Back to Journals » Nanotechnology, Science and Applications » Volume 7
Effects of spray-drying on w/o/w multiple emulsions prepared from a stearic acid matrix
Authors Mlalila N , Swai H , Kalombo L, Hilonga A
Received 1 August 2014
Accepted for publication 8 September 2014
Published 1 December 2014 Volume 2014:7 Pages 105—112
DOI https://doi.org/10.2147/NSA.S72083
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Israel (Rudi) Rubinstein
Nichrous Mlalila,1 Hulda Swai,2 Lonji Kalombo,2 Askwar Hilonga3
1School of Life Sciences and Bioengineering, Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania; 2Materials Science and Manufacturing, Council for Scientific and Industrial Research, Pretoria, South Africa; 3Department of Materials Science and Engineering, Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania
Abstract: The goal of this study was to explore the effects of spray-drying on w/o/w double emulsions of methyltestosterone (MT) loaded in a stearic acid matrix. MT-loaded nanoparticles were formulated by a water-in-oil-in-water emulsion technique using 50, 75, and 100 mg of stearic acid, 2% and 3% w/v polyvinyl alcohol, 5% w/v lactose, and 0.2% w/v chitosan. The emulsions were immediately spray-dried based on an optimized model of inlet temperature and pump rate, and characterized for optimized responses with regard to particle size, polydispersity index, and zeta potential, for both emulsion and powder samples. Dynamic light scattering analysis shown that the nanoparticles increased in size with increasing concentrations of polyvinyl alcohol and stearic acid. Scanning electron microscopy indicated that the MT-loaded nanoparticles were spherical in shape, had a smooth surface, and were in an amorphous state, which was confirmed by differential scanning calorimetry. These MT-loaded nanoparticles are a promising candidate carrier for the delivery of MT; however, further studies are needed in order to establish the stability of the system and the cargo release profile under normal conditions of use.
Keywords: double emulsions, nanoparticles, pump rate, spray-drying, testosterone
Introduction
In this era of nanotechnology, spray-drying technology is being increasingly used to transform liquids into solid powders in various manufacturing industries. Its main applications are in the food, chemical, and materials industries to enhance conservation of ingredients, particle properties, powder handling, and storage. Spray-drying enables the drying of temperature-sensitive products without degradation using controlled process parameters. The spray-dried products are suitable for various applications, especially in the pharmaceutical industry, due to their spherical or hollowed morphology, and are generally degradable and more compatible with biological systems. However, the nature of the products has been shown to be dependent on various process and formulation parameters, including inlet temperature, solid concentration, gas flow rate, and feed rate of the formulations.1
Additionally, nanoparticles can be prepared using various methods and molecular building blocks to suit specific applications2,3 and have an influence in spray-drying. Successful design of the desired nanoparticles depends on the instrumental and operational parameters/variables used, and these need to be tuned correctly.4 Parameters such as stirring rate,5 temperature, organic solvent evaporation rate, amount of surfactant,6,7 aqueous-to-organic phase volume ratio,8,9 viscosity,10–12 and pH can influence the physical properties of the final emulsion.13,14 Furthermore, bulk physicochemical and chemical structure characteristics are also important for formation and stability of the emulsions. For example, oil polarity can affect interfacial tension with the aqueous phase and partitioning of the components at the interface, as well as fatty acid chain length, degree of unsaturation, and molecular configuration, and have an effect on the stability of the emulsion.
Hence, many drugs and other bioactive compounds have been tuned for specific scientific applications in life science fields based on a pragmatic knowledge of formulation and process parameters. Androgens and their metabolites, including testosterone, have been widely investigated for various applications coincident with increased development of innovative preparations.15 The preparation has been mainly centered on controlled release delivering and reducing their adverse effects in biological systems using nanocarriers. Molecular building blocks, including polymeric nanoparticles and their block copolymers,2,16,17 lipid nanoparticles,18,19 nanoemulsions,20 and cyclodextrins13,21 have been investigated for use as nanodelivery systems for testosterone and other steroids. In the pharmaceutical industry, testosterone and other androgen drugs are used in hormonal therapy,22 transgender medicine,23 prevention of cardiovascular disease and osteoporosis,24–26 gynecology,20 and gerontology.27,28 Free testosterone is also emerging as a significant possibility for improvement of quality of life in patients with human immunodeficiency virus.26,29 The drawbacks of using testosterone drugs and their derivatives, as with many lipophilic drugs, include poor solubility, stability, and high clearance from the biological system, which reduces their efficacy and efficiency.13,14,21
In this research, 17α-methyltestosterone (MT)-loaded nanoparticles were designed using a double-emulsion technique. Our aim was to investigate the effects of spray-drying parameters on the particle size (PS) and storage stability of these nanoparticles in emulsion. Stearic acid (SA) as the lipid material, polyvinyl alcohol (PVA), and chitosan, a cosurfactant, were used in the preparation of these nanocarrier systems. The prepared emulsions and powders were characterized for their structural and physicochemical properties, including PS and zeta potential (ZP).
