Back to Journals » Drug Design, Development and Therapy » Volume 16
Effects of Super-Activated Platelet Lysate on Early Healing of Tooth Extraction Sockets in Rats
Authors Guo X , Lu H, Liu C, Zhang Y, Bi L
Received 2 March 2022
Accepted for publication 30 June 2022
Published 13 July 2022 Volume 2022:16 Pages 2213—2227
DOI https://doi.org/10.2147/DDDT.S363766
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Xiaorui Guo,1,2 Huiying Lu,3 Chunxiang Liu,3 Yi Zhang,3 Liangjia Bi1
1Department of Stomatology, The Fourth Affiliated Hospital, Harbin Medical University, Harbin, 150001, People’s Republic of China; 2State Key Laboratory of Military Stomatology and National Clinical Research Center for Oral Diseases and Shaanxi Key Laboratory of Oral Diseases, Department of Operative Dentistry and Endodontics, School of Stomatology, The Fourth Military Medical University, Xi’an, People’s Republic of China; 3National and Local Joint Stem Cell Research & Engineering Center for Aging Diseases, Tian Qing Stem Cell Co., Ltd, Harbin, 150028, People’s Republic of China
Correspondence: Liangjia Bi, Department of Stomatology, The Fourth Affiliated Hospital, Harbin Medical University, Harbin, 150001, People’s Republic of China, Tel/Fax +86 451 82576565, Email [email protected]
Purpose: To evaluate the effect of super-activated platelet lysate (sPL) on wound healing of tooth extraction sockets in rats.
Methods: Rat models of the tooth extraction socket were established. Thirty-six rats were divided into control and sPL groups and sacrificed on days 7, 14, and 28 after tooth extraction. Bone formation in tooth extraction sockets were observed by microscopic computed tomography (micro-CT) and hematoxylin and eosin (HE) staining; osteoprotegerin (OPG), receptor activator of nuclear factor kappa-&Bgr; ligand (RANKL), interleukin 6(IL-6), and tumor necrosis factor-alpha (TNF-α) proteins were detected by immunohistochemistry; and chemokine and osteogenic gene expressions were detected by polymerase chain reaction (PCR).
Results: sPL accelerated soft tissue wound healing in the extraction socket of rats. Micro-CT showed that the amount of bone formation and bone volume fraction were higher in the sPL group than the control 14 days after extraction. HE staining showed promotion of the formation of bony trabeculae by sPL in the apical third of the extraction socket 7 days after extraction and more mature and organized bony trabeculae in the sPL group than the control 14 days after extraction; mature bony trabeculae filling most of the fossa with lesser bone porosity in the socket in the sPL group than the control 28 days after extraction. Immunohistochemistry showed that sPL induced OPG expressions 7 and 14 days after tooth extraction but did not affect the RANKL expression while transiently promoting the IL-6 expression 7 days after extraction. PCR showed that sPL promoted chemokine expressions 7 and 14 days after extraction. The expressions of osteogenesis-related factors were higher in the sPL group than the control 7 and 28 days after extraction, while the opposite trend was observed 14 days after extraction.
Conclusion: sPL has a transient pro-inflammatory effect and promotes soft tissue healing and bone formation during early wound healing of extraction sockets in rats.
Keywords: sPL, blood product, wound healing, bone formation, cytokines
Introduction
In dental science, the following conditions require tooth extraction: periodontitis, malposed third molars, dental trauma, orthodontic treatment, tumors, and other local or systemic factors. Early complications, such as pain, excessive bleeding, and dry socket, often occur because of damage to local tissues and disruption of blood microcirculation. Long-term complications are due to persistent alveolar bone resorption or residual ridge resorption.1 Inflammation may exacerbate alterations in the local microenvironment, further enhancing bone resorption. The demand for esthetics and comfort in oral treatment is increasing, and dentists are required to design restorations matching the adjacent native dentition after tooth extractions. Adequate alveolar bone volume and complete alveolar ridge morphology are indispensable for achieving esthetic and functional reconstructions.2
Tooth extraction is followed by an orderly series of biological events, involving soft and hard tissue repair and regeneration, that ensure wound healing in extraction sockets and restoration of lost tissues.3,4 Signaling pathways are activated by spatially and temporally dependent interactions of cytokines, chemokines, and growth factors that regulate the expressions of genes and transcription factors, thereby determining the fate of cells in a healing environment.5
Biomaterials used to preserve the alveolar ridge after tooth extraction include bone grafts, barrier membranes, biological agents or matrices, such as bone morphogenic proteins (BMPs), enamel matrix protein, demineralized bone matrix, and their combinations.6–8 Grafts are at a risk of infection, presenting as delayed new bone formation.9,10 To reduce post-extraction complications, the development of a topical therapeutic agent capable of promoting bone regeneration and early wound healing in tooth extraction sockets is necessary.
