Back to Journals » Drug Design, Development and Therapy » Volume 16
Effects of the Different Doses of Esketamine on Postoperative Quality of Recovery in Patients Undergoing Modified Radical Mastectomy: A Randomized, Double-Blind, Controlled Trial
Authors Zhu M, Xu S, Ju X, Wang S, Yu X
Received 9 October 2022
Accepted for publication 7 December 2022
Published 16 December 2022 Volume 2022:16 Pages 4291—4299
DOI https://doi.org/10.2147/DDDT.S392784
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Min Zhu, Siqi Xu, Xia Ju, Shengbin Wang, Xitong Yu
Department of Anesthesiology, The Anqing Medical Center of Anhui Medical University, The Fifth Clinical Medical College of Anhui Medical University, Anqing, People’s Republic of China
Correspondence: Xia Ju, Department of Anesthesiology, The Anqing Medical Center of Anhui Medical University, The Fifth Clinical Medical College of Anhui Medical University, Anqing, 246003, People’s Republic of China, Tel +86 13685563136, Email [email protected]
Purpose: This study aims to investigate the effects of the different doses of esketamine on postoperative quality of recovery in patients undergoing modified radical mastectomy.
Methods: Ninety-nine female patients were randomly allocated to three groups: the low-dose esketamine group (group E1) (0.5 mg/kg loading, 2 μg/kg/h infusion), the high-dose esketamine group (group E2) (0.5 mg/kg loading, 4 μg/kg/h infusion), the control group (group C) (received normal saline). The primary outcome was the quality of recovery-15 (QoR-15) scores on postoperative day 1 (POD1) and days 3 (POD3). The secondary outcomes were the sleep quality scores on POD1, bispectral index (BIS) value at 10, 30, and 60 min after operation, numeric rating scale (NRS) pain scores within 24 h after surgery, nausea, vomiting, drowsiness, nightmare, and intraoperative awareness.
Results: The total QoR-15 scores were higher in group E1 and group E2 than in group C on POD1 and POD3 (P< 0.05). The sleep quality scores on POD1 and BIS value at 10, 30, and 60 min after operation were higher in group E1 and group E2 than in group C (P< 0.05). The NRS pain scores at 2, 4 and 6 h after surgery in group E1 and at 2, 4, 6, 12 and 24 h after surgery in group E2 were lower than in group C (P< 0.05). The NRS pain scores at 6, 12 and 24 h after surgery in group E2 were lower than in group E1 (P< 0.05). The incidence of drowsiness was higher in group E1 and group E2 than in group C (P< 0.05).
Conclusion: Esketamine infusion improved to some extent the quality of recovery on POD1 and POD3 in patients undergoing modified radical mastectomy, especially 4 μg/kg/h esketamine was better, but the BIS value and incidence of drowsiness were significantly increased.
Keywords: esketamine, postoperative quality of recovery, radical mastectomy
A Letter to the Editor has been published for this article.
A Response to Letter by Dr Cheng has been published for this article.
Introduction
Breast cancer is one of the most common malignant tumors in women with a high mortality rate. Modified radical mastectomy is recommended as most effective clinical treatment options for breast cancer. Due to the large extent of surgical resection, nerve injury and inflammatory stimulation, most of the patients undergoing modified radical mastectomy were experienced postoperative pain after surgery, the inadequate pain management affects quality of life and causes reduced physical function,1,2 so adequate pain control is necessary for improving the quality of recovery after surgery.
