Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 11 » Issue 1
Effects of roflumilast in COPD patients receiving inhaled corticosteroid/long-acting β2-agonist fixed-dose combination: RE2SPOND rationale and study design
Authors Rennard S, Martinez F , Rabe K, Sethi S , Pizzichini E, McIvor A, Siddiqui S , Anzueto A, Zhu H
Received 1 April 2016
Accepted for publication 17 June 2016
Published 17 August 2016 Volume 2016:11(1) Pages 1921—1928
DOI https://doi.org/10.2147/COPD.S109661
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Stephen I Rennard,1,2 Fernando J Martinez,3,4 Klaus F Rabe,5–7 Sanjay Sethi,8 Emilio Pizzichini,9 Andrew McIvor,10 Shahid Siddiqui,11 Antonio Anzueto,12 Haiyuan Zhu13
1Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE, USA; 2AstraZeneca, Cambridge, UK; 3Joan and Sanford I Weill Department of Medicine, Weill Cornell University, New York, NY, 4Department of Internal Medicine, Michigan Health System, Ann Arbor, MI, USA; 5LungenClinic Grosshansdorf, Großhansdorf, 6Department of Medicine, University Kiel, Kiel, 7Airway Research Center North, German Center for Lung Research, Großhansdorf, Germany; 8Department of Medicine, University at Buffalo, State University of New York, Buffalo, NY, USA; 9Department of Medicine, Universidade Federal de Santa Catarina, Santa Catarina, Brazil; 10Firestone Institute of Respiratory Health, St Joseph’s Healthcare, McMaster University, Hamilton, ON, Canada; 11AstraZeneca, Gaithersburg, MD, 12South Texas Veterans Health Care System at San Antonio, University of Texas Health Science Center, San Antonio, TX, 13Allergan plc, Jersey City, NJ, USA
Background: Roflumilast, a once-daily, selective phosphodiesterase-4 inhibitor, reduces the risk of COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations. The RE2SPOND study is examining whether roflumilast, when added to an inhaled corticosteroid/long-acting β2-agonist (ICS/LABA) fixed-dose combination (FDC), further reduces exacerbations. The methodology is described herein.
Methods: In this Phase IV, multicenter, double-blind, placebo-controlled, parallel-group trial, participants were randomized 1:1 (stratified by long-acting muscarinic antagonist use) to receive roflumilast or placebo, plus ICS/LABA FDC, for 52 weeks. Eligible participants had severe COPD associated with chronic bronchitis, had two or more moderate–severe exacerbations within 12 months, and were receiving ICS/LABA FDC for ≥3 months. The primary efficacy measure is the rate of moderate or severe COPD exacerbations per participant per year. The secondary efficacy outcomes include mean change in prebronchodilator forced expiratory volume in 1 second (FEV1) over 52 weeks, rate of severe exacerbations, and rate of moderate, severe, or antibiotic-treated exacerbations. Additional assessments include spirometry, rescue medication use, the COPD assessment test, daily symptoms using the EXACT-Respiratory symptoms (E-RS) questionnaire, all-cause and COPD-related hospitalizations, and safety and pharmacokinetic measures.
Results: Across 17 countries, 2,354 participants were randomized from September 2011 to October 2014. Enrollment goal was met in October 2014, and study completion occurred in June 2016.
Conclusion: This study will further characterize the effects of roflumilast added to ICS/LABA on exacerbation rates, lung function, and health of severe–very severe COPD participants at risk of further exacerbations. The results will determine the clinical benefits of roflumilast combined with standard-of-care inhaled COPD treatment.