Human blood is a source for many therapeutic products. The World Health Organization has recognized some blood products as “essential medicines.”11 Platelet concentrates (PCs) are a blood product with a higher platelet concentration than physiological whole blood. They are an innovative formulation in regenerative medicine and widely used in oral and maxillofacial surgery.12,13 The preparation protocols of PCs differ and lack standardization, resulting in differences in their composition and application. PCs have different nomenclatures, such as platelet-rich plasma (PRP), platelet-rich fibrin(PRF), and platelet lysate. Super-activated platelet lysate (sPL) is a novel platelet lysate obtained using a patented platelet-induced high activation culture technology. During preparation of sPL, residual solid cellular components and white cells are removed, while immunogenicity is reduced. sPL rapidly release highly concentrated bioactive factors, such as the platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), transforming growth factor-beta (TGF-β), BMP-2, BMP-4, BMP-6, interleukin (IL)-1, etc.14,15 As sPL can repair bone defects without activating platelets, they can be directly injected into the bone defect,16 and their easy manipulation and application have increased their use in orthopedics. The efficacy of sPL combined with allografts is superior to that of allografts alone and comparable to that of autogenous bone grafts. Therefore, sPL combined with allografts are an alternative therapy to repair long bone defects.17 sPL also prevent glucocorticoid-related femoral head necrosis by upregulating the expressions of osteogenic and angiogenic genes through autophagy.18
PCs have not shown reliable or consistent results in alveolar ridge preservation.19,20 Most previous studies were limited to the combined application of PC with other biomaterials and did not describe the method of PC preparation or biological factors. Further, studies on the effect of PC products alone on the alveolar sockets are lacking. Therefore, the aim of the present study was to explore the effects of the novel platelet lysate sPL on wound healing in noncritical bone defects, such as tooth extraction sockets, in the maxillofacial region.
Materials and Methods
Animals
We obtained 45 healthy male Sprague–Dawley rats from the animal experimental center of the Second Affiliated Hospital of Harbin Medical University, Harbin, People’s Republic of China. The rats were at an average age of 6 weeks and weighed 180–200 g. Nine rats were used for sPL preparation, and 36 rats underwent tooth extraction. Each rat was placed in a separate cage with free access to water and rat food and acclimatized to the environment for 7 days preoperatively.
sPL Preparation
According to the patented technical method described previously,21 10 mL of sPL was obtained from 90 mL blood harvested from the 9 rats. Blood was repeatedly centrifuged at low temperature at an ultra-high speed with the method conventionally used for platelet-rich lysates. Repeated freeze-thaw with patented cytokine culture technologies were used to activate and collect sPL.
Establishment of the Rat Tooth Extraction Model
A sterile 20× 60-mm gelatin sponge was removed from the outer tray, placed in a Petri dish, and cut in half (20×30-mm). Subsequently, 2 mL sPL or normal saline was uniformly dispensed over the entire gelatin sponge surface. Following 30 min of incubation at 37°C, the soaked gelatin sponge was cut into 3 mm× 5-mm segments (1 piece/defect) for subsequent use. Each segment contained 50 µL (42.375 mg) of sPL.
The 36 rats were randomly divided into sPL and control groups, with 18 rats per group. Under general anesthesia with ketamine (80 mg/kg) and dexmedetomidine (0.6 mg/kg), bilateral maxillary first molars were carefully extracted to avoid root fracture, and gelatin sponge segments (5 mm × 3 mm) absorbed with normal saline and sPL were placed into the extraction sockets in the control and sPL groups, respectively. Finally, the cyanoacrylate adhesive was used to close the surrounding mucosal tissue. (See the Supplement 1 for the schematic of the tooth extraction process.)
The extraction wounds in the sPL group were treated with 100 µL sPL (50 µL for the extraction wound and 25 µL each for the buccal and lingual sides of the extraction site) using a small 26-G needle every other day until complete closure of the mucosa overlying the extraction sites was achieved (approximately 14 days after tooth extraction). The extraction wounds in the control group were treated with normal saline simultaneously. The accommodation and diet of the rats in the sPL and control groups were the same.
The rats were sacrificed 7, 14, and 28 days after tooth extraction, with 6 rats sacrificed in each group at each time point. The alveolar fossa and process of the right maxilla were harvested immediately and stored in liquid nitrogen until the molecular analysis, while those of the left maxilla were fixed in 4% paraformaldehyde before micro-CT and histological analyses. The same operator (Xiaorui Guo) performed all these procedures.
Micro–CT Assessment
Micro-CT was performed 14 days after tooth extraction. The harvested specimens were scanned with micro-CT (SCANCO Medical AG) in high resolution using a tube current of 200 µA, voltage of 90 kV, and slice thickness of 20 µm. The maxillary specimens were reconstructed from two- and three-dimensional images with the center of the extraction socket defect as the region of interest. The percentage of bone present in the tooth extraction sockets was calculated using the bone volume fraction (bone volume divided by total tissue volume, BV/TV) and, trabecular number (Tb.N), trabecular thickness (Tb.Th), and trabecular separation (the distance between the two bony trabeculae, Tb.Sp). Quantitatively and statistically analyses were performed for the percentage of bone present in the sPL and control groups.
Histological Analysis
The maxillary specimens were harvested 7, 14, and 28 days after tooth extraction, fixed in 4% paraformaldehyde, and stored overnight at 4 °C. After rinsing the samples with phosphate-buffered saline (PBS), they were decalcified with a 10% ethylenediaminetetraacetic acid (EDTA) solution at 7.4 pH and stored at 4 °C for 6–8 weeks, depending on the degree of mineralization, with EDTA being renewed every 3 days. The samples were then dehydrated in a range of concentrations of ethanol, transferred to xylene, and embedded in paraffin. Sections of 4–5-µm thickness were cut in sagittal section along the long axis of the extraction socket using a slicer, stained with hematoxylin and eosin, and imaged at 40× and 100× magnifications with a light microscope (Olympus Manual System Microscope BX43; Olympus) fitted with a camera (Diagnostic Instruments).