Materials and methods
Materials
SA, low-viscous chitosan, hydrolyzed PVA (87%–89%), and D-lactose monohydrate were purchased from Sigma-Aldrich (Aston Manor, South Africa) and MT was supplied by Aquaculture Innovation (Grahamstown, South Africa). All other chemical products were of analytical grade.
Preparation of nanoparticles
The MT-containing nanoparticles were prepared using a water-oil-water (w/o/w) multiple/double emulsion technique followed by high-speed homogenization using the same ingredients. Briefly, in the internal (primary) phase, a defined amount of SA was mixed in 10 mL of ethyl acetate until completely dissolved, and 100 mg of MT and 2 mL of aqueous 2% or 3% w/v PVA were added (Table 1) and mixed thoroughly. The mixture was homogenized in ice blocks at 8,000 rpm for 5 minutes. The primary emulsions were then added to an external phase containing 10 mL of surfactant (PVA), 5 mL of chitosan, and 5 mL of lactose, and homogenized again at 8,000 rpm for 20 minutes. The resulting MT-containing emulsions were used to investigate PS, polydispersity index (PDI), and ZP, and then spray-dried to produce powders.
  | Table 1 Composition ingredients for preparation of nanoparticles |
Analysis of particle size and zeta potential
The nanosuspensions were diluted with distilled water before analysis of PS and ZP, which were determined by dynamic light scattering (Zetasizer Nano ZS, Malvern Instruments, Malvern, UK) at 25°C. The average PS and ZP of the nanoparticle suspensions were measured by dynamic light scattering and electrophoretic light scattering, respectively, using the same instrument. The measurements were taken by cuvette cells. The dynamic light scattering data were analyzed at 25°C and with a fixed light incidence angle of detection of 173 degrees using the backscattering technique, while the ZP was calculated from the electrophoretic mobility of the nanoparticles using the Helmholtz-Smoluchowski equation. The mean hydrodynamic diameter (PS, Z-average) and PDI of the analyzed samples were obtained by calculating the average of 11 runs, with 20 runs for ZP. We analyze the data by the cumulant method, with the assumption that the particles were spherical. Measurements were performed in triplicate.
Spray-dried nanoparticles
The emulsions were then used to prepare the powdered nanoparticles in the spray-dryer. The emulsions were spray-dried using a laboratory-scale spray-dryer (Buchi®, B-290, Flawil, Switzerland) in closed mode under ultra-high purity nitrogen gas. Different inlet temperatures and pump rates were used to optimize PS during the spray-drying process. The emulsion formulations were taken in a closed beaker with laboratory parafilm or aluminum foil to the spray-dryer and spray-dried at 70°C, 80°C, 90°C, or 100°C inlet temperatures and at pump rates of 1%, 2%, or 3%, in an optimization model. Other parameters, such as aspirator and processing pressure, were set at 100% and 5.1 bar, respectively. The dried samples were analyzed for PS, PDI, and ZP, and then placed in amber bottles and stored at 4°C for about 30 days until further analysis.
Analysis of nanoparticle stability
The stored powders were dispersed and characterized in terms of PS, PDI, and ZP. Mean diameter was measured on the basis of fluctuations in the intensity of the scattered light due to particle movement by monitoring size as a function of time (for up to 1 month) under given storage conditions.
