Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Effects Of PPARγ2 Pro12Ala Variant On Adipocyte Phenotype Dependent Of DHA
Authors Wan R, Ding Z, Xia S, Zheng L, Lu J
Received 5 May 2019
Accepted for publication 3 October 2019
Published 1 November 2019 Volume 2019:12 Pages 2273—2279
DOI https://doi.org/10.2147/DMSO.S214526
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Renhui Wan,* Zhengping Ding,* Sheng Xia, Longyi Zheng, Jin Lu
Department of Endocrinology, Changhai Hospital, Second Military Medical University, Shanghai 200433, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jin Lu; Longyi Zheng
Department of Endocrinology, Changhai Hospital, Second Military Medical University, No.168, Changhai Road, Shanghai 200433, People’s Republic of China
Email [email protected]; [email protected]
Background: Peroxisome proliferator-activated receptor γ2 (PPARγ2) plays a critical role in the regulation of adipocyte differentiation and adipocytokine production. The Pro12Ala variant is the most common mutation in the PPARγ2 gene. Its effect appears to be sensitive to dietary factors, such as docosahexaenoic acid (DHA) level. The purpose of this study was to investigate the interaction effect between PPARγ2 Pro12Ala variant and DHA on the phenotypes of adipocytes.
Methods: We generated stable 3T3-L1 cell lines expressing wild-type PPARγ2 or PPARγ2 Pro12Ala variant. These two cell lines were cultured with different concentrations of DHA (0, 50, 200 umol/L). Then Oil red O staining was used to observe cell differentiation and the degree of lipid accumulation, TUNNEL assay was used to detect cell apoptosis, and ELISA assays were used to detect the changes of TNF-α, resistin and adiponectin levels in cell culture supernatant.
Results: PPARγ2 Pro12Ala variant reduced lipid droplet accumulation in 3T3-L1 preadipocytes treated with or without 50 μmol/L DHA, but not with 200 μmol/L DHA, compared to that of wild-type PPARγ2. PPARγ2 reduced resistin production and increased adiponectin production in 3T3-L1 adipocytes, whereas PPARγ2 Pro12Ala variant diminished these effects. However, the absence of DHA blocked PPARγ2 Ala12 variant-induced effects on adiponectin production. There was no significant difference in TNF-α secretion between wild-type PPARγ2 and PPARγ2 Pro12Ala cells whether with or without DHA.
Conclusion: These results indicated that the effects of PPARγ2 Pro12Ala variant were dependent on DHA concentration.
Keywords: peroxisome proliferator-activated receptor γ2, Pro12Ala variant, docosahexaenoic acid, adipocyte, differentiation, adiponectin
Introduction
Peroxisome proliferator-activated receptor gamma (PPARγ), particularly the PPARγ2 isoform, is a ligand-dependent nuclear receptor highly expressed in adipose tissue. Activation of PPARγ2 regulates adipocyte differentiation, triglyceride storage, and some metabolic effects, including glucose homeostasis and insulin sensitivity.1,2
The Pro12Ala variant of the PPARγ2 gene is common (3–14%)3 and can cause a moderate decrease in its transcriptional activity and adipogenic potential.4 Human population studies on PPARγ2 gene Pro12Ala polymorphism showed the association between this variant and reduced weight gain and reduced risk of type 2 diabetes.5,6 Moreover, the Pro12Ala variant appears to be sensitive to environmental effects, such as dietary factors.7–9
Long-chain n-3 polyunsaturated fatty acids, such as docosahexaenoic acid (DHA), have been reported to reduce adiposity by preventing fat accretion and improve glycolipid metabolic disorders in high-fat diet-fed rats.10 Additionally, a randomized clinical trial showed that DHA exhibited anti-inflammatory effects.11,12
Thus, in this study, we aimed to examine the gene-environment interactions between PPARγ2 Pro12Ala variant and DHA in 3T3-L1 preadipocytes. We generated stable 3T3-L1 cell lines expressing wild-type PPARγ2 or PPARγ2 Pro12Ala variant. Then, the effects of wild-type PPARγ2 and PPARγ2 Pro12Ala variant on the differentiation, apoptosis, and adipocytokine levels were investigated in 3T3-L1 preadipocytes treated with or without different concentrations of DHA.