Immunohistochemical Analysis
OPG, RANKL, IL-6, and TNF-α expressions were evaluated for 6 rats each on days 7, 14, and 28 after tooth extraction. Slides were obtained as described above. Sections were dewaxed with xylene, dehydrated with a graded series of alcohols, washed with PBS, and incubated in 3% H2O2 with deionized water to neutralize the endogenous peroxidase activity. Non-specific binding sites were blocked with 2% bovine serum albumin in PBS for 1 h. The sections were incubated overnight at 4°C with one of the following primary antibodies: goat anti-rat polyclonal OPG (1:100; Abcam), RANKL (1:100; Abcam), IL-6 (1:100; Abcam), or TNF-α (1:100; Abcam). After three rinses with PBS, the sections were incubated with the biotinylated anti-goat antibody for 30 min at 37 °C. Additionally, following three rinses with PBS again, the sections were incubated with the enhanced enzyme-conjugated goat anti-mouse IgG polymer for 20 min at 37 °C. Finally, following three rinses with PBS again, the sections were colored with the freshly configured DAB chromogenic solution, and the nuclei were restained with hematoxylin. The cells were placed in 1% hydrochloric acid alcohol differentiation solution and washed under running water. After placing in the solution returned to blue, hydrated with a graded series of alcohol, and transparent with xylene, the samples were sealed with neutral gum and observed under a microscope as follows:
The sections were placed under a microscope for double-blind observation. The whole section was observed at 100× magnification, and the OPG/RANKL/IL-6/TNF-α protein expression in the tooth socket was photographed. The criteria for determining a positive result for the OPG/RANKL/IL-6/TNF-α expression in the tooth socket were brownish yellow particles at the site of secreted proteins, with the plasma or cell membranes used as the positive criteria for staining. Quantitative and statistical analyses were performed for the OPG/RANKL/IL-6/TNF-α expression in the extraction socket.
Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
Gingival specimens were carved out from the alveolar crest above the healing site of the tooth extraction sockets to analyze chemokine expressions. The socket tissues occupying the alveolus were collected to analyze osteogenic gene expressions.
The TRIzol® reagent protocol (Invitrogen) was used to isolate the total mRNA, which was then reverse-transcribed. Table 1 lists the primer sequences and reference genes (β-actin). Reactions were performed using a 20-µL mixed system containing 7.2 µL of sterile distilled water, 10 µL of qPCR SYBR Green Master Mix (Yeasen Biotechnology), 0.8 µL of each gene-specific primer, and 2 µL of the cDNA template. Each sample was analyzed in three replicates. RT-qPCR was performed using the ABI Prism 7500 Real-Time PCR system (Applied Biosystems).
 |
Table 1 Primer List |
Statistical Analysis
The analysis of variance followed by the t-test was performed to analyze the differences. For non-normally distributed data, the Mann–Whitney U-test was performed. Data are expressed as mean ± standard deviation. A p-value < 0.05 indicates statistical significance. Statistical analyses were performed using GraphPad Prism 8.0.
Results
Micro-CT Results and Effect of sPL on Soft Tissue Healing in Extraction Wounds
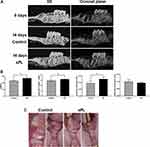
Micro-CT showed no bone deposition in the tooth extraction socket immediately after tooth extraction. Fourteen days after tooth extraction, micro-CT showed slightly more bone deposition and greater alveolar ridge height in the sPL group than in the control group (Figure 1A). The bone volume fraction (BV/TV; %) and trabecular number (Tb.N) and thickness (Tb.Th) of the tooth extraction socket were significantly higher in the sPL group than in the control group (p <0.05), but trabecular separation did not differ significantly between the two groups (Figure 1B).
Ten days after tooth extraction, most rats in the sPL group had reached complete closure of the extraction wound, while those in the control group had not (Figure 1C). Wound healing was approximately 2–4 days faster in the sPL group than in the control group.
Histological Findings
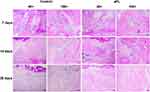
Seven days after tooth extraction, the tooth extraction socket in both groups showed no residual periodontal ligament, shrunken blood clots, and more fibroblasts. Both groups showed new trabecular bone formation, but the new trabecular bone was less abundant and discontinuous with fragility in the control group than in the sPL group (Figure 2). The new trabeculae bone in sPL-treated sockets transitioned from the apical third to the middle third of the root, was also found at the margin of the extraction socket, and radiated toward the center of the extraction socket. The margin of the extraction socket showed greater quantity and quality of the new trabeculae bone in the sPL group than in the control group (Figure 2) but was occupied by granulation tissue in the remaining area above the extraction socket in both groups.
Fourteen days after tooth extraction, no obvious interalveolar septum structure was observed in either group, and blood clots were scant. The bony trabeculae in maturation filled most of the extraction sockets, and sinus capillaries occasionally regenerated near the lateral wall of the alveolar fossa. Bone formation started from the middle third of the alveolar fossa and gradually transitioned to the alveolar ridge. The sPL group showed more organized trabecular bone and less trabecular separation in the alveolar fossa compared to the control group (Figure 2).
Twenty-eight days after tooth extraction, mature bony trabeculae filled most of the extraction sockets. Thick and clear bony trabeculae with a small pulp space and connective tissue islands were found in the alveolar fossa. The trabecular bones with medullary cavities, bone matrix, and cells did not differ significantly between the two groups, but bone porosity was less in the sPL group than in the control group (Figure 2).