Opioids have been widely used for the postoperative analgesia due to their powerful analgesic effect. However, opioids also inevitably produce some adverse effects, such as respiratory depression, nausea, vomiting, and postoperative hyperalgesia.3 With the development of the concept of opioid-free anesthesia (OFA), multimodal analgesia is considered the optimal strategy for postoperative pain management through nerve blockade4,5 and non-opioid medications.6–8
Ketamine is an antagonist of the N-methyl-D-aspartate (NMDA) receptor, which has a powerful sedative and analgesic effects for clinical anesthesia for many years. Esketamine, the right-handed split, has a faster metabolism, stronger potency, and fewer side effects.9,10 Some evidence states that ketamine or esketamine administration has an analgesic effect.11–13 Currently, existing evidence has shown that esketamine via intranasal way is more effective and safe than via intravenous way for major resistant depression disorder because the pharmacokinetics of nasal spray and intravenous administration were similar, but the former had a greater antidepressant effect and less overall adverse reactions.14–16 In addition, several studies showed that esketamine promoted postoperative recovery by reducing postoperative pain, and there was no significant difference in the incidence of postoperative nausea and vomiting.17–19 Therefore, we hypothesized that the esketamine infusion provides better the quality of recovery in patients undergoing modified radical mastectomy.
Materials and Methods
The current study was approved by the Ethics Committee of Anqing Municipal Hospital and prospectively registered at www.clinicaltrials.gov (NCT05289440, date of registration: March 19, 2022). All methods were performed in accordance with the relevant guidelines and regulations in our present study. We enrolled 99 female patients who received elective modified radical mastectomy under general anaesthesia from March 2022 to September 2022. Written informed consent was obtained from all patients. The inclusion criteria included the American Society of Anesthesiologists (ASA) physical status I (A normal healthy patient) and II (A patient with mild systemic disease), and aged 32–72 years. The exclusion criteria were as follows: severe pulmonary hypertension, arrhythmia, liver and kidney dysfunction, uncontrolled hypertension, take analgesics and sedatives recently, history of chronic pain, psychosocial abnormalities, history of alcohol abuse, pregnant, and lactating.
Patients were randomly divided into 3 groups using computer-generated random numbers. The low-dose esketamine group (group E1) received 0.5 mg/kg esketamine diluted with normal saline to 20 mL by intravenous injection over 1 minute before surgical incision, followed by at a rate of 2 µg/kg esketamine diluted with normal saline to 20 mL every hour until closure of surgical incisions; the high-dose esketamine group (group E2) received 0.5 mg/kg esketamine diluted with normal saline to 20 mL by intravenous injection over 1 minute before surgical incision, followed by at a rate of 4 µg/kg esketamine diluted with normal saline to 20 mL every hour until closure of surgical incisions; the control group (group C) received equal volume of normal saline. All participants including enrolled patients, anesthesiologists, surgeons, and the follow-up personnel were kept blind to the drug and the group assignments.
All patients were abstained from food and water 6 hours before surgery. After entering the operation room, the non-operative peripheral vein was opened and the sodium lactate Ringer injection was infused. Routine monitoring, including systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), peripheral pulse oximeter (SpO2), electrocardiogram (ECG), temperature, and end-tidal CO2 pressure (PetCO2), bispectral index (BIS) were performed after the patients entered the operation room.
Patients in the three groups received inhalation of pure oxygen (100%) 3 minutes before induction of anesthesia, followed by intravenous administration of dexamethasone 10 mg and penehyclidine 0.5 mg. The induction of anesthesia was as follows: midazolam 0.05 mg/kg, sufentanil 0.4 µg/kg, etomidate 0.3 mg/kg, and cisatracurium 0.15 mg/kg. Flurbiprofen 0.1 mg/kg was injected before skin incision. After loss of corneal and palpebral reflexes, BIS<60 and muscle relaxation was perfect enough to achieve intubation conditions, endotracheal intubation was performed with video laryngoscope (endotracheal intubation diameter was 6.5–7.0 mm, distance from incisor 21–23 cm). After successful intubation, respiratory parameters such as tidal volume and respiratory rate were set at 6–8 mL/kg and 12–14 beat/min (bpm) to maintain the PetCO2 between 35 and 45 mmHg during the intraoperative period, respectively. Remifentanil and propofol were continuously infused at a rate of 0.15 µg/kg/min and 4–6 mg/kg/h during the anesthesia period, respectively. BIS values were kept between 45 and 75 by adjusting the infusion dose of propofol during the anesthesia period. Atropine (0.5 mg) was intravenously given when the HR<50 beats/min. Ephedrine (6 mg) was intravenously given when the mean blood pressure (MBP)<60 mmHg. During the operation period, 1–2 mg cis-atracurium was injected intermittently, and sufentanil was injected intermittently 5–10 µg according to the hemodynamic parameters to maintain the blood pressure and HR at 20% of the basal value. At the end of the operation, the infusion of propofol and remifentanil was stopped. And the endotracheal tube was removed and transferred to the post-anesthesia care unit (PACU) after the patient was naturally awake. If the postoperative numeric rating scale (NRS) pain score>3, non-steroidal anti-inflammatory drugs could be given for remedial analgesia.