Materials And Methods
Cell Culture
HEK293T cells were obtained from the American type culture collection (ATCC). 3T3-L1 preadipocytes were obtained from the Chinese Academy of Sciences Cell Bank. All cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (Gibco), penicillin (100 U/mL), and streptomycin (100 mg/mL) in a humidified incubator with 5% CO2 (v/v) at 37 °C.
Construction Of Lentivirus Plasmids Containing PPARγ2 Pro Or PPARγ2 Ala Gene
Wild-type PPARγ2 and PPARγ2 Pro12Ala genes were polymerase chain reaction (PCR)-amplified from human adipose tissue cDNA library. The PCR products were subcloned into the pMD18-T vectors (Takara), and the Pro12Ala variant was confirmed by sequencing (Shanghai Boshang Biotechnology Co. Ltd.). Then, the products were cloned into pLVTHM via restriction digestion (EcoRI/XhoI) and T4 DNA ligase (TOYOBO) to construct the pLVTHM-PPARγ2 Pro and pLVTHM-PPARγ2 Ala plasmids.
Preparation Of Lentivirus
Lentiviral supernatants were produced in HEK293T cells by co-transfection of 20 μg of transfer vectors harboring the indicated genes (empty vector, pLVTHM-PPARγ2 Pro, or pLVTHM-PPARγ2 Ala), 10 μg of pRsv-REV, 15 μg of pMDlg-pRRE, and 7.5 μg of pMD2G, according to the manufacturer’s protocol. Supernatants containing lentivirus were collected at 48 h after transfection. Cell debris was removed by centrifugation at 4000 ×g for 10 min at 4 °C, followed by filtration through a 0.45 μm polyethersulfone membrane filter unit. Then, the virus was concentrated at 72,000 ×g for 120 min at 4 °C.
Generation Of Stable Cell Lines
3T3-L1 cells were seeded on a 24-well plate at a density of 5 × 104 cells/well. Then, in the presence of polybrene (1 μg/mL), cells were infected with pLVTHM-GFP lentivirus (LV-vector group), pLVTHM-PPARγ2 Pro lentivirus (Lv-PPARG2 group), or pLVTHM-PPARγ2 Ala lentivirus (Lv-PPARG2 P12A group). After 24 h incubation at 37 °C, the culture medium was replaced with a fresh complete medium without polybrene. At 72 h post-infection, stably transfected cell lines were selected using puromycin (Sigma-Aldrich; Merck Millipore) at a concentration of 1 μg/mL for 7 days.
Quantitative RT-PCR Assay
Total RNA was extracted using Trizol (Invitrogen), according to the manufacturer’s instructions. cDNA was synthesized from 1 μg of RNA using a cDNA reverse transcription kit with RNase inhibitor (MBI Fermentas). Real-time PCR was performed in a thermal cycler (ABI) using Quant qRT-PCR (SYBR Green I) kit (TIANGEN). The △CT method was used to measure the relative expression. Results were normalized to that of a reference gene (β-actin). The primer sequences were as follows: PPARγ2 (5ʹ-GGAGCCCAAGTTTGAGTTTGCTGT-3ʹ, 5ʹ-AGGGCTTGTAGCAGGTTGTCTTGA3ʹ) and β-actin (5ʹ-TGTGATGGTGGGAATGGGTCAGAA-3ʹ, 5ʹ-TGTGGTGCCAGATCTTCTCCATGT-3ʹ).
Fatty Acid Treatments
On the first day of cell differentiation, the same number of 3T3-L1 cells were inoculated into 24-well plates. 3T3-L1 cells were treated with different concentrations of DHA (0, 50, or 200 μmol/L) and MDI (Sigma) containing 0.5 mM 3-isobutyl-1-methylxanthine, 1 μM dexamethasone, and 1 μg/mL insulin.