Differential scanning calorimetry
Differential scanning calorimetry (DSC) was performed using a model Q200™ device equipped with an automated, computer-controlled, refrigerated cooling system (RSC-90) with Tzero™ capability (TA Instruments, New Castle, DE, USA). The DSC thermograms were collected using a sample weight of 5 mg powder and placed in Tzero alodine-coated aluminum DSC pans, which were then hermetically sealed with a Tzero hermetic sealer (TA Instruments). The phase transition temperatures and melt points of the samples were measured under a 40 mL per minute dry ultra-high purity nitrogen gas (Scott-Gross Company Inc., Campbellsville, KY) purge in DSC. The samples were heated at 5°C per minute from 10°C to 200°C. The samples were kept at 200°C for 5 minutes and then cooled to room temperature at a rate of 5°C per minute. The measured DSC data were analyzed using a coupled DSC Q200-1740 data station (TA Instruments).
Scanning electron microscopy
The shape and surface morphology of the powders were investigated using a JSM-7500F field emission scanning electron microscope (JEOL Ltd, München, Germany). Briefly, a carbon tape was stuck on field emission scanning electron microscope snubs, and a very small amount of sample was sprinkled onto the carbon tape. The sample was then carbon-coated and introduced into the microscope for imaging.
Results and discussion
MT-loaded nanoparticles were successfully prepared by double emulsion technique using a high-speed Silverson L 4R homogenizer. During the process, various physical and chemical assays were used to observe the effects of the process on PS, PDI, and ZP before and after spray-drying.
Synthesis of nanoparticles
PVA has a lipophilic/hydrophobic balance value of 18, with polyhydric alcohol, a hydrophilic group, and a fatty acid lipophilic group.30 The lipid nanoparticles were prepared from 2% and 3% w/v PVA with an SA matrix, and showed variations in the PS formed. The lower weight lipid matrix, ie, containing SA 50 mg and 2% w/v PVA, yielded smaller PS compared with SA 75 mg and 100 mg at 3% w/v PVA and constant 100 mg (Table 2). The smaller PS observed is in contrast with other reports on the effects of increased concentrations of surfactants and matrices.14,30 The PS of nanoparticles was shown to be the attribute of amount SA and concentration of PVA. The higher contents of SA and PVA, increased the viscosity of solutions and reduced their solubility in aqueous solution, which contributed to observed larger PS.
In practical terms, viscosity contributes most to disruption and break-up of molecules in emulsion during homogenization. It has been reported that the PS of nanoparticles is mainly determined by the viscosity4 of the organic phase and the water phase used in preparation of the nanoparticles. The difference in PS between 25% and 3% w/v PVA could account for the interfacial films of the PVA in the emulsion molecules that cover the surface of exposed hydrophobic patches, as previously reported.31 PVA concentrations higher than 3% w/v could account for the greater stability of the nanoparticles under process conditions, such as during spray-drying.
Our results are in agreement with the literature regarding the effects of homogenization of emulsions on PS.30,32,33 The higher the lipid content, the higher the mean PS. To form stable w/o/w emulsions, the oil phase should ideally have high viscosity and low water solubility.9,10 In this regard, PVA is widely used in the fabrication of polymeric micro/nanospheres due to its high viscosity in aqueous solution and strong adsorption around the surfaces of the emulsion droplets.30 Higher emulsifier concentrations decrease the interfacial tension and amount of energy by facilitating particle disruption, resulting in stable PS during homogenization34,35 and an increased surface area in the dispersed hydrophilic phase.32 Additionally, an increase in the surfactant concentration leads to stable small PS due to stabilization of oil droplets as a result of localization of the surfactant molecules at the oil–water interface.14,30
Emulsification of fish oil with pectin surfactants weighing between 0.05% and 0.35%5 has shown an increasing trend of nanoparticle size with increasing concentrations of surfactant. However, it should be noted that an increase in the surfactant concentration can lead to poor drug entrapment efficiency in the oil phases because some drugs tend to bind to excessive emulsifier molecules.33 The emulsifiers recommended for w/o/w emulsions should have a lipophilic/hydrophobic balance value between 8 and 1830,36 and optimal viscosity,6 creating kinetic and steric stability with low initial interfacial tension and a constant viscosity with time.36 The use of an inappropriate emulsifier could lead to an inverted emulsion. Selection of the most appropriate emulsifier7 or combination of emulsifiers is the most important factor to consider during production.11,35,37
Spray-dried MT-loaded nanoparticles
For effective spraying-drying, certain process parameters, such as inlet temperature, drying gas, feed rate, compressed air flow rate, and pressure, should be considered. The formulation parameters to be taken into account include the feed composition, type of solvent, viscosity, and surface tension of the drying solutions.4 In this study, the experiments were carried at 70°C, 80°C, 90°C, and 100°C, which could evaporate the ethyl acetate and water used as the solvent in the atomization cone, leading to a more stable and pure formulation. In addition, pump rates of 1%, 2%, and 3% were considered for a PS optimization model. The PS data showed that the PS obtained at pump rates of 1% and 2% was larger compared with that at a pump rate of 3%. The 3% pump rate generated a smaller PS (Table 3), regardless of the temperature used. Another observable attribute of spray-dried nanoparticles was the change in the range of the ZP during the spray-drying process, which may be attributable to degradation of the cross-linked chitosan backbone. The negatively charges of the ZP could account for the reduced stability of the nanoparticles during storage.