Primary and Secondary Outcomes
Anesthesiologist who was not participated in this study evaluated the total postoperative recovery quality scores based on the QoR-15 scale on POD1 and POD3. The QoR-15 questionnaire is composed of 15 questions, including physical comfort (5 items), emotional state (4 items), physical independence (2 items), psychological support (2 items), and pain (2 items). The higher of the QoR-15 scores, the better of the quality of recovery after surgery (range is 0 to 150 points).20
NRS pain scores were assessed for all patients at 2, 4, 6, 12 and 24 h after surgery (0 points: painless; 1–3 points: mild pain; 4–6 points: moderate pain; 7–10 points: severe pain). The quality of sleep was evaluated using a 10-point rating scale (0 = terrible sleep, 10 = excellent sleep).21 If severe nausea requiring antiemetics and retching or vomiting (greater than or equal 2 times) occurred, ondansetron 0.1 mg/kg was given intravenously or metoclopramide 10 mg was injected intramuscularly. BIS values were recorded at 10, 30 and 60 min after the surgical incision. The adverse effects, including nausea, vomiting, drowsiness, nightmares, and intraoperative awareness were also recorded.
Statistical Analysis
Based on our pilot study, the results indicated that the mean values of total QoR-15 scores were 106.2, 110.5, and 116.2 in the three groups on POD1; the standard deviations (SD) were 6.8, 8.2, and 10.5, respectively. The sample size was calculated by PASS 11.0. Eventually, we selected 33 patients in each group with a power of 0.9 and an α of 0.05, allowing for a 10% drop-out rate.
We completed statistical analyses based on SPSS v.20 (IBM Corp., Armonk, NY, USA) in the present study. Categorical data analysis were adopted χ2 or Fisher’s exact test as appropriate and were presented as numbers. Continuous data were evaluated normality and homogeneity using Kolmogorov–Smirnov test and Levene’s test, respectively. Normally distributed data were expressed as the mean (SD) and were analyzed by one-way analysis of variance (ANOVA). When a significant difference was found among the three groups, Dunnett’s T3 analysis was performed. Nonparametric distribution of data were expressed as median (interquartile range [IQR]) and analyzed by the Kruskal–Wallis tests. The P value <0.05 was viewed as statistical significance.
Results
A total of 105 patients were enrolled in this study. Five patients did not conform to inclusion criteria. One patient did not consent to participate research. Eventually, 33 patients were included in each group. Three patients had an intraoperative HR<50 beats/min and were given atropine 0.5 mg intravenously. Thirteen patients had MBP<60 mmHg and were given ephedrine 6 mg intravenously. All patients did not injected sufentanil during the perioperative period. No awareness occurred in all patients during the intraoperative period (Figure 1).