Keywords: exacerbation, RE2SPOND, phosphodiesterase-4, ICS/LABA, methodology, study design
Introduction
COPD is characterized by irreversible, progressive airflow limitation often associated with pulmonary inflammation.1–3 In a recent study, $32 billion USD were attributed to COPD-related medical costs, with a projected increase to $49 billion by 2020.4 Acute worsening of respiratory symptoms (exacerbations) contributes to morbidity and mortality in patients with COPD, is associated with chronic and acutely worsened airway inflammation,5 and further increases the cost of care.6 Patients with acute COPD exacerbations have nearly double the all-cause quarterly incremental US health care costs than patients without exacerbations.6 They also have a greater prevalence of cardiovascular disease, gastroesophageal reflux, depression, and cognitive impairment.5,7 Roflumilast, an orally administered selective phosphodiesterase-4 inhibitor, increases the levels of intracellular 3′,5′-cyclic adenosine monophosphate in inflammatory cells and in the epithelial cells of the airways,1,2 which may contribute to the reduction of pulmonary inflammation.8
Roflumilast has been shown to reduce the risk of COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of COPD exacerbations.9–12 Studies have shown that combinations of roflumilast with long-acting β2-agonists (LABAs) or long-acting muscarinic antagonists (LAMAs) are effective in reducing COPD exacerbations,10,13,14 and the Global Initiative for Obstructive Lung Disease recommends that roflumilast be prescribed in conjunction with one or more long-acting bronchodilators.3 Similar to inhaled corticosteroids (ICS), roflumilast may decrease inflammation,8,15 which may account for its ability to reduce the frequency of COPD exacerbations.10,13,16
Although studies evaluating the concomitant use of roflumilast with long-acting bronchodilators have generally excluded concomitant ICS treatment,10,13,14 a post hoc analysis of pooled data from two Phase III studies showed significant reductions in moderate to severe COPD exacerbation rates with roflumilast versus placebo in patients with severe, stable COPD who were receiving concurrent ICS (19% reduction; P=0.01).11 Subsequent clinical trials that formed the basis for approval of roflumilast required subjects to discontinue concurrent ICS.10 Because roflumilast and ICS may reduce inflammation via different mechanisms, the combination of these drugs could have an additive or synergistic effect.17 Whether roflumilast added to an ICS/LABA combination would offer greater benefit for reducing COPD exacerbations and to what degree are important questions for clinicians managing patients who experience frequent COPD exacerbations. To address this need, a commitment was made at the time of roflumilast approval to pursue this question via two clinical trials. One was the recently completed Roflumilast and Exacerbations in participants receiving Appropriate Combination Therapy study (REACT), a randomized, controlled trial conducted in 21 countries (NCT0132902918). In this study of 1,945 participants, the European formulation of roflumilast (film-coated tablets) reduced moderate to severe COPD exacerbations and hospitalizations when added to an ICS/LABA combination treatment in participants with severe COPD.18 The second study (RE2SPOND; NCT01443845) is evaluating the efficacy and safety of the US formulation of roflumilast (noncoated tablets) in a separate population of participants with severe COPD. A proportion of participants enrolled in RE2SPOND are allowed to use concomitant LAMA, which enables examination of the efficacy of roflumilast in reducing the rate of COPD exacerbations when added to ICS/LABA/LAMA triple therapy. The methodology, design, and study population of the ongoing RE2SPOND trial are described herein.
Methods
Study design
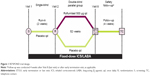
In this multicenter, double-blind, placebo-controlled, parallel-group trial (NCT01443845), 2,354 participants were randomized 1:1 to receive once-daily roflumilast 500 μg or placebo, plus fixed-dose combination (FDC) ICS/LABA (fluticasone 250 μg/salmeterol 50 μg [one inhalation bid] or budesonide 160 μg/formoterol 4.5 μg [two inhalations bid]), for 52 weeks of double-blind treatment following a 2-week single-blind placebo run-in phase (Figure 1). Randomization was stratified by LAMA use. A safety follow-up conducted by telephone occurs either 4 weeks after the last visit of the double-blind treatment phase or following early termination. Participants who terminate the study early are followed to the scheduled Week 52 visit. The follow-up period includes one or two on-site visits (depending on the time of discontinuation) to perform vital sign and spirometry assessments and telephone contacts to collect information regarding COPD exacerbations, concomitant medication use, and adverse effects (AEs). This clinical study is conducted in accordance with the ethical principles of the Declaration of Helsinki and in compliance with the International Council for Harmonization Guidance on General Considerations for Clinical Trials and Good Clinical Practice. The study protocol was approved by the institutional review board at each study center in the United States and by the Independent Ethics Committee at each study center outside of the United States. All participants provided written informed consent prior to study participation. Although similar to the REACT trial, RE2SPOND has some notable differences in both design and conduct described in Table 1.
