, may be needed to further confirm these findings. On the other hand, considering difficulties associated with performance of this valuable exercise by the elderly, further studies are needed to modify the HLE protocol to make it easier to perform.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Easterling C, Grande B, Kern M, Shaker R. Attaining and maintaining isometric and isokinetic goals of the Shaker exercise. Dysphagia. 2005;20(2):133–138. doi:10.1007/s00455-005-0004-2
2. Seidler RD, Bernard JA, Burutolu TB, et al. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34(5):721–733. doi:10.1016/j.neubiorev.2009.10.005
3. Booth FW, Weeden SH, Tseng BS. Effect of aging on human skeletal muscle and motor function. Med Sci Sports Exerc. 1994;26(5):556–560.
4. Easterling C. Does an exercise aimed at improving swallow function have an effect on vocal function in the healthy elderly? Dysphagia. 2008;23(3):317–326. doi:10.1007/s00455-008-9158-z
5. Caserotti P. Strength training in older adults: changes in mechanical muscle function and functional performance. Open Sports Sci J. 2010;3(1):62–66. doi:10.2174/1875399X01003010062
6. Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. J Speech Hear Res. 2000;43(5):1264.
7. Rademaker AW, Pauloski BR, Colangelo LA, Logemann JA. Age and volume effects on liquid swallowing function in normal women. J Speech Lang Hear Res. 1998;41(2):275–284.
8. Robbins J, Hamilton JW, Lof GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103(3):823.
9. Alfonsi E, Cosentino G, Mainardi L, et al. Electrophysiological investigations of shape and reproducibility of oropharyngeal swallowing: interaction with bolus volume and age. Dysphagia. 2015;30(5):540–550.
10. Shaker R, Kern M, Bardan E, et al. Augmentation of deglutitive upper esophageal sphincter opening in the elderly by exercise. Am J Physiol Gastrointest Liver Physiol. 1997;272(6):G1518–G1522. doi:10.1152/ajpgi.1997.272.6.G1518
11. Aydoğdu İ, Kiylioğlu N, Tarlaci S, Pehlivan M, Ertekin C. Physiological changes in oropharyngeal swallowing with age: an electrophysiological study. J Neurol Sci Turk. 2007;24(2):144–154.
12. Ding R, Logemann JA, Larson CR, Rademaker AW. The effects of taste and consistency on swallow physiology in younger and older healthy individuals – a surface electromyographic study. J Speech Lang Hear Res. 2003;46(4):977–989. doi:10.1044/1092-4388(2003/076)
13. Vaiman M, Eviatar E, Segal S. Surface electromyographic studies of swallowing in normal subjects: a review of 440 adults. Report 1. Quantitative data: timing measures. Otolaryngol Head Neck Surg. 2004;131(4):548–555. doi:10.1016/j.otohns.2004.03.013
14. Poorjavad M, Talebian S, Ansari NN, Soleymani Z. Surface electromyographic assessment of swallowing function. Iran J Med Sci. 2017;42(2):194.
15. Cabre M, Serra-Prat M, Palomera E, Almirall J, Pallares R, Clavé P. Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing. 2009;39(1):39–45. doi:10.1093/ageing/afp100
16. Rofes L, Arreola V, Almirall J, et al. Diagnosis and management of oropharyngeal dysphagia and its nutritional and respiratory complications in the elderly. Gastroenterol Res Pract. 2010;2011.1–13.
17. Roy N, Stemple J, Merrill RM, Thomas L. Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann Otol Rhinol Laryngol. 2007;116(11):858–865. doi:10.1177/000348940711601112
18. Park J, Oh J, Lee HJ, Park S, Yoon T, Kwon BS. Effortful swallowing training coupled with electrical stimulation leads to an increase in hyoid elevation during swallowing. Dysphagia. 2009;24(3):296–301. doi:10.1007/s00455-008-9205-9
19. Shaker R, Easterling C, Kern M, et al. Rehabilitation of swallowing by exercise in tube-fed patients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology. 2002;122(5):1314–1321.