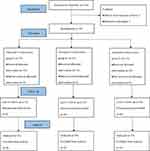 |
Figure 1 Consolidated Standards of Reporting Trials (CONSORT) flow diagram. Adapted from Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. Open Access.40 |
There were no significant differences in all three groups with regard to age, height, weight, body mass index (BMI), and ASA grade (Table 1).
 |
Table 1 Clinical Characteristics of All Patients |
The QoR-15 Scores on POD1 and POD3 and Sleep Quality Scores Between Groups
Compared with group C, The total QoR-15 scores were significantly higher on POD1 and POD3 in group E1 and group E2 (P<0.001, P<0.001, P = 0.016, P<0.001, respectively). The total QoR-15 scores were the highest in group E2 on POD1 and POD3. There were no significant differences with regard to total QoR-15 scores at POD1 and POD3 in group E1 and group E2 (P = 0.263 and P = 0.221). The sleep quality scores were higher on POD1 in group E1 and group E2 compared to group C (P = 0.001 and P<0.001). The sleep quality scores were no significant differences between group E1 and group E2 (P = 0.230) (Table 2).
 |
Table 2 The Comparison of QoR-15 Scores and Sleep Quality Scores Between Groups |
The BIS Values at Any Time Points After Surgical Incision
The BIS values were higher in group E1 and group E2 than in group C at 10, 30, and 60 min after surgical incision (P<0.001, P<0.001, P<0.001, P<0.001, P<0.001, P<0.001, respectively). The BIS values were no significant differences in group E1 and group E2 10, 30, and 60 min after surgical incision (P = 0.890, P = 0.993, P = 0.398) (Table 3).
 |
Table 3 The BIS Values at Any Time Points After Surgical Incision |
The NRS Pain Scores During the First 24 h After Surgery
The NRS scores were lower at 2, 4 and 6 hours after surgery in group E1 than in group C (P<0.001, P = 0.003, P = 0.027, respectively), the NRS pain scores were lower at 2, 4, 6, 12 and 24 hours after surgery in group E2 than in group C (P<0.001, P<0.001, P<0.001, P<0.001, P = 0.001, respectively); the NRS pain scores were lower at 6, 12 and 24 hours after surgery in group E2 than in group E1 (P = 0.027, P = 0.003, P = 0.042). The NRS scores were no significant differences at 2 and 4 hours after operation in group E1 and group E2 (P = 0.317 and P = 0.103). The NRS scores were also no significant differences at 12 and 24 hours after surgery in group C and group E1 (P = 0.181 and P = 0.727) (Table 4).
 |
Table 4 The NRS Pain Scores During the First 24 h After Surgery |
The Incidence of Adverse Effects at 24 h After Surgery and Remedial Treatment
The incidence of drowsiness was higher in group E1 and group E2 than in group C (P<0.001 and P<0.001). There were no significant differences with respect to drowsiness between the group E1 and group E2 (P = 0.378). Compared with group C, postoperative nausea, vomiting, nightmares, intraoperative awareness, rescue analgesia, and anti-emetics required were not statistically significant in groups E1 and E2 (all P>0.05) (Table 5).
 |
Table 5 The Incidence of Adverse Effects at 24 h After Surgery and Remedial Treatment |
Discussion
The results of our study indicated that esketamine infusion especially 4 µg/kg/h esketamine provided better quality of recovery 1 and 3 days after surgery in patients undergoing modified radical mastectomy. In addition, our results also found that 4 µg/kg/h esketamine administration significantly alleviated postoperative 24 h intensity of pain, but esketamine infusion could increase BIS values and the incidence of drowsiness.