  | Table 1 Comparison of the REACT18,27 and RE2SPOND trials |
Study population
The planned enrollment is 2,300 males and females ≥40 years of age with a history of COPD for ≥12 months prior to screening associated with chronic productive cough for 3 months in each of 2 consecutive years (with other causes of productive cough excluded). Participants must have had two or more documented moderate or severe COPD exacerbations in the 12 months prior to screening. They are required to be using FDC ICS/LABA treatment for ≥3 months prior to screening; participants must remain on the same COPD maintenance treatment between screening and randomization. Participants must have a postbronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio <70% and postbronchodilator FEV1 ≤50% of predicted. Additionally, participants can be current or former smokers (smoking cessation ≥1 year prior to enrollment) with a smoking history of ≥20 pack-years.
Participants were excluded if, within the 4 weeks prior to enrollment, they had a moderate or severe COPD exacerbation and/or COPD exacerbation treated with antibiotics or systemic corticosteroids or a lower respiratory tract infection. Other exclusionary criteria include diagnoses of other lung diseases, moderate-to-severe liver impairment (Child-Pugh B or C), HIV or hepatitis infection, current diagnosis of asthma, cancer in the past 5 years, α1-antitrypsin deficiency, a clinically significant cardiovascular condition, a resting QTc interval >470 ms, or a body mass index ≥45 kg/m2. Participants must not have used theophylline or add-on theophylline derivatives 2 weeks prior to screening or have any clinically relevant abnormalities in clinical laboratory tests. Participants must not have participated in an acute pulmonary rehabilitation program in the previous 3 months (except if on a stable pulmonary rehabilitation exercise regimen for ≥6 weeks).
Concomitant medications
In addition to background FDC ICS/LABA treatment as part of the study treatment, up to 60% of enrolled participants are permitted to use a LAMA (eg, tiotropium) if treatment is stable (taking the drug for ≥3 months prior to screening with no change in dose). For participants not using a LAMA, treatment with a short-acting muscarinic antagonist (eg, ipratropium and oxitropium) is allowed. Corticosteroids (oral, parenteral, and intranasal) or H1-antihistamines (eg, loratadine) are also permitted as needed, and sponsor-provided albuterol/salbutamol (rescue medication) is supplied as needed.
Outcome and safety measures
As the objective of this study is to determine whether roflumilast added to an ICS/LABA combination could offer greater benefit for reducing COPD exacerbations, the primary efficacy measure for this study is the rate of moderate or severe COPD exacerbations per participant per year. Moderate COPD exacerbations are defined as those requiring corticosteroid treatment; severe COPD exacerbations are those that require hospitalization and/or those that lead to death. Secondary efficacy outcomes include mean change in prebronchodilator FEV1 from randomization to Week 52 of double-blind treatment, the rate of severe COPD exacerbations, and the rate of moderate, severe, or antibiotic-treated COPD exacerbations. Additional efficacy assessments include spirometry (FVC, FEV1/FVC ratio, and forced expiratory volume in the first 6 seconds [FEV6]), rescue medication use, the COPD Assessment Test, daily symptoms as measured by the EXAcerbation of Chronic Pulmonary Disease Tool – Patient Reported Outcomes (EXACT-PRO) tool, and all-cause and COPD-related hospitalizations.