Oil Red O Staining
After incubation with DHA for 6 h, the cells were washed three times with phosphate-buffered saline (PBS), fixed with 10% formaldehyde for 30 min, and then stained with 0.5% Oil Red O (Sigma) for 20 min at 20 °C. Red-stained adipocytes were observed under a phase-contrast microscope (Olympus). To quantify Oil Red O levels, 2.5 mL of 100% isopropanol was added to each well. After shaking at room temperature for 5 min, the optical density (OD) of samples was measured at 500 nm using a spectrophotometer (UV-765).
TUNEL Assay
A one-step TUNEL cell apoptosis detection kit (Ggreen fluorescence) was purchased from Beyotime Biotechnology Research Institute. Briefly, cells cultured with DHA for 2 h were fixed with 4% paraformaldehyde for 30–60 min at room temperature. After washing with PBS, the cells were permeabilized with PBS containing 0.1% Triton X-100 on ice for 2 min. Then, they were stained with a freshly-prepared TUNEL detection solution (20 μL of TdT enzyme, 480 μL of the fluorescent labeling solution, and 500 μL of the TUNEL detection solution) in dark at 37 °C for 60 min, followed by sealing using the antifluorescence quenching sealing fluid. Finally, apoptosis rates were evaluated using a fluorescence microscope.
Enzyme-Linked Immunosorbent Assay (ELISA)
Adiponectin ELISA kit was purchased from B-Bridge International Corporation. Tumor necrosis factor-α (TNF-α) and resistin ELISA kits were obtained from Quantikine Corporation. Cytokine levels were measured by taking cell medium, according to the manufacturer’s instructions.
Statistical Analysis
Values are expressed as the means ± standard error of the mean (SEM). Data were analyzed using SPSS package program version 22.0. One-way analysis of variance followed by Student-Newman-Keuls (SNK) post-hoc test was used to compare the responses among different groups. A P-value < 0.05 was considered statistically significant.
Results
Generation Of Stable Cell Lines
To investigate the effects of PPARγ2 genotypes in 3T3-L1 cells, we generated stable cell lines expressing wild-type PPARγ2 (Lv-PPARG2) or PPARγ2 Pro12Ala variant (Lv-PPARG2 P12A). Plasmids containing the Pro12Ala variant were confirmed by sequencing. The presence of the CCA→GCA mutation (Pro/Ala) at codon 12 of PPARγ2 exon B without additional changes in the coding sequence was verified. The expression of PPARγ2 in stable cell lines was verified by real-time PCR assay. The expression of PPARγ2 in 3T3-L1 cells transfected with Lv-PPARG2 or Lv-PPARG2 P12A increased by 10 fold, compared to that in 3T3-L1 cells transfected with Lv-vector.
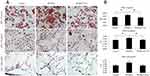
Effects Of PPARγ2 Pro12Ala Variant On Lipid Droplet Formation In DHA-Treated 3T3-L1 Adipocytes
The effects of PPARγ2 genotypes on 3T3-L1 preadipocyte differentiation were investigated. Lv-vector, Lv-PPARG2, or Lv-PPARG2 P12A-transfected 3T3-L1 cells were treated with different concentrations of DHA. Cells were stained with Oil Red O to evaluate the degree of lipid accumulation. As shown in Figure 1, overexpression of PPARγ2 increased lipid accumulation in 3T3-L1 cells treated with 0 or 50 μmol/L DHA, whereas overexpression of PPARγ2 Pro12Ala variant reduced lipid accumulation, compared to that of PPARγ2. However, at a concentration of 200 μmol/L DHA, there was no statistical difference among the control, PPARγ2 Pro12, and PPARγ2 Pro12Ala-expressing adipocytes.
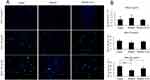
Effects Of PPARγ2 Pro12Ala Variant On Adipocyte Apoptosis In DHA-Treated 3T3-L1 Adipocytes
We then evaluated apoptosis ratios in different groups. As shown in Figure 2, overexpression of wild-type PPARγ2 or PPARγ2 Pro12Ala variant did not affect apoptosis in 3T3-L1 adipocytes treated with 0 or 50 μmol/L DHA. However, in both wild-type PPARγ2 and PPARγ2 Pro12Ala variant-expressing adipocytes treated with 200 μmol/L DHA, apoptosis ratios were reduced, compared to that in the control group. At all DHA concentrations, there was no statistical difference in apoptosis ratios between 3T3-L1 adipocytes overexpressing wild-type PPARγ2 and PPARγ2 Pro12Ala variant.