Modified radical mastectomy is one of the method for treatment of breast cancer, most of patients usually experience postoperative acute pain, which may be affect endocrine function, immune function, and lead to some adverse events such as atelectasis and pneumonia.22,23 Acute pain after surgery affects quality of recovery, moreover, if it is not adequately controlled, it may be develop into chronic pain, which affects quality of life of patients.24 Miziara et al reported that S(+)-ketamine infusion alleviated postoperative pain and reduced morphine requirement undergoing laparoscopic cholecystectomy.25 Su et al found that esketamine administration significantly decreased the dosage of remifentanil and reduced the incidence of severe pain with liver tumor ablation.26 However, Brinck et al revealed that intraoperative S(+)-ketamine administration did not alleviate postoperative pain and oxycodone consumption undergoing major lumbar fusion surgery.27 In the present study, our results indicated that low-dose esketamine infusion relieved NRS pain scores during the first postoperative 6 h period and high-dose esketamine alleviated NRS pain scores during the first postoperative 24 h period. It might be related to the analgesia property of esketamine infusion in a dose-dependent manner. In addition, the results of our study also showed that high-dose esketamine further decreased postoperative pain intensity at 6, 12, and 24 h after surgery. It might be associated with high-dose esketamine infusion prolong the analgesic time and inhibit opioid-induced hyperpathia.
BIS value is usually used for monitoring the depth of anesthesia in clinical practice. Some studies found that ketamine administration could increase BIS value by affecting the relative power of slow wave (θ) and fast wave (γ). Therefore, BIS value can no longer objectively reflect the depth of anesthesia during the ketamine administration period.28,29 A study has proved that esketamine injection increase BIS value at 1 and 5 min after intubation, but it was not affect the intraoperative BIS value.10 In present study, we found that esketamine continuous infusion increased BIS value during the intraoperative period. The results of our study were inconsistent with the results of above-mentioned study, it might attribute to the various method of esketamine administration.
The QoR-15 questionnaire is sensitive and reliable tool, which is usually used for assessing the quality of recovery after surgery and easily complete in clinical practice.30,31 Zhao et al revealed that low-dose ketamine did not enhanced the overall quality of postoperative recovery undergoing breast cancer surgery, but it provided better emotional state and pain scores.32 Cheng et al indicated that S-ketamine administration improved quality of postoperative recovery, postoperative analgesia, and depression after operation in patients undergoing video-assisted thoracic surgery.19 In our study, we found that low-dose esketamine infusion was higher total QoR-15 scores on POD1 and POD3. Moreover, our results also demonstrated that high-dose esketamine infusion had the highest overall QoR-15 scores on POD1 and POD3. This suggested that esketamine infusion enhanced quality of recovery on POD1 and POD3 in patients undergoing modified radical mastectomy in a dose-dependent manner, which was associated with lower postoperative NRS pain scores and better the quality of sleep. On the contrary, Moro et al proved that ketamine administration did not enhanced the quality of postoperative recovery undergoing laparoscopic cholecystectomy.33 These inconsistent results might be associated with the dose of ketamine or esketamine, the type of surgery, and the duration of ketamine or esketamine.
Nausea and vomiting is the most common complication after surgery with general anesthesia. The higher incidence of postoperative nausea and vomiting affects the quality of recovery, which may be prolong the length of stay and decrease the patients’ satisfaction. Some studies showed that esketamine or S-ketamine did not the occurrence of postoperative nausea and vomiting.34–36 However, Brinck et al revealed that ketamine reduced the nausea and vomiting after operation.11 In the present study, our results showed that the different dose of esketamine did not decrease the incidence of postoperative nausea and vomiting. It might be attributed to the same rate of remifentanil during the perioperative period and non-steroidal anti-inflammatory drugs could be given for remedial analgesia. Racemic ketamine with an loading followed by continuous infusion (0.04–0.25 mg/kg/h) was used in most studies.37–39 Brinck et al indicated that intraoperative high-dose S-ketamine (0.5 mg/kg loading, 0.6 mg/kg/h infusion) administration had higher rate of sedation than low-dose S-ketamine (0.5 mg/kg loading, 0.12 mg/kg/h infusion).27 We selected esketamine with an loading (0.5 mg/kg) followed by continuous infusion (2 µg/kg/h and 4 µg/kg/h) was used in the present study. We also observed that esketamine administration significantly increased the incidence of drowsiness. It could be related to sedation effect of esketamine.