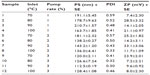  | Table 3 Powdered nanoparticles size from spray dryer |
A similar observation was reported for production of spray-dried cyclosporin A, in the form of multifunctional particles for dry powder inhalation aerosol delivery, when spray-dried at pump rates of 10%, 25%, 50%, and 75%.38 That study concluded that the spray-dried sample with a pump rate of 75% produced a smaller PS when compared with unprocessed samples and a lower pump rate. On other hand, the concentrated feed solution led to smaller dried particles. The phenomenon of decreasing PS and a tendency for crystallization when the pump rate is increased has been reported by Paudel et al and Serfert et al in the production of spray-dried artemisinin-maltodextrin.4,5 Additionally, increasing the surfactant concentration leads to small PS being produced by spray-drying because of stabilization of oil droplets when the surfactant molecules localize at the oil–water interface.14,30 This contributes to produce more stable nanoparticles at any temperature during spray-drying.
Finally, we investigated the PDI and ZP of the MT-loaded nanoparticles. The PDI of the MT-loaded nanoparticles was virtuous (virtuous implies acceptable ranges or conforming to acceptable ranges, for stability of emulsions) but the ZP was in the marginal range of stability for nanoparticles spray-dried at a pump rate of 3%. The decrease in ZP for the spray-dried nanoparticles might be contributed to by degradation of intrachain hydrogen bonds in chitosan,39 with a critical temperature at 40°C.40 Similarly, it has been reported that chitosan nanoparticles formulated from pentasodium tripolyphosphate by ionotropic gelation show different PS by varying the processing temperatures and time. At 37°C, chitosan nanoparticles aggregated after 1 hour and partial aggregation occurred after 2 hours at room temperature or 24 hours at 4°C.41 This indicates a loss of the cross-linked network of chitosan,42 which promotes interfacial interactions, to aggregates into large PS. In general, the PDI and ZP give an indication of the susceptibility of colloidal systems to aggregation under specific storage conditions. Particles can be considered stably dispersed when the absolute value of the ZP is above positive or negative 30 mV, due essentially due to electric repulsion between particles. Therefore, the MT-loaded nanoparticles developed in the present work may be considered relatively unstable due to a low ZP value.
After successfully optimizing the dried powder nanoparticles and achieving for PS within the administration range for delivery, we set out to characterize the prepared nanoparticles for their stability, crystalline structure, and morphology we set out to characterize critically the prepared nanoparticles for their stability, crystal structure, and morphology, as well as other constituents in the composition of nanoparticles. Just as importantly, the studies yielded range estimates for key factors that allow confident construction of future experimental designs.
Analysis of nanoparticle stability
The solid spray-dried MT-loaded nanoparticles were stored in a desiccator for 1 month and then characterized for their stability based on PS, PDI, and ZP analysis. The mean diameter was measured based on fluctuations in the intensity of scattered light due to particle movement by monitoring PS as a function of time and storage conditions. The PDI and ZP values for the MT-loaded nanoparticles indicated poor stability. The stored nanoparticles indicated that there were fluctuations of PS, PDI and ZP, although in nonlinear fashion to the temperature used in spray-drying process. The formulations spray-dried at 70°C and 100°C showed some stability in ZP, whereas at 80°C and 90°C, the ZP became negative (Table 4). For all samples, there was an increase in PDI values, indicating a high potential for aggregation. In addition, the ZP for all samples was decreased, and was associated with increased PS. The greater the change in ZP, the more the change in PS. These observations account for the need to optimize the formulation and process parameters to control the stability of the nanoparticles during storage.