Immunohistochemical Results for the Immunolabeling Patterns of OPG and RANKL
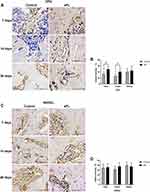
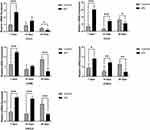
Seven days after tooth extraction, OPG staining was moderate-to-intense in the sPL group and mild to moderate in the control group. Fourteen days after tooth extraction, OPG staining was moderate-to-intense in the sPL group and mild in the control group. The quantitative analysis showed that 7 and 14 days after tooth extraction, OPG immunomarkers were higher in the sPL group than in the control group (p<0.05). Twenty-eight days after tooth extraction, OPG staining was moderate-to-intense in both groups. The quantitative analysis showed no statistical difference in OPG immunomarkers between the sPL and control groups (Figure 3A and B).
At all time points, RANKL staining was moderate in both groups. The quantitative analysis showed no significant difference in the RANKL expression between the sPL and control groups at any time point (Figure 3C and D).
Immunohistochemical Results for the Immunolabeling Patterns of IL-6 and TNF-α
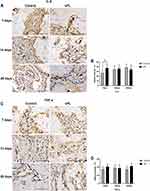
IL-6 and TNF-α stained positive in the cytoplasm and extracellular matrix. Seven days after tooth extraction, IL-6 staining was moderate in the sPL group and mild to moderate in the control group. The quantitative analysis showed a higher IL-6 expression in the sPL group than in the control group (p<0.05). On days 14 and 28 after tooth extraction, IL-6 staining was moderate in both groups. The quantitative analysis showed no significant differences in the IL-6 expression between the sPL and control groups (Figure 4A and B). At all time points, TNF-α staining was mild to moderate in both groups. The TNF-α expression did not differ significantly between the sPL and control groups at any time point (Figure 4C and D).
Effect of sPL on Chemokine Expressions During Wound Healing in the Tooth Extraction Socket
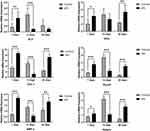
Chemokine expressions showed the effect of sPL on the immune response to tooth extraction (Figure 5). Seven days after tooth extraction, expressions of the chemokines CCL3, CCL5, CCR9, CCR10, and CXCL2 in the healing mucosa of the alveolar ridge above the extraction socket were 2.8-, 4.4-, 2.1-, 2.1-, and 2.6-folds higher in the sPL group than in the control group, respectively (p<0.05). 14 days after tooth extraction, the expression of the chemokine CCL3 or CCR9 did not differ significantly between the sPL and control groups, while those of the chemokines CCL5, CCR10, and CXCL2 were 4.6-, 2.7-, and 3.0-folds higher in the sPL group than in the control group, respectively (p<0.05). In contrast, 28 days after tooth extraction, expressions of the chemokines CCL3, CCL5, CCR9, CCR10, and CXCL2 were 2.1-, 1.4-, 3.4-, 2.6-, and 2.5-folds higher in the control group that in the sPL group, respectively.
Effect of sPL on Osteogenic Gene Expressions During Wound Healing in the Tooth Extraction Socket
Seven days after tooth extraction, expressions of the osteogenic factors ALP, OCN, COL-1, BMP-2, Runx2, and Osterix were 1.6-, 3.0-, 2.0-, 2.5-, 3.1-, and 3.0-folds higher in sPL group than in the control group, respectively (p<0.05). In contrast, 14 days after tooth extraction, expressions of the osteogenic factors ALP, COL-1, BMP-2, Runx2, and Osterix were 6.4-, 4.2-, 2.4-, 3.1-, and 2.9-folds higher in the control group than in the sPL group, respectively (p<0.05), while that of OCN did not differ significantly between the sPL and control groups. Twenty-eight days after tooth extraction, expressions of the osteogenic factors OCN, COL-1, BMP-2, Runx2, and Osterix were 2.3-, 3.0-, 1.7-, 5.4-, and 5.0-folds higher in the sPL group than in the control group (p<0.05), while that of ALP did not differ significantly between the two groups (Figure 6).
Discussion
In the present study, sPL has a transient pro-inflammatory effect and promotes soft tissue healing and bone formation during early wound healing of extraction sockets in rats. Soft tissue wound healing achieved in approximately 2 weeks after tooth extraction in rats.22 sPL can accelerate soft tissue wound healing in the extraction socket of rats by an average of 2–4 days. Platelet-derived factors can regulate wound healing in refractory skin ulcers and avoid skin flap necrosis by forming fibrin clots, promoting fibroblast proliferation, and up-regulating collagen synthesis in the early healing stage.23 Further, faster soft tissue wound healing provides a good internal environment homeostasis and protection for bone reconstruction in tooth extraction sockets.