Safety assessments include AE reporting, vital signs, physical examinations, body weight, electrocardiograms, clinical laboratory measures, major adverse cardiac events, and the Columbia Suicide Severity Rating Scale (C-SSRS). Any AE not present before the first dose of study drug that occurred during the double-blind phase or increased in severity during treatment with the investigational product is classified as a treatment-emergent AE.
Pharmacokinetics
For serial pharmacokinetic (PK) analyses, blood samples will be taken from 40 participants at several study sites to determine roflumilast and roflumilast N-oxide plasma concentration–time profiles. Samples will be collected on Visit 7 at the following time points: predose (0 hour) and 0.25 hour, 0.5 hour, 1 hour, 2 hours, 4 hours, 6 hours, 8 hours, 12 hours, and 24 hours postdose. For sparse population PK analyses, predose blood samples will be collected from ~20% of the total study population (n=420) on Visits 5, 7, and 8.
Statistical analyses
Efficacy analyses will be performed on the intention-to-treat (ITT) population, consisting of all randomized participants who received one or more dose of double-blind study drug and based on the treatment to which they were randomized. Safety analyses will be analyzed for the safety population, consisting of all randomized participants who received one or more dose of study drug and based on the treatment received. Demographic and other baseline characteristics will be summarized by treatment and pooled across treatment groups for the safety and ITT populations. The primary outcome will be analyzed using a negative binomial regression model with number of COPD exacerbations as a dependent variable, treatment and stratum (LAMA or no LAMA) as independent variables, and logarithm of exposure time (in years) as an offset. Change in prebronchodilator FEV1 and other spirometry measures will be assessed via repeated measurement models, with the dependent variable being change from randomization at each scheduled postrandomization visit, and treatment, stratum (LAMA or no LAMA), baseline, time, treatment by time and baseline by time interactions as independent variables. All statistical tests will be two sided and performed at the 5% significance level for the main effects; confidence intervals will be two sided with 95% confidence. Based on assumptions of a mean of 1.35 COPD exacerbations per participant per year in the placebo group and a mean exposure time of 287 days across both treatment arms, a sample size of 2,300 randomized participants has ≥90% power to detect an 18% reduction in COPD exacerbation rates with roflumilast treatment versus placebo.
Additionally, a sensitivity analysis will be performed to determine whether missing data from participants after premature treatment discontinuation have an effect on the primary outcome measure (moderate–severe exacerbation rates). A negative binomial regression will be applied on the pre- and postdiscontinuation data (collected from follow-up calls and visits). Exposure time will be recalculated only for early-terminating participants based on the last data collected. A second analysis will be performed to examine the potential effect of participants in the no LAMA stratum who initiate LAMA after study randomization. Exacerbation data collected after these participants begin LAMA use will not be analyzed, and exposure calculations will only include the number of days of pre-LAMA treatment.
All safety data will be summarized, and no formal hypothesis tests will be used. The serial PK parameters of peak plasma concentration (Cmax), area under the curve, time to peak plasma concentration (Tmax), and half-life (T1/2) will be calculated and summarized. Sparse PK and serial PK data will be added to an existing roflumilast population PK model combining data from several studies.
Results
Across 17 countries, 2,354 participants were randomized between September 2011 and October 2014. The study enrollment goal was met in October 2014 (Figure 2), and study completion occurred in June 2016. Demographics of the study population are described in Table 2.