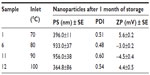  | Table 4 Nanoparticle stability under storage conditions |
Therefore, the decrease for ZP below ±30 mV of spray-dried nanoparticles led to instability of stored nanoparticles, as reported in previous section. Long-term stability studies of these nanoparticles need to be carried out to be able to develop a stable dynamic system suitable for controlled release of testosterone. This observation can be explained in terms of various stabilizing scenarios, such as the moisture content and sorption of the spray-dried samples.43 The sorption effect is an important consideration for storage. A high moisture content in nanoparticles is undesirable after the spray-drying process, and causes some dissolution of the dried bioactive ingredients or a change in state, resulting in caking. High moisture content was also observed to cause rearrangement of nanoparticles from a high-energy level (amorphous) to a low energy level (crystalline) after spray-drying, leading to formation of large clumps rather than a free-flowing powder.
The amount of surfactant used during the process of spray-drying and the degree of crystallization are functions of nanoparticle stability. This depends to a large extent on the concentration of the surfactant relative to surface area, which is governed by the shape and type of the crystal structures generated. A low surfactant concentration may be insufficient to cover the exposed hydrophobic patches in relation to the rate of particle–particle collision. This may influence the rearrangement of the nanoparticles, leading to aggregation.31
Differential scanning calorimetry
Polymorphism is the term used to describe the occurrence of different molecular conformations and molecular packing.33 Polymorphs have striking thermodynamic properties, including melting point, X-diffraction patterns, and solubility, reflecting their capacity for drug incorporation and their release rate.33 Polymorphs are caused by a substantial transformation in structure at a low temperature, driven by surface interactions, indicating that the internal strain depends on the nature of the surroundings as well as size. In this study, DSC analysis of physical mixtures of MT and SA, bulk SA, and the formulations was performed to confirm if MT was solubilized in the matrix carrier, using bulk MT as the reference.
Figure 1 shows the melting point and crystallization behavior of the bulk MT compound, physical mixtures of MT and SA, and SA-loaded and MT-loaded nanoparticles. At baseline, the heat flux of the reaction or transition or both is equal to zero. The upper section of Figure 1 shows respective exothermic (crystallization/cooling) isotherms, while the lower part corresponds to the endothermic (heating/melting point) enthalpy of the sample. The melting point and crystallization spectra for MT were 165°C and 138°C, respectively. SA had a stable melting point at 69°C (β-polymorphic form) and an insignificant metastable spectra (α-polymorphic form) at 174°C, with a crystallization point at 68°C.
  | Figure 1 Melting points and crystallization of MT, physically mixed MT and SA nanoparticles. |
The physical mixtures of SA and MT did not show a melting point, although in the third cycle there is much lower intensity, which may be explained by the presence of a small amount of MT in SA (Figure 1). The melting points and crystallinity spectra for the nanosuspensions disappeared. Disappearance of the DSC endothermic spectra for the solid dispersions may reflect the lower crystallinity of MT in the polymers. This suggests that most of the MT was converted to an amorphous state in the solid dispersions after spray-drying.
Heating and cooling of the majority of lipids leads to appearance of transitional states between their multiple polymorphic states, ie, unstable (α), metastable (β′), and stable (β) forms.33 The transformation in state to crystalline structures is contributed to mainly by the partial formation of lower energy lipid modifications and surfactants that can lower the onset temperature, maximum temperature, and melting enthalpy, in turn reducing crystallinity. Rapid quenching of solid lipid nanoparticles containing hydrocortisone and progesterone prevented the drug molecules’ nucleation to the crystal lattice, and consequently the drug molecules remained dispersed in the lipid matrix of the solid lipid nanoparticles in an amorphous state.44 If the there is no existence of melting points for physical mixtures of the bulk materials, the formulations appear as amorphous in spray-drying, which is the function of lattice imperfection.45
Solvent evaporation kinetics also contribute to formation of amorphous solid dispersions, and the higher the evaporation rate the less the crystalline formation. Other factors thought to facilitate formation of an amorphous state include the solution state of the active ingredients, formulation parameters, and process parameters, such as the drying gas and flow rates.4 Transformation of nanoparticles from α to β′ to β results in transformation of the spherically shaped lipid crystalline particles to needle-like or platelet-shaped particles,31 this was not observed with these experiments (Figure 1). The physical stability of the metastable amorphous state is the most important form for sustained drug release from nanoparticles.4
Moreover, imperfections (eg, lattice defects) in nanoparticles allow a greater amount of drug to be accommodated, resulting in a high drug-loading capacity. The lipid within nanoparticles should be in a less ordered arrangement than that in crystalline bulk materials.46 Low crystallinity in lipid nanoparticles results in the highest “compatibility” and higher drug loading.19 In this regard, the prepared nanoparticles had an amorphous structure. Amorphous thermoplastics undergo step-like changes in their properties in the glass transition range. The characteristics in glass transition are a function of the physical forces responsible for bonding in the polymers used.