There are some limitations in our study. Firstly, remifentanil was continuous infusion at a rate of 0.15 µg/kg/min in the three groups during the anesthesia period, which did not reflect opioid-sparing effect of esketamine, therefore, we should adjust the rate of remifentanil based on changes of hemodynamic parameters. Secondly, esketamine may be cause hypertension and tachycardia, however, in the present study, we did not observe changes of hemodynamic parameters during the esketamine administration period. Thirdly, esketamine can produce dissociative symptoms and worsening of psychiatric symptoms (like anxiety or agitation), but we did not observe the incidence of anxiety or agitation. Fourthly, in the present study, we did not perform multivariate analysis for quality of the recovery, level of pain, and quality of sleep. Our results could be more forceful if we would have used multivariate analysis for quality of the recovery, level of pain, and quality of sleep. Hence we will perform multivariate analysis in the future study. Finally, we selected 33 cases in each group based on Power analysis of our preliminary results, but we thought that the sample size was small for clinical study. Therefore, we need large sample, multi-center, randomized, double-blinded controlled study to further explore effect of esketamine infusion on the quality of postoperative recovery in the future clinical research.
Conclusions
The different doses of esketamine infusion especially 4 µg/kg/h esketamine improved to some extent the quality of recovery 1 and 3 days after surgery, decreased the intensity of postoperative pain in patients undergoing modified radical mastectomy. However, esketamine administration could increase BIS values and the incidence of drowsiness.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
This study was supported by Clinical Research Fund of Hubei Chen Xiaoping Science and Technology Development Foundation (CXPJJH12000005-07-44).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bozzuto LM, Bartholomew AJ, Tung S, et al. Decreased postoperative pain and opioid use following prepectoral versus subpectoral breast reconstruction after mastectomy: a retrospective cohort study: pain after pre- versus subpectoral reconstruction. J Plast Reconstr Aesthet Surg. 2021;74(8):1763–1769. doi:10.1016/j.bjps.2020.12.009
2. Altıparmak B, Korkmaz Toker M, Uysal Aİ, Turan M, Gümüş Demirbilek S. Comparison of the effects of modified pectoral nerve block and erector spinae plane block on postoperative opioid consumption and pain scores of patients after radical mastectomy surgery: a prospective, randomized, controlled trial. J Clin Anesth. 2019;54:61–65. doi:10.1016/j.jclinane.2018.10.040
3. Mendonça FT, Pellizzaro D, Grossi BJ, Calvano LA, de Carvalho LSF, Sposito AC. Synergistic effect of the association between lidocaine and magnesium sulfate on peri-operative pain after mastectomy: a randomised, double-blind trial. Eur J Anaesthesiol. 2020;37(3):224–234.
4. Nedeljkovic SS, Kett A, Vallejo MC, et al. Transversus abdominis plane block with liposomal bupivacaine for pain after cesarean delivery in a multicenter, randomized, double-blind, controlled trial. Anesth Analg. 2020;131(6):1830–1839.
5. Yildiz M, Kozanhan B, Iyisoy MS, Canıtez A, Aksoy N, Eryigit A. The effect of erector spinae plane block on postoperative analgesia and respiratory function in patients undergoing laparoscopic cholecystectomy: a double-blind randomized controlled trial. J Clin Anesth. 2021;74:110403.
6. Ramli RA, Hassan WM, Ali S, Othman AK, Zaini RHM, Hassan MH. Comparison of combination between ketamine and parecoxib as multimodal preemptive analgesia with ketamine alone for elective laparotomy. Asian J Anesthesiol. 2021;59(4):161–168.
7. Murphy GS, Avram MJ, Greenberg SB, et al. Perioperative methadone and ketamine for postoperative pain control in spinal surgical patients: a randomized, double-blind, placebo-controlled trial. Anesthesiology. 2021;134(5):697–708.
8. Gasbjerg KS, Hägi-Pedersen D, Lunn TH, et al. Effect of dexamethasone as an analgesic adjuvant to multimodal pain treatment after total knee arthroplasty: randomised clinical trial. BMJ. 2022;376:e067325.