The inflammatory response is transiently enhanced in the initial phase of regeneration by platelet lysates and is then subsided by the nuclear factor kappa B signaling pathway and cyclooxygenase-2.24,25 This explains the higher expression of IL-6 in the sPL group compared to the control group 7 days after tooth extraction but lack of significant difference between the two groups 14 or 28 days after tooth extraction. In other words, sPL enhanced the inflammatory response in the early stages of wound healing. Further, regulating inflammatory responses is key to tissue repair, as it induces initial tissue remodeling and allows recovery of tissue structure and function.26
The balance between osteoblasts and osteoclasts plays a crucial role in intra-tissue homeostasis and bone remodeling. Cytokines and growth factors regulate the proliferation, differentiation, and function of osteoblasts and osteoclasts and coordinate communication between them.27 After tooth extraction, healing of the alveolar fossa involves bone remodeling, resulting in significant volume contraction of the soft and hard tissues of the alveolar ridge horizontally and vertically. Bone grafts and barrier membranes can reduce these tissue changes for alveolar ridge preservation, but no graft material can inhibit bone remodeling in the alveolar sockets or completely prevent bone resorption after tooth extraction.4,28
The balance between OPG and RANKL determines the amount of new bone formation.29 The nuclear factor kappa B ligand receptor activator RANKL activates osteoclasts and stimulates bone resorption by binding to its receptor RANK. The osteoblast-produced OPG is a soluble antagonist of the RANKL/RANK binding that prevents osteoclast formation and bone resorption.30 In the present study, sPL induced OPG expression after tooth extraction but did not affect RANKL expression, consistent with Sumida et al’s and Yu et al’s studies.31,32 IL-6 is a regulator of RANKL/OPG that stimulates the differentiation of mesenchymal cells to the osteoblast lineage, acts as an anti-apoptotic agent of osteoblasts, and promotes bone formation.33 TNF-α enhances the RANKL expression in osteocytes and promotes the formation of osteoclasts.34 In the present study, the IL-6 expression was higher in the sPL group than in the control group in the early stage of wound healing. This finding suggests that IL-6 may be involved in early alveolar bone formation. However, the TNF-α expression did not differ between the two groups during wound healing. This finding suggests sPL did not elevate the RANKL expression by increasing the TNF-α expression, further explaining the increased OPG/RANKL ratio.
Platelet lysates resume the proliferation of quiescent osteoblasts without affecting their differentiation potential, but their removal returns osteoblasts to a state of no or slow division.25 During natural healing of the extraction socket, resting cells with osteogenic potential are exposed to platelet-releasing factors, resume proliferation, and participate in the bone repair cascade events. In the present study, in addition to the participation of endogenous platelets in wound healing, the administration of exogenous sPL amplified osteoblast activation, increased expressions of the cell cycle regulators, accelerated entry into the cell cycle, and enhanced osteoblast proliferation, differentiation, and bone formation. Therefore, 7 days after tooth extraction, histological results showed that a greater quantity and quality of the new trabecular bone had transitioned from the apical third to the middle third of the root in the sPL group compared to the control group and that the relative mRNA expressions of the osteogenic factors ALP, OCN, COL-1, Runx2, BMP-2, and Osterix were higher in the sPL group than in the control group.
However, with removal of platelet lysates, osteoblasts return to the state of no division or slow division.13 sPL treatment was stopped approximately 10 days after tooth extraction, possibly influencing the activity of osteoblasts. Due to the high activity of osteoblasts in the early stage of wound healing in the sPL group, to balance bone remodeling in the extraction socket, osteoclast differentiation was prompted when the number of osteocytes reached a specific limit.35 Osteoblasts in the sPL group responded more strongly to the osteocyte-generated or endocrine-activated signals compared to the control group, had a greater ability to recruit osteoclasts to the remodeling sites, showed enhanced osteoclast activity, and inhibited osteogenic differentiation. Therefore, mRNA expressions of the osteogenic factors were reduced in the sPL group than in the control group 14 days after tooth extraction. Due to the cumulative effect of osteogenic differentiation in the early stage, 14 days after tooth extraction, the sPL group showed more evident bone deposition on micro-CT and more mature bone structures in the alveolar fossa on hematoxylin and eosin staining compared to the control group.
Accumulated osteoclasts promote osteoblast differentiation and activity in addition to acting as bone resorbing cells.36,37 Therefore, 28 days after tooth extraction, the differentiation of osteoblasts was stronger than that of osteoclasts in the sPL group, and expressions of osteogenic factors were conversely higher in the sPL group than in the control group. Further, hematoxylin and eosin staining showed that most of the alveolar fossa had been filled with mature bony trabeculae at this time point but that bone porosity was less in the sPL group than in the control group.
Osteoclasts that interface the oral barrier tissues remain active in the alveolar crest during wound healing in the tooth extraction socket,38,39 and various cytokines and chemokines are involved in the immune response after tooth extraction.40 Therefore, chemokine expressions in the healing gingival tissue at the crest of the alveolar ridge should be investigated.
Activated platelets secrete various chemokines, such as CCL5, CXCL4, CXCL12, CXCL16, platelet factor 4, and stromal cell-derived factor-1α, which initiate and facilitate local inflammatory processes at the site of blood vessel injury.41,42 Therefore, in the early stage of wound healing in the tooth extraction socket, chemokines secreted by exogenous platelets increased because of repeated injections of sPL into the extraction socket, resulting in a significant difference from the control group. In addition, multiple injections of sPL increased fibrin, also leading to increased chemokine expressions in the local environment. Nevertheless, fibrin is an exogenous activator of the bone remodeling unit, which may be conducive to bone resorption.43 The local effects of fibrin accumulation on bone healing and specific role of increased chemokines in alveolar bone reconstruction should be further investigated.