Scanning electron microscopy analysis
Particle morphology can be described in terms of PS, shape, internal structure, and surface properties. With few exceptions, spray-dried particles are spherical, and their size can be described by their geometric diameter, which influences the forces on particles during fluid flows and the packing of particles when they form a powder.39 The particle and surface morphology of the formulations were imaged, and the SEM images are shown in Figure 2. The spray-dried powders were observed to comprise smooth-surfaced, spherical particles with some having a “doughnut” or concave shape and a maximum diameter of 1 μm. These results are in agreement with the dynamic light scattering results obtained after 1 month under storage conditions, although the instability phenomena were not well displayed in the SEM analysis. The smoothness of the MT-loaded nanoparticle surfaces implies that no or limited aggregation occurred after 2 months of storage.
  | Figure 2 Scanning electron micrograph of spray-dried, methyltestosterone-loaded nanoparticles. |
The smooth-surface and spherical morphology of the nanoparticles is due to denser packing of the surfactant (PVA) on the surface, indicating a higher entrapment efficacy and good kinetic release of the bioactive ingredients from the carrier systems.30 These types of nanoparticles have been reported to facilitate minimal contacts with the aqueous environments, hence increasing their bioavailability and efficacy for delivering bioactive ingredients in biological systems. Additionally, they require a small amount of surfactant to have a high potential for protecting the incorporated drug.47 During the spray-drying process, several factors contribute to formation of the amorphous surface. These include the solvent evaporation kinetics, carrier, formulation parameters, and process parameters, such as drying gas, flow rate, and solution spray rate.4 SA serves as an inhibitor of crystallization by decreasing the molecular mobility (anti-plasticization) of the amorphous state of the drugs dispersed in the matrix. For these reasons, spray-drying is used as a particle engineering technique and, for nanosuspensions, to generate particles and dispersion.4
Conclusion
The results obtained in the present work show that lipid nanoparticles are suitable carrier systems for incorporation of androgens intended for oral administration. Furthermore, the homogenization method and w/o/w multiple emulsion techniques are both appropriate for production of lipid nanoparticles. These findings represent an advance in simplification of the methods needed to produce testosterone-loaded nanoparticles. However, ongoing long-term stability studies are comparing the PS and physicochemical stability of placebo-loaded versus MT-loaded formulations for longer periods. Additional in vitro/in vivo correlation studies are also underway to assess the encapsulation parameters and the profile of drug release from nanoparticles.
Acknowledgments
The authors appreciate the support of the Nelson Mandela African Institution of Science and Technology in Arusha, Tanzania, and the Council for Scientific and Industrial Research in Pretoria, South Africa, for providing the research platform and facilities. Finally, we acknowledge the contributions of Aeysha Jakoet, Brendon Naicker, Patric Nkuna, and Vincent Mandla Khumalo from the Council for Scientific and Industrial Research, Pretoria, South Africa.
Disclosure
The authors report no conflicts of interest in this work.