9. Wang J, Huang J, Yang S, et al. Pharmacokinetics and safety of esketamine in Chinese patients undergoing painless gastroscopy in comparison with ketamine: a randomized, open-label clinical study. Drug Des Devel Ther. 2019;13:4135–4144.
10. Li J, Wang Z, Wang A, Wang Z. Clinical effects of low-dose esketamine for anaesthesia induction in the elderly: a randomized controlled trial. J Clin Pharm Ther. 2022;47(6):759–766. doi:10.1111/jcpt.13604
11. Brinck EC, Tiippana E, Heesen M, et al. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Syst Rev. 2018;12(12):CD012033. doi:10.1002/14651858.CD012033.pub4
12. Lei Y, Liu H, Xia F, et al. Effects of esketamine on acute and chronic pain after thoracoscopy pulmonary surgery under general anesthesia: a multicenter-prospective, randomized, double-blind, and controlled trial. Front Med. 2021;8:693594. doi:10.3389/fmed.2021.693594
13. Wang Y, Zhang Q, Dai X, Xiao G, Luo H. Effect of low-dose esketamine on pain control and postpartum depression after cesarean section: a retrospective cohort study. Ann Palliat Med. 2022;11(1):45–57. doi:10.21037/apm-21-3343
14. Bahji A, Vazquez GH, Zarate CA. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2021;278:542–555. doi:10.1016/j.jad.2020.09.071
15. Canuso CM, Ionescu DF, Li X, et al. Esketamine nasal spray for the rapid reduction of depressive symptoms in major depressive disorder with acute suicidal ideation or behavior. J Clin Psychopharmacol. 2021;41(5):516–524. doi:10.1097/JCP.0000000000001465
16. Kryst J, Kawalec P, Pilc A. Efficacy and safety of intranasal esketamine for the treatment of major depressive disorder. Expert Opin Pharmacother. 2020;21(1):9–20. doi:10.1080/14656566.2019.1683161
17. Wang J, Wang Y, Xu X, Peng S, Xu F, Liu P. Use of various doses of S-ketamine in treatment of depression and pain in cervical carcinoma patients with mild/moderate depression after laparoscopic total hysterectomy. Med Sci Monit. 2020;26:e922028. doi:10.12659/MSM.922028
18. Brinck ECV, Virtanen T, Mäkelä S, et al. S-ketamine in patient-controlled analgesia reduces opioid consumption in a dose-dependent manner after major lumbar fusion surgery: a randomized, double-blind, placebo-controlled clinical trial. PLoS One. 2021;16(6):e0252626. doi:10.1371/journal.pone.0252626
19. Cheng X, Wang H, Diao M, Jiao H. Effect of S-ketamine on postoperative quality of recovery in patients undergoing video-assisted thoracic surgery. J Cardiothorac Vasc Anesth. 2022;36(8):3049–3056. doi:10.1053/j.jvca.2022.04.028
20. Shen Y, Lv F, Min S, et al. Impact of enhanced recovery after surgery protocol compliance on patients’ outcome in benign hysterectomy and establishment of a predictive nomogram model. BMC Anesthesiology. 2021;21(1):289. doi:10.1186/s12871-021-01509-0
21. Snyder E, Cai B, DeMuro C, Morrison MF, Ball W. A new single-item sleep quality scale: results of psychometric evaluation in patients with chronic primary insomnia and depression. J Clin Sleep Med. 2018;14(11):1849–1857. doi:10.5664/jcsm.7478
22. Kaiser U, Kopkow C, Deckert S, Sabatowski R, Schmitt J. Validation and application of a core set of patient-relevant outcome domains to assess the effectiveness of multimodal pain therapy (VAPAIN): a study protocol. BMJ Open. 2015;5(11):e008146. doi:10.1136/bmjopen-2015-008146
23. Prabhakar A, Mancuso KF, Owen CP, et al. Perioperative analgesia outcomes and strategies. Best Pract Res Clin Anaesthesiol. 2014;28:105–115.
24. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625.
25. Miziara LE, Simoni RF, Esteves LO, Cangiani LH, Grillo-Filho GF, Paula AG. Efficacy of continuous S(+)-ketamine infusion for postoperative pain control: a randomized placebo-controlled trial. Anesthesiol Res Pract. 2016;2016:6918327.
26. Su Y, Zhang J, Wang H, Gu Y, Ouyang H, Huang W. The use of Esketamine in CT-guided percutaneous liver tumor ablation reduces the consumption of remifentanil: a randomized, controlled, double-blind trial. Ann Transl Med. 2022;10(12):704.
27. Brinck ECV, Maisniemi K, Kankare J, Tielinen L, Tarkkila P, Kontinen VK. Analgesic effect of intraoperative intravenous S-ketamine in opioid-naïve patients after major lumbar fusion surgery is temporary and not dose-dependent: a randomized, double-blind, placebo-controlled clinical trial. Anesth Analg. 2021;132(1):69–79.
28. Ballesteros JJ, Huang P, Patel SR, Eskandar EN, Ishizawa Y. Dynamics of ketamine-induced loss and return of consciousness across primate neocortex. Anesthesiology. 2020;132:750–762.
29. Amat FM, Jensen AA, Plath N, Herrik KF, Celada P, Artigas F. Temporally dissociable effects of ketamine on neuronal discharge and gamma oscillations in rat thalamo-cortical networks. Neuropharmacology. 2018;137:13–23.
30. Chazapis M, Walker EMK, Rooms MA, Kamming D, Moonesinghe SR. Measuring quality of recovery-15 after day case surgery. Br J Anaesth. 2016;116(2):241–248.
31. Kleif J, Waage J, Christensen KB, Gögenur I. Systematic review of the QoR-15 score, a patient-reported outcome measure measuring quality of recovery after surgery and anaesthesia. Br J Anaesth. 2018;120(1):28–36.
32. Zhao Z, Xu Q, Chen Y, et al. The effect of low-dose ketamine on postoperative quality of recovery in patients undergoing breast cancer surgery: a randomised, placebo-controlled trial. Int J Clin Pract. 2021;75(12):e15010.
33. Moro ET, Feitosa IM, de Oliveira RG, et al. Ketamine does not enhance the quality of recovery following laparoscopic cholecystectomy: a randomized controlled trial. Acta Anaesthesiol Scand. 2017;61(7):740–748.
34. Zhang C, He J, Shi Q, Bao F, Xu J. Subanaesthetic dose of esketamine during induction delays anaesthesia recovery a randomized, double-blind clinical trial. BMC Anesthesiol. 2022;22(1):138.
35. Wang X, Lin C, Lan L, Liu J. Perioperative intravenous S-ketamine for acute postoperative pain in adults: a systematic review and meta-analysis. J Clin Anesth. 2021;68:110071.
36. Eberl S, Koers L, van Hooft J, et al. The effectiveness of a low-dose esketamine versus an alfentanil adjunct to propofol sedation during endoscopic retrograde cholangiopancreatography: a randomised controlled multicentre trial. Eur J Anaesthesiol. 2020;37(5):394–401.
37. Loftus RW, Yeager MP, Clark JA, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113:639–646.
38. Garg N, Panda NB, Gandhi KA, et al. Comparison of small dose ketamine and dexmedetomidine infusion for postoperative analgesia in spine surgery-A prospective randomized double-blind placebo controlled study. J Neurosurg Anesthesiol. 2016;28:27–31.
39. Kim SH, Kim SI, Ok SY, et al. Opioid sparing effect of low dose ketamine in patients with intravenous patient-controlled analgesia using fentanyl after lumbar spinal fusion surgery. Korean J Anesthesiol. 2013;64:524–528.
40. Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