20. Pearson WG
21. Crary MA, Groher ME. Biomechanical correlates of surface electromyography signals obtained during swallowing by healthy adults. J Speech Hear Res. 2006;49(1):186. doi:10.1044/1092-4388(2006/015)
22. Watts CR. Measurement of hyolaryngeal muscle activation using surface electromyography for comparison of two rehabilitative dysphagia exercises. Arch Phys Med Rehabil. 2013;94:2542–2548. doi:10.1016/j.apmr.2013.04.013
23. Yoon WL, Khoo JKP, Liow SJR. Chin tuck against resistance (CTAR): new method for enhancing suprahyoid muscle activity using a Shaker-type exercise. Dysphagia. 2014;29(2):243–248. doi:10.1007/s00455-013-9502-9
24. Antunes EB, Lunet N. Effects of the head lift exercise on the swallow function: a systematic review. Gerodontology. 2012;29(4):247–257. doi:10.1111/j.1741-2358.2012.00638.x
25. Logemann JA, Rademaker A, Pauloski BR, et al. A randomized study comparing the Shaker exercise with traditional therapy: a preliminary study. Dysphagia. 2009;24(4):403–411. doi:10.1007/s00455-009-9217-0
26. Mishra A, Rajappa A, Tipton E, Malandraki GA. The recline exercise: comparisons with the head lift exercise in healthy adults. Dysphagia. 2015;30(6):730–737. doi:10.1007/s00455-015-9651-0
27. Yoshida M, Groher ME, Crary MA, Mann GC, Akagawa Y. Comparison of surface electromyographic (sEMG) activity of submental muscles between the head lift and tongue press exercises as a therapeutic exercise for pharyngeal dysphagia. Gerodontology. 2007;24(2):111–116. doi:10.1111/j.1741-2358.2007.00164.x
28. Kraaijenga SA, van der Molen L, Stuiver MM, Teertstra HJ, Hilgers FJ, van den Brekel MW. Effects of strengthening exercises on swallowing musculature and function in senior healthy subjects: a prospective effectiveness and feasibility study. Dysphagia. 2015;30(4):392–403.
29. Kraaijenga SAC, van der Molen L, Stuiver MM, et al. Efficacy of a novel swallowing exercise program for chronic dysphagia in long-term head and neck cancer survivors. Head Neck. 2017;39(10):1943–1961. doi:10.1002/hed.24710
30. Glinsky J, Harvey L, van Es P. Efficacy of electrical stimulation to increase muscle strength in people with neurological conditions: a systematic review. Physiother Res Int. 2007;12(3):175–194. doi:10.1002/pri.375
31. Caggiano E, Emrey T, Shirley S, Craik RL. Effects of electrical stimulation or voluntary contraction for strengthening the quadriceps femoris muscles in an aged male population. J Orthop Sports Phys Ther. 1994;20(1):22–28. doi:10.2519/jospt.1994.20.1.22
32. Freed ML, Freed L, Chatburn RL, Christian M. Electrical stimulation for swallowing disorders caused by stroke. Respir Care. 2001;46(5):466–474.
33. Rofes L, Arreola V, Lopez I, et al. Effect of surface sensory and motor electrical stimulation on chronic poststroke oropharyngeal dysfunction. J Neurogastroenterol Motil. 2013;25(11):888–e701. doi:10.1111/nmo.12211
34. Bülow M, Speyer R, Baijens L, Woisard V, Ekberg O. Neuromuscular electrical stimulation (NMES) in stroke patients with oral and pharyngeal dysfunction. Dysphagia. 2008;23(3):302–309.