References
Li X, Anton N, Arpagaus C, Belleteix F, Vandamme TF. Nanoparticles by spray drying using innovative new technology: the Büchi Nano Spray Dryer B-90. J Control Release. 2010;147(2):304–310. | |
Chen S, Pederson D, Oak M, Singh J. In vivo absorption of steroidal hormones from smart polymer based delivery systems. J Pharm Sci. 2010;99(8):3381–3388. | |
Chen S, Singh J. Controlled delivery of testosterone from smart polymer solution based systems: in vitro evaluation. Int J Pharm. 2005;295(1):183–190. | |
Paudel A, Worku ZA, Meeus J, Guns S, Van den Mooter G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: formulation and process considerations. Int J Pharm. 2013;453(1):253–284. | |
Serfert Y, Schröder J, Mescher A, et al. Spray drying behaviour and functionality of emulsions with β-lactoglobulin/pectin interfacial complexes. Food Hydrocoll. 2013;31(2):438–445. | |
Jafari SM, Assadpoor E, He Y, Bhandari B. Re-coalescence of emulsion droplets during high-energy emulsification. Food Hydrocoll. 2008;22(7):1191–1202. | |
Dalgleish DG. Food emulsions – their structures and structure-forming properties. Food Hydrocoll. 2006;20(4):415–422. | |
Desplanques S, Renou F, Grisel M, Malhiac C. Impact of chemical composition of xanthan and acacia gums on the emulsification and stability of oil-in-water emulsions. Food Hydrocoll. 2012;27(2):401–410. | |
Dokic L, Krstonošic V, Nikolic I. Physicochemical characteristics and stability of oil-in-water emulsions stabilized by OSA starch. Food Hydrocoll. 2012;29(1):185–192. | |
Su J, Flanagan J, Hemar Y, Singh H. Synergistic effects of polyglycerol ester of polyricinoleic acid and sodium caseinate on the stabilisation of water-oil-water emulsions. Food Hydrocoll. 2006;20(2006):261–268. | |
Cornacchia L, Roos YH. Lipid and water crystallization in protein-stabilised oil-in-water emulsions. Food Hydrocoll. 2011;25(7):1726–1736. | |
Qian C, McClements DJ. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: factors affecting particle size. Food Hydrocoll. 2011;25(5):1000–1008. | |
Memisoglu-Bilensoy E, Sen M, Hincal AA. Effect of drug physicochemical properties on in vitro characteristics of amphiphilic cyclodextrin nanospheres and nanocapsules. J Microencapsul. 2006;23(1):59–68. | |
Balakumar K, Vijaya Raghavan C, Tamil Selvan N, Habibur Rahman SM. Self emulsifying drug delivery system: optimization and its prototype for various compositions of oils, surfactants and co-surfactants. J Pharm Res. 2013;6(5):510–514. | |
de Ziegler D, Fanchin R. Progesterone and progestins: applications in gynecology. Steroids. 2000;65(2000):671–679. | |
Allen C, Han J, Yu Y, Maysinger D, Eisenberg A. Polycaprolactone-b-poly(ethylene oxide) copolymer micelles as a delivery vehicle for dihydrotestosterone. J Control Release. 2000;63(3):275–286. | |
Misra A, Raghuvanshi RS, Ganga S, Diwan M, Talwar GP, Singh O. Formulation of a transdermal system for biphasic delivery of testosterone. J Control Release. 1996;39(1):1–7. | |
Hathout RM, Woodman TJ, Mansour S, Mortada ND, Geneidi AS, Guy RH. Microemulsion formulations for the transdermal delivery of testosterone. Eur J Pharm Sci. 2010;40(3):188–196. | |
Yuan H, Wang L-L, Du Y-Z, You J, Hu F-Q, Zeng S. Preparation and characteristics of nanostructured lipid carriers for control-releasing progesterone by melt-emulsification. Colloids Surf B Biointerfaces. 2007;60(2):174–179. | |
Cserháti T, Forgács E. Modification of the apparent lipophilicity of steroidal drugs with gamma-cyclodextrin. Eur J Pharm Biopharm. 1998;46(2):153–159. | |
Rodriguez-Tenreiro C, Alvarez-Lorenzo C, Rodriguez-Perez A, Concheiro A, Torres-Labandeira JJ. Estradiol sustained release from high affinity cyclodextrin hydrogels. Eur J Pharm Biopharm. 2007;66(1):55–62. | |
Bhatti HN, Khera RA. Biological transformations of steroidal compounds: a review. Steroids. 2012;77(12):1267–1290. | |
Moreno-Pérez ó, Esteva De Antonio I. [Clinical practice guidelines for assessment and treatment of transsexualism. SEEN Identity and Sexual Differentiation Group (GIDSEEN)]. Endocrinol Nutr. 2012;59(6):367–382. Spanish. | |