The content of growth factors in human platelet lysates obtained using the sPL preparation method proposed by Huang et al were higher than those obtained by other preparation methods,18,44 results are consistent with Huang in our other study (unpublished). PDGF initiates wound healing through chemotaxis and mitosis of mesenchymal stem and connective tissue cells with production of the cell adhesion molecule fibronectin and participates in angiogenesis by promoting the proliferation of vascular endothelial cells.45 VEGF binds to the relevant receptors expressed on endothelial cells, inducing a cellular response that releases matrix metalloproteinases, digesting the surrounding extracellular matrix and allowing vascular endothelial cells to migrate and proliferate, which are essential for neointimal formation.46 TGF-β1 and TGF-β2 stimulate fibroblast chemotaxis, produce collagen and fibronectin, inhibit collagen degradation by reducing protease and increasing the protease inhibitor, and participate in the apoptosis and inhibition of osteoclasts to accelerate bone regeneration.47 FGF stimulates the signal transmission of bone marrow mesenchymal stem cells to the osteogenic pathway, particularly the formation of osteoblasts.48 IGF, which plays a role in the later stages of bone maturation and remodeling, is derived from osteocytes, endothelial cells, and platelets, and its secretion may be stimulated by BMPs.49 The BMP family is a powerful osteo-inducible protein family related to bone formation and regeneration. sPL induces bone formation probably owing to BMP-2 and BMP-6, which stimulate mesenchymal stem cells to initiate osteoblast differentiation and calcification.50,51
The platelet lysate preparation method is not uniform or standardized, and platelet counts and centrifugation settings vary across studies, resulting in wide variations in the biochemical and functional properties of platelet lysate preparations, with potential implications for therapeutic efficacy and safety in certain clinical conditions. In this present study, sPL was prepared using a patented method to induce and activate sPL using a biofactor culture technique. The activation of CaCl2 results in a less concentrated and more persistent fibrin matrix, which slows the release of growth factors. Therefore, sPL contain more growth factors compared to PRP or platelet lysates. Leukocytes can be harmful because of release of pro-inflammatory and catabolic mediators, such as metalloproteinases.52 The removal of leukocytes, platelet fragments, and immunoglobulins from sPL reduces concerns of immunogenicity and allows for allogeneic application. Cryopreservation of sPL allows for future use, while PRP and platelet-rich fibrin are temperature-sensitive mixtures and cannot be stored below 4°C.
The gelatin sponge is used as a carrier for sPL and acts as a hemostatic and clot-fixing agent. Similar to PRP,53 sPL shows a sharp release of growth factors in the first 12 h, accounting for approximately 25% of the total release, and reduced release of growth factors to approximately half of the initial release after 24 h. Therefore, in the present study, local injections of sPL were required at 1-day intervals during wound healing in the extraction sockets until complete wound healing was achieved to enhance the effect of sPL.
In the future, we aim to explore a standardized method for sPL preparation, considering the use of healthy donor pools to minimize individual differences, maximize consistency and reproducibility of sPL product composition, and ensure that growth factor levels are within the optimal range. The use of allogeneic sPL for “off-the-shelf” application would reduce the psychological burden on patients with trypanophobia. The development of an sPL slow-release system would ensure that the delivery rate of growth factors remains constant over a long time period (eg, 28 days) to enhance the biological effects of sPL.
Conclusions
In the present study, sPL did not alter bone remodeling in the extraction sockets but transiently promoted inflammation, soft tissue healing, and osteoblast differentiation, thus promoting bone formation in the early stages of wound healing in the tooth extraction sockets of rats.
Ethics Approval Statement
Animal care and experimental protocols were performed in according to the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications Number 8023, revised 1978). The protocol was approved by the Experimental Animal Ethics Committee of Harbin Medical University.
Acknowledgments
This study was funded by the Natural Science Foundation of China [grant number 81670994].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Klemetti EA. review of residual ridge resorption and bone density. J Prosthet Dent. 1996;75(5):512–514. doi:10.1016/s0022-3913(96)90455-2
2. Zaidi M, Moonga BS, Abe E. Calcitonin and bone formation: a knockout full of surprises[J]. J Clin Invest. 2002;110(12):1769–1771. doi:10.1172/JCI17425
3. Pagni G, Pellegrini G, Giannobile WV, Rasperini G. Postextraction alveolar ridge preservation: biological basis and treatments. Int J Dent. 2012;2012:151030. doi:10.1155/2012/151030
4. Araújo MG, Silva CO, Misawa M, Sukekava F. Alveolar socket healing: what can we learn? Periodontol 2000. 2015;68(1):122–134. doi:10.1111/prd.12082
5. Berglundh T, Abrahamsson I, Lang NP, Lindhe J. De novo alveolar bone formation adjacent to endosseous implants. Clin Oral Implants Res. 2003;14:251–262. doi:10.1034/j.1600-0501.2003.00972.x
6. Deppe H, Stemberger A. Effects of laser-modified versus osteopromotively coated titanium membranes on bone healing: a pilot study in rat mandibular defects. Lasers Med Sci. 2004;18(4):190–195. doi:10.1007/s10103-003-0279-1
7. Oryan A, Alidadi S, Moshiri A, Bigham-Sadegh A. Bone morphogenetic proteins: a powerful osteoinductive compound with non-negligible side effects and limitations. BioFactors. 2014;40(5):459–481. doi:10.1002/biof.1177
8. Daigo Y, Daigo E, Fukuoka H, Fukuoka N, Ishikawa M, Takahashi K. Wound healing and cell dynamics including mesenchymal and dental pulp stem cells induced by photobiomodulation therapy: an example of socket-preserving effects after tooth extraction in rats and a literature review. Int J Mol Sci. 2020;21(18):18. doi:10.3390/ijms21186850
9. Rakhmatia YD, Ayukawa Y, Furuhashi A, Koyano K. Carbonate apatite containing statin enhances bone formation in healing incisal extraction sockets in rats. Materials. 2018;11(7):12. doi:10.3390/ma11071201
10. Roffi A, Matteo DB, Krishnakumar GS, Kon E, Filardo G. Platelet- rich plasma for the treatment of bone defects: from pre-clinical rational to evidence in the clinical practice. A systematic review. Int Orthop. 2017;41(2):221–237. doi:10.1007/s00264-016-3342-9
11. World Health Organization model list of essential medicines. Geneva, Switzerland: WHO; 2019. Available from:https://www.who.int/medicines/publications/essentialmedicines/en/.
12. Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet- rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009;17(10):602–608. doi:10.5435/00124635-200910000-00002
13. Nguyen VT, Nardini M, Ruggiu A, Cancedda R, Descalzi F, Mastrogiacomo M. Platelet lysate induces in human osteoblasts resumption of cell proliferation and activation of pathways relevant for revascularization and regeneration of damaged bone. Int J Mol Sci. 2020;21(14):20. doi:10.3390/ijms21145123
14. Andia I, Abate M. Platelet-rich plasma: underlying biology and clinical correlates. Regen Med. 2013;8(5):645–658. doi:10.2217/rme.13.59
15. Huang Z, Wang W, Wang Q, et al. Coaxial nanofiber scaffold with super- active platelet lysate to accelerate the repair of bone defects. RSC Adv. 2020;10(59):35776–35786. doi:10.1039/d0ra06305c
16. Strandberg G, Sellberg F, Sommar P, et al. Standardizing the freeze-thaw preparation of growth factors from platelet lysate. Transfusion. 2017;57(4):1058–1065. doi:10.1111/trf.13998
17. Wang Q, Huang Z, Huang X, Zhang T, Wang W. Reparative effect of super active platelet combined with allogeneic bone for large bone defects. Artif Organs. 2021;45(10):1219–1228. doi:10.1111/aor.14002
18. Huang ZP, Wang QL, Zhang T, Fu YS, Wang WB. Hyper-activated platelet lysates prevent glucocorticoid-associated femoral head necrosis by regulating autophagy. Biomedicine. 2021;139:111711. doi:10.1016/j.biopha.2021.111711
19. Gomes PS, Daugela P, Poskevicius L, Mariano L, Fernandes MH. Molecular and cellular aspects of socket healing in the absence and presence of graft materials and autologous platelet concentrates: a focused review. J Oral Maxillofac Res. 2019;10(3):e2. doi:10.5037/jomr.2019.10302
20. Anitua E, Fernández-de-Retana S, Alkhraisat MH. Platelet rich plasma in oral and maxillofacial surgery from the perspective of composition. Platelets. 2021;32(2):174–182. doi:10.1080/09537104.2020.1856361
21. Zhang Y, Zhuang DZ, Zhang Y, et al. Super activated platelet lysate, a novel autologous platelet lysate, regulates the expression of inflammasome and cytokine in the experimental periodontitis in rats. Drug Des Devel Ther. 2020;14:5535–5543. doi:10.2147/DDDT.S289753
22. Lin Z, Rios HF, Volk SL, Sugai JV, Jin Q, Giannobile WV. Gene expression dynamics during bone healing and osseointegration. J Periodontol. 2011;82(7):1007–1017. doi:10.1902/jop.2010.100577
23. Man D, Plosker H, Winland-Brown JE. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg. 2001;107(1):229–237. doi:10.1097/00006534-200101000-00037
24. Pereira RC, Scaranari M, Benelli R, et al. Dual effect of platelet lysate on human articular cartilage: a maintenance of chondrogenic potential and a transient proinflammatory activity followed by an inflammation resolution. Tissue Eng Part A. 2013;19(11–12):1476–1488. doi:10.1089/ten.TEA.2012.0225
25. Ruggiu A, Ulivi V, Sanguineti F, Cancedda R, Descalzi F. The effect of Platelet Lysate on osteoblast proliferation associated with a transient increase of the inflammatory response in bone regeneration. Biomaterials. 2013;34(37):9318–9330. doi:10.1016/j.biomaterials.2013.08.018
26. Luu HH, Song WX, Luo X, et al. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25(5):665–677. doi:10.1002/jor.20359
27. Jann J, Gascon S, Roux S, Faucheux N. Influence of the TGF-β superfamily on osteoclasts/osteoblasts balance in physiological and pathological bone conditions. Int J Mol Sci. 2020;21(20):20. doi:10.3390/ijms21207597
28. Canellas JS, Soares BN, Ritto FG, et al. What grafting materials produce greater alveolar ridge preservation after tooth extraction? A systematic review and network meta-analysis. J Craniomaxillofac Surg. 2021;49(11):1064–1071. doi:10.1016/j.jcms.2021.06.005
29. Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–319. doi:10.1016/S0092-8674(00)80209-3
30. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. doi:10.1038/nature01658
31. Sumida R, Maeda T, Kawahara I, Yusa J, Kato Y. Platelet-rich fibrin increases the osteoprotegerin/receptor activator of nuclear factor-κB ligand ratio in osteoblasts. Exp Ther Med. 2019;18(1):358–365. doi:10.3892/etm.2019.7560
32. Yu DM, Zhang T, Liu JH, Wang WT, Wang WB. The molecular mechanism of platelet lysate promotes transformation of non-union cells into osteoblasts. Transl Cancer Res. 2020;9(3):1985–1992. doi:10.21037/tcr.2019.12.95