  | Figure 2 Timeline of participant enrollments and discontinuations. |
Discussion
Acute exacerbations of COPD are detrimental to patient health and pose a great economic burden to society.5–7 One way to reduce this disease burden is by using optimum treatments to reduce the number of recurring COPD exacerbations. Several medications, such as inhaled ICS and LABA, have been shown to reduce COPD exacerbations.19,20 While both of these drugs are more effective than placebo when given on their own,19,20 they are more efficacious when given in combination.21 Inhaled LAMAs have also been shown to reduce COPD exacerbation frequency,22 and combining them with ICS/LABA treatments may further improve efficacy.23,24 While this triple combination is frequently prescribed in clinical practice,23,25 data on its efficacy are limited, and its use remains controversial.23,24 Monotherapy with the orally administered phosphodiesterase-4 inhibitor, roflumilast, can decrease the frequency of COPD exacerbations in patients with severe COPD and a history of bronchitis and COPD exacerbations.10,13 Whether there are added benefits when roflumilast is combined with either ICS/LABA therapy or ICS/LABA/LAMA is an important clinical question that the REACT18 and RE2SPOND studies were designed to address.
As both roflumilast and ICS may reduce airway inflammation through different mechanisms of action,8,15 combining roflumilast, ICS, and long-acting bronchodilators may maximize anti-inflammatory effects while providing the additional benefit of bronchodilation. In the REACT trial, roflumilast added to concomitant ICS/LABA therapy reduced moderate–severe COPD exacerbations by 13%–14% and decreased the rates of severe exacerbations and exacerbation-related hospital admissions by 24% in participants with severe COPD.18 Similar to the REACT trial, participants in the RE2SPOND study must remain on ICS/LABA treatment throughout the study. The RE2SPOND protocol limits concurrent LAMA use to 60% of participants to ensure robust analyses of the effects of roflumilast when administered with concurrent ICS/LABA or triple therapy. In the REACT study, 70% of enrolled participants were on concurrent LAMA as no such restrictions on LAMA use were imposed.18 Notably, significant reductions in exacerbation rates were observed in participants using roflumilast added to ICS/LABA treatment, regardless of concomitant LAMA use. In the RE2SPOND trial, 47% of enrolled participants were on ICS/LABA/LAMA triple therapy at baseline, which should provide the opportunity to assess any potential additive effects of roflumilast with concurrent dual or triple therapy in distinct patient populations. If the results of this study confirm that these combinations are safe and effective in decreasing COPD exacerbation rates, this would present strong evidence supporting roflumilast as an added option for patients who continue to experience COPD exacerbations while on ICS/LABA or ICS/LABA/LAMA.
RE2SPOND will also allow us to address additional questions of clinical relevance. As the roflumilast knowledge base grows, it will be possible to determine whether there are beneficial effects or AEs in specific groups of COPD participants, such as those with common comorbidities such as diabetes and heart disease.5,26 Compared with the REACT study,18 the RE2SPOND study had slightly fewer males (68.7% vs 74.6%), current smokers (39.4% vs 43.6%), and a higher percentage of patients with very severe COPD (39.3% vs 29.1%). However, both studies share similar methodologies and generally similar baseline patient characteristics, potentially allowing these data sets to be pooled for more robust analyses.
Roflumilast was approved based on data that demonstrated its efficacy and acceptable safety profile, but questions remained about its role when added to other therapies, especially ICS/LABA combinations. Results from RE2SPOND, a study conducted as part of a postmarketing commitment, will address this gap and provide important information to help guide clinical practice.
Conclusion
This Phase IV study will further characterize the effects of roflumilast in combination with common ICS/LABA fixed-dose drugs on COPD exacerbation rates, lung function, and health status of patients with severe–very severe COPD who are at risk of further COPD exacerbations. Results from this study will be vital in determining the clinical benefits of the US formulation of roflumilast (uncoated tablet) when combined with standard-of-care inhaled COPD treatments.
Acknowledgments
The authors thank Kristen Andersen, PhD, and Lynn Anderson, PhD, of Prescott Medical Communications Group, Chicago, IL, USA, for medical writing support funded by AstraZeneca LP (Wilmington, DE, USA). This information was previously presented in poster format at the 2015 American Thoracic Society Annual Meeting, May 15–20, 2015, Denver, CO, USA. This study was supported by Forest Laboratories LLC, an affiliate of Actavis, Inc (Parsippany, NJ, USA) and AstraZeneca LP (Wilmington, DE, USA).