Seal LJ. Testosterone replacement therapy. Medicine. 2009;37(9):445–449. | |
Yoo J-W, Lee CH. Drug delivery systems for hormone therapy. J Control Release. 2006;112(1):1–14. | |
Cotter AG, Powderly WG. Endocrine complications of human immunodeficiency virus infection: hypogonadism, bone disease and tenofovir-related toxicity. Best Pract Res Clin Endocrinol Metab. 2011;25(3):501–515. | |
Yialamas MA, Hayes FJ. Androgens and the ageing male and female. Best Pract Res Clin Endocrinol Metab. 2003;17(2):223–236. | |
Bhowmik BB, Sa B, Mukherjee A. Preparation and in-vitro characterization of slow release testosterone nanocapsules in alginates. Acta Pharm. 2006;56(4):417–429. | |
Falutz J. Growth hormone and HIV infection: Contribution to disease manifestations and clinical implications. Best Pract Res Clin Endocrinol Metab. 2011;25(3):517–529. | |
Feng S-S, Huang G. Effects of emulsifiers on the controlled release of paclitaxel (Taxol®) from nanospheres of biodegradable polymers. J Control Release. 2001;71(1):53–69. | |
Helgason T, Awad TS, Kristbergsson K, McClements DJ, Weiss J. Effect of surfactant surface coverage on formation of solid lipid nanoparticles (SLN). J Colloid Interface Sci. 2009;334(1):75–81. | |
Triplett MD II, Rathman JF. Optimization of β-carotene loaded solid lipid nanoparticles preparation using a high shear homogenization technique. J Nanopart Res. 2009;11(3):601–614. | |
Parhi R, Suresh P. Preparation and characterization of solid lipid nanoparticles – a review. Curr Drug Discov Technol. 2012;9(1):2–16. | |
Chanasattru W, Decker EA, McClements DJ. Influence of glycerol and sorbitol on thermally induced droplet aggregation in oil-in-water emulsions stabilized by β-lactoglobulin. Food Hydrocoll. 2009;23(2):253–261. | |
Aoki T, Decker EA, McClements DJ. Influence of environmental stresses on stability of O/W emulsions containing droplets stabilized by multilayered membranes produced by a layer-by-layer electrostatic deposition technique. Food Hydrocoll. 2005;19(2):209–220. | |
Ushikubo FY, Cunha RL. Stability mechanisms of liquid water-in-oil emulsions. Food Hydrocoll. 2012;34:145–153. | |
Bonnet M, Cansell M, Berkaoui A, Ropers MH, Anton M, Leal-Calderon F. Release rate profiles of magnesium from multiple w/o/w emulsions. Food Hydrocoll. 2009;23(1):92–101. | |
Wu X, Zhang W, Hayes D Jr, Mansour H. Physicochemical characterization and aerosol dispersion performance of organic solution advanced spray-dried cyclosporine A multifunctional particles for dry powder inhalation aerosol delivery. Int J Nanomedicine. 2013;8:1269–1283. | |
Vehring R. Pharmaceutical particle engineering via spray drying. Pharm Res. 2008;25(5):999–1022. | |
Rinaudo M. Chitin and chitosan: properties and applications. Prog Polym Sci. 2006;31(7):603–632. | |
De Salamanca AE, Diebold Y, Calonge M, et al. Chitosan nanoparticles as a potential drug delivery system for the ocular surface: toxicity, uptake mechanism and in vivo tolerance. Invest Ophthalmol Vis Sci. 2006;47(4):1416–1425. | |
Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J Control Release. 2004;100(1):5–28. | |
Chiou D, Langrish TAG. Development and characterisation of novel nutraceuticals with spray drying technology. J Food Eng. 2007;82(1):84–91. | |
Cavalli R, Peira E, Caputo O, Gasco MR. Solid lipid nanoparticles as carriers of hydrocortisone and progesterone complexes with β-cyclodextrins. Int J Pharm. 1999;182(1):59–69. | |
Silva A, González-Mira E, García M, et al. Preparation, characterization and biocompatibility studies on risperidone-loaded solid lipid nanoparticles (SLN): high pressure homogenization versus ultrasound. Colloids Surf B Biointerfaces. 2011;86(1):158–165. | |
Neves AR, Lúcio M, Martins S, Lima J, Reis S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int J Nanomedicine. 2013;8:177–187. | |
Kumar S, Randhawa JK. Preparation and characterization of paliperidone loaded solid lipid nanoparticles. Colloids Surf B Biointerfaces. 2013;102:562–568. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