35. Nam HS, Beom J, Oh B, Han TR. Kinematic effects of hyolaryngeal electrical stimulation therapy on hyoid excursion and laryngeal elevation. Dysphagia. 2013;28(4):548–556. doi:10.1007/s00455-013-9465-x
36. Oh B, Kim D, Paik N. Recovery of swallowing function is accompanied by the expansion of the cortical map. Int J Dev Neurosci. 2007;117(9):1215–1227. doi:10.1080/00207450600936254
37. Park J, Kim Y, Oh J, Lee H. Effortful swallowing training combined with electrical stimulation in post-stroke dysphagia: a randomized controlled study. Dysphagia. 2012;27(4):521–527. doi:10.1007/s00455-012-9403-3
38. Kim SJ, Han TR. Effect of surface electrical stimulation of suprahyoid muscles on hyolaryngeal movement. Neuromodulation. 2009;12(2):134–140. doi:10.1111/j.1525-1403.2009.00200.x
39. Kim SH, Oh B, Han TR, Jeong HJ, Sim YJ. Different movement of hyolaryngeal structures by various application of electrical stimulation in normal individuals. Ann Rehabil Med. 2015;39(4):535–544. doi:10.5535/arm.2015.39.4.535
40. Suiter DM, Leder SB, Ruark JL. Effects of neuromuscular electrical stimulation on submental muscle activity. Dysphagia. 2006;21:56–60. doi:10.1007/s00455-005-9010-7
41. Eddy BS. The Effects of Neuromuscular Electrical Stimulation Training on the Electromyographic Power Spectrum of Suprahyoid Musculature: 2015. Available from:
42. Daniels SK, Brailey K, Priestly DH, Herrington LR, Weisberg LA, Foundas AL. Aspiration in patients with acute stroke. Arch Phys Med Rehabil. 1998;79(1):14–19.
43. Daniels SK, McAdam CP, Brailey K, Foundas AL. Clinical assessment of swallowing and prediction of dysphagia severity. Am J Speech Lang Pathol. 1997;6(4):17. doi:10.1044/1058-0360.0604.17
44. Beom J, Oh B, Choi KH, et al. Effect of electrical stimulation of the suprahyoid muscles in brain-injured patients with dysphagia. Dysphagia. 2015;30:1–7.
45. Beom J, Kim SJ, Han TR. Electrical stimulation of the suprahyoid muscles in brain-injured patients with dysphagia: a pilot study. Ann Rehabil Med. 2011;35(3):322–327. doi:10.5535/arm.2011.35.3.322
46. Dreibati B, Lavet C, Pinti A, Poumarat G. Influence of electrical stimulation frequency on skeletal muscle force and fatigue. Ann Phys Rehabil Med. 2010;53(4):266–277. doi:10.1016/j.rehab.2010.03.004
47. Archer SK, Garrod R, Hart N, Miller S. Dysphagia in duchenne muscular dystrophy assessed objectively by surface electromyography. Dysphagia. 2012;28:1–11.
48. Ding R, Larson CR, Logemann JA, Rademaker AW. Surface electromyographic and electroglottographic studies in normal subjects under two swallow conditions: normal and during the Mendelsohn manuever. Dysphagia. 2002;17(1):1–12.
49. Crary MA, Baldwin BO. Surface electromyographic characteristics of swallowing in dysphagia secondary to brainstem stroke. Dysphagia. 1997;12(4):180–187. doi:10.1007/PL00009534
50. Ertekin C, Pehlivan M, Aydoǧgdu I, Ertaş M, Uludaǧ B, Çelebi G. PS-24-6 An electrophysiological investigation of deglutition in man. Electroencephalogr Clin Neurophysiol. 1995;97(4):S143. doi:10.1016/0924-980X(95)92952-I
51. Cohen J. Statistical Power Analysis for the Behaviors Science.
52. Azola AM, Greene LR, Taylor-Kamara I, Macrae P, Anderson C, Humbert IA. The relationship between submental surface electromyography and hyo-laryngeal kinematic measures of Mendelsohn Maneuver duration. J Speech Lang Hear Res. 2015;58(6):1627–1636.
53. Alfonsi E, Bergamaschi R, Cosentino G, et al. Electrophysiological patterns of oropharyngeal swallowing in multiple sclerosis. Clin Neurophysiol. 2013;124(8):1638–1645.
54. Bloch F, Charasz N. Attitudes of older adults to their participation in clinical trials: a pilot study. Drugs Aging. 2014;31(5):373–377.
55. Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–98.
56. Buckles VD. Age-related slowing. In Stelmach GE, Hömberg V (eds) Sensorimotor Impairment in the Elderly. NATO ASI Series (Series D: Behavioural and Social Sciences); 1993;75:73–87.
57. Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81(2):929–969.
58. Burkhead LM, Sapienza CM, Rosenbek JC. Strength-training exercise in dysphagia rehabilitation: principles, procedures, and directions for future research. Dysphagia. 2007;22:251–256.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