Author contributions
All authors provided substantial contributions to the convention or design of the work, acquisition, analysis, or interpretation of data for the work. All authors drafted the work or revised it critically for important intellectual content. All authors provided final approval of the version to be published and have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
SI Rennard has received personal fees from the American Board of Internal Medicine, Able Associates, Advantage Healthcare, Align2Action, Almirall, Association of Polysomnography Technologist, American Thoracic Society, AstraZeneca, Baxter, Boehringer-Ingelheim, Chiesi, CIPLA, ClearView Healthcare, Cleveland Clinic, CME Incite, Complete Medical Group, COPDFoundation, Cory Paeth, CSA, CSL, California Thoracic Society Carmel, Daiichi Sankyo, Decision Resources, Dunn Group, Easton Associates, Elevation Pharma, FirstWord, Forest, Frankel Group, Gerson, GlaxoSmithKline, Gilead, Grifols, GroupH, Guidepoint Global, Haymarket, HealthStar, Huron Consulting, Incite, Inthought, IntraMed (Forest), Johnson & Johnson, LEK, McKinsey, Medical Knowledge, MedImmune, Methodist Health System (Dallas), Navigant, NCI Consulting, Novartis, Nuvis, Pearl, Penn Technology, Pfizer, PlanningShop, Prescott, Pro Ed Comm, ProiMed, PSL FirstWord, Pulmatrix, Quadrant, Qessential, Regeneron, Saatchi and Saatchi, Schlesinger Associates, Strategic North, Synapse, Takeda, Theron, and WebMD. He also received grant support from the National Heart, Lung, and Blood Institute, the Nebraska Department of Health and Human Services, Otsuka, Pfizer, GlaxoSmithKline, Boehringer-Ingelheim, Nycomed, AstraZeneca, Centocor, and Almirall and is an employee of AstraZeneca. FJ Martinez has received personal fees from Forest, Janssen, GlaxoSmithKline, Nycomed/Takeda, Amgen, AstraZeneca, Boehringer-Ingelheim, Carden Jennings, CSA Medical, Ikaria/Bellerophon, Genentech, Merck, Novartis, Pearl, Pfizer, Roche, Sunovion, Theravance, Axon Communication, CME Incite, California Society for Allergy and Immunology, Annenberg, Inova Health System, Integritas, InThought, Miller Medical, National Association for Continuing Education, Paradigm, PeerVoice, UpToDate, Haymarket Communications, St John’s Hospital, St Mary’s Hospital, Western Society of Allergy and Immunology, Informa, Bioscale, Unity Biotechnology, Kadmon, Vertex, American Thoracic Society, Academic CME, Falco, MedScape, Genzyme, Johnson & Johnson, University of Texas Southwestern, and Spectrum Health System. He also received nonfinancial support from Boehringer-Ingelheim, Centocor, Gilead, Promedior, Roche/Genentech, Bayer, Veracyte, and Biogen and a grant from the National Institutes of Health. KF Rabe received a grant from the German Federal Ministry of Education and Research, Novartis, and Boehringer-Ingelheim. He has also received personal fees from AstraZeneca, Boehringer-Ingelheim, Chiesi Pharmaceutical, Novartis, Takeda, and Intermune. S Sethi has received personal fees from AstraZeneca, Boehringer-Ingelheim, Forest, GlaxoSmithKline, Novartis, Pearl, and Sunovion. He also received grant support from AstraZeneca, GlaxoSmithKline, Pearl, and Dey. E Pizzichini has nothing to disclose. A McIvor has received personal fees for honoraria CME from AstraZeneca, Novartis, Merck, and Boehringer-Ingelheim. S Siddiqui is an employee of AstraZeneca. A Anzueto has received a grant from GlaxoSmithKline and consultant fees from GlaxoSmithKline, AstraZeneca, Novartis, and Boehringer-Ingelheim. H Zhu is an employee of Allergan. The authors report no other conflicts of interest in this work.