33. Canciani E, Dellavia C, Marazzi MG, et al. RNA isolation from alveolar bone and gene expression analysis of RANK, RANKL and OPG: a new tool to monitor bone remodeling and healing in different bone substitutes used for prosthetic rehabilitation. Arch Oral Biol. 2017;80:56–61. doi:10.1016/j.archoralbio.2017.03.011
34. Kitaura H, Marahleh A, Ohori F, et al. Osteocyte-related cytokines regulate osteoclast formation and bone resorption. Int J Mol Sci. 2020;21(14):5169. doi:10.3390/ijms21145169
35. Gao M, Gao W, Papadimitriou JM, Zhang C, Gao J, Zheng M. Exosomes-The enigmatic regulators of bone homeostasis. Bone Res. 2018;6(1):36. doi:10.1038/s41413-018-0039-2
36. Henriksen K, Karsdal MA, Martin TJ. Osteoclast‐ derived coupling factors in bone remodeling. Calcif Tissue Int. 2014;94(1):88–97. doi:10.1007/s00223-013-9741-7
37. Ota K, Quint P, Ruan M, et al. TGF‐beta induces Wnt10b in osteoclasts from female mice to enhance coupling to osteoblasts. Endocrinology. 2013;154(10):3745–3752. doi:10.1210/en.2013-1272
38. Kondo T, Kanayama K, Egusa H, Nishimura I. Current perspectives of residual ridge resorption: pathological activation of oral barrier osteoclasts. J Prosthodont Res. 2022. doi:10.2186/jpr.JPR_D_21_00333
39. Araujo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005;32(2):212–218. doi:10.1111/j.1600-051X.2005.00642.x
40. Horuk R. Chemokine receptors. Cytokine Growth Factor Rev. 2001;12(4):313–335. doi:10.1016/s1359-6101(01)00014-4
41. Hundelshausen PV, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res. 2007;100(1):27–40. doi:10.1161/01.RES.0000252802.25497.b7
42. Bakogiannisa C, Sachseb M, Stamatelopoulose K, Stellos K.Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine. 2019;122:154157. doi:10.1016/j.cyto.2017.09.013
43. Cole HA, Ohba T, Nyman JS, et al. Fibrin accumulation secondary to loss of plasmin-mediated fibrinolysis drives inflammatory osteoporosis in mice. Arthritis Rheumatol. 2014;66(8):2222–2233. doi:10.1002/art.38639
44. Shiga Y, Kubota G, Orita S, et al. Freeze-dried human platelet-rich plasma retains activation and growth factor expression after an eight-week preservation period. Asian Spine J. 2017;11(3):329–336. doi:10.4184/asj.2017.11.3.329
45. Rasubala L, Yoshikawa H, Nagata K, Iijima T, Ohishi M. Platelet-derived growth factor and bone morphogenetic protein in the healing of mandibular fractures in rats. Br J Oral Maxillofac Surg. 2003;41(3):173–178. doi:10.1016/S0266-4356(03)00075-5
46. Wei B, Huang C, Zhao M, et al. Effect of mesenchymal stem cells and platelet-rich plasma on the bone healing of ovariectomized rats. Stem Cells Int. 2016;2016:9458396. doi:10.1155/2016/9458396
47. Fei Y, Xiao L, Doetschman T, Coffin DJ, Hurley MM. Fibroblast growth factor 2 stimulation of osteoblast differentiation and bone formation is mediated by modulation of the Wnt signaling pathway. J Biol Chem. 2011;286(47):40575–40583. doi:10.1074/jbc.M111.274910
48. Marie PJ, Miraoui H, Severe N.FGF/FGFR signaling in bone formation: progress and perspectives. Growth Factors. 2012;30(2):117–123. doi:10.3109/08977194.2012.656761
49. Zhou FH, Foster BK, Sander G, Xian CJ.Expression of proinflammatory cytokines and growth factors at the injured growth plate cartilage in young rats. Bone. 2004;35(6):1307–1315. doi:10.1016/j.bone.2004.09.014
50. Tomoyasu A, Higashio K, Kanomata K, et al. Platelet-rich plasma stimulates osteoblastic differentiation in the presence of BMPs. Biochem Biophys Res Commun. 2007;361(1):62–67. doi:10.1016/j.bbrc.2007.06.142
51. Lavery K, Swain P, Falb D, Alaoui-Ismaili MH. BMP-2/4 and BMP-6/7 differentially utilize cell surface receptors to induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. J Biol Chem. 2008;283(30):20948–20958. doi:10.1074/jbc.M800850200
52. Braun HJ, Kim HJ, Chu CR, et al. The effect of platelet-rich plasma formulations and blood products on human synoviocytes: implications for intra-articular injury and therapy. Am J Sports Med. 2014;42(5):1204–1210. doi:10.1177/0363546514525593
53. Zhang MY, Zhen J, Zhang X, et al. Effect of autologous platelet-rich plasma and gelatin sponge for tendon-to-bone healing after rabbit anterior cruciate ligament reconstruction. Arthroscopy. 2019;35(5):1486–1497. doi:10.1016/j.arthro.2018.11.014
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.