References
Tashkin DP. Roflumilast: the new orally active, selective phophodiesterase-4 inhibitor, for the treatment of COPD. Expert Opin Pharmacother. 2014;15(1):85–96. | ||
Hatzelmann A, Morcillo EJ, Lungarella G, et al. The preclinical pharmacology of roflumilast – a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2010;23(4):235–256. | ||
Heron M. Deaths: Leading Causes for 2010. National Vital Statistics Reports. Vol. 62, No. 6. Hyattsville, MD: National Center for Health Statistics; 2013. | ||
Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged ≥ 18 years in the United States for 2010 and projections through 2020. Chest. 2015;147(1):31–45. | ||
Wouters EF, Groenewegen KH, Dentener MA, Vernooy JH. Systemic inflammation in chronic obstructive pulmonary disease: the role of exacerbations. Proc Am Thorac Soc. 2007;4(8):626–634. | ||
Yu AP, Yang H, Wu EQ, Setyawan J, Mocarski M, Blum S. Incremental third-party costs associated with COPD exacerbations: a retrospective claims analysis. J Med Econ. 2011;14(3):315–323. | ||
Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 2013;11:181. | ||
Grootendorst DC, Gauw SA, Verhoosel RM, et al. Reduction in sputum neutrophil and eosinophil numbers by the PDE4 inhibitor roflumilast in patients with COPD. Thorax. 2007;62(12):1081–1087. | ||
Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(2):154–161. | ||
Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ; M2-124 and M2-125 Study Groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374(9691):685–694. | ||
Rennard SI, Calverley PM, Goehring UM, Bredenbroker D, Martinez FJ. Reduction of exacerbations by the PDE4 inhibitor roflumilast – the importance of defining different subsets of patients with COPD. Respir Res. 2011;12:18. | ||
Daliresp® (roflumilast tablets). US prescribing information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015. | ||
Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374(9691):695–703. | ||
Hanania NA, Calverley PM, Dransfield MT, et al. Pooled subpopulation analyses of the effects of roflumilast on exacerbations and lung function in COPD. Respir Med. 2014;108(2):366–375. | ||
Cazzola M, Picciolo S, Matera MG. Roflumilast in chronic obstructive pulmonary disease: evidence from large trials. Expert Opin Pharmacother. 2010;11(3):441–449. | ||
Drummond MB, Dasenbrook EC, Pitz MW, Murphy DJ, Fan E. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300(20):2407–2416. | ||
Moodley T, Wilson SM, Joshi T, et al. Phosphodiesterase 4 inhibitors augment the ability of formoterol to enhance glucocorticoid-dependent gene transcription in human airway epithelial cells: a novel mechanism for the clinical efficacy of roflumilast in severe chronic obstructive pulmonary disease. Mol Pharmacol. 2013;83(4):894–906. | ||
Martinez FJ, Calverley PMA, Goehring U-M, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet. 2015;385(9971):857–866. | ||
Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7):Cd002991. | ||
Kew KM, Mavergames C, Walters JA. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;(10):Cd010177. | ||
Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. | ||
Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. | ||
Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146(8):545–555. | ||
Cazzola M, Page C. Long-acting bronchodilators in COPD: where are we now and where are we going? Breathe. 2014;10(2):110–120. | ||
Rennard S, Thomashow B, Crapo J, et al. Introducing the COPD foundation guide for diagnosis and management of COPD, recommendations of the COPD foundation. COPD. 2013;10(3):378–389. | ||
Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(8):631–639. | ||
Calverley PM, Martinez FJ, Fabbri LM, Goehring UM, Rabe KF. Does roflumilast decrease exacerbations in severe COPD patients not controlled by inhaled combination therapy? The REACT study protocol. Int J Chron Obstruct Pulmon Dis. 2012;7:375–382. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.