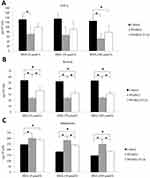
Effects Of PPARγ2 Pro12Ala Variant On Inflammatory Cytokine Levels In DHA-Treated 3T3-L1 Adipocytes
Furthermore, we examined the effects of PPARγ2 genotypes on the secretion of inflammatory cytokines in 3T3-L1 adipocytes. As shown in Figure 3, overexpression of wild-type PPARγ2 decreased the secretion of proinflammatory cytokines and resistin from 3T3-L1 adipocytes treated with or without DHA. Moreover, overexpression of PPARγ2 Pro12Ala variant partially abolished these effects (Figure 3B). On the contrary, overexpression of wild-type PPARγ2 increased the secretion of adiponectin from 3T3-L1 adipocytes treated with or without DHA. Similarly, overexpression of PPARγ2 Pro12Ala variant in DHA-treated cells attenuated these effects. However, the absence of DHA blocked PPARγ2 Ala12 variant-induced effects on adiponectin production (Figure 3C). However, there was no significant difference in TNF-α secretion between wild-type PPARγ2 and PPARγ2 Pro12Ala-expressing adipocytes, regardless of DHA treatment (Figure 3A).
Dissussion
PPARγ2 is a nuclear receptor involved in lipid metabolism, adipocyte differentiation, and proliferation.13 A missense mutation (CCGPro-GCGAla) at codon 12 is a common variant of PPARγ2 gene. Meta-analyses showed that PPARγ2 Pro12Ala variant correlated with improved insulin sensitivity.14,15 Another clinical study verified that PPAR-γ2 Pro12Ala variant had modest protective effects against the development of type 2 diabetes.16
In our study, overexpression of wild-type PPARγ2 gene resulted in increased lipid droplet accumulation and adipocyte differentiation. PPARγ2 Pro12Ala variant with or without 50 μmol/L DHA treatment attenuated these effects, suggesting that PPARγ2 Pro12Ala variant might correlate with lower risk of obesity. However, there was no significant difference in lipid droplet accumulation between wild-type PPARγ2 and PPARγ2 Pro12Ala1-expressing adipocytes treated with 200 μmol/L DHA, indicating that the effects of PPARγ2 Pro12Ala variant was dependent on the concentration of DHA to some extent. Meantime, our results showed that DHA can induce apoptosis of adipocytes and this effect becomes more obvious with the increase of DHA concentration. This may also be the reason why there is no statistically significant difference in 200μmol/L DHA group.
However, overexpression of PPARγ2 did not affect apoptosis of adipocytes except when incubated with high concentration (200μmol/L) of DHA. And PPARγ2 Pro12Ala variant showed no effect on apoptosis of adipocytes.
It has been reported that some adipocytokines, such as resistin, TNFα, and adiponectin, are under the transcriptional control of PPARγ2. Resistin belongs to the family of cysteine-rich proteins, called resistin-like molecules.17 The expression of resistin positively correlated with body fat, insulin, glucose, and triglyceride levels in high-fat diet-fed mice.18 Additionally, a PPARγ agonist was shown to improve insulin sensitivity via downregulation of resistin expression.19 PPARγ also regulates the circulating levels of adiponectin, where the effects of PPARγ on adiponectin production are partially attributed to the direct activation of adiponectin gene transcription.20 Adiponectin expression and its signaling pathway are directly regulated by PPARγ.
In line with the results of previous studies, we showed that overexpression of PPARγ2 gene reduced TNF-α and resistin production and increased adiponectin production. However, overexpression of PPARγ2 Pro12Ala variant attenuated these effects. It is noteworthy that a significant difference in adiponectin level between wild-type PPARγ2 and PPARγ2 Pro12Ala-expressing cells was only observed in cells treated with DHA (50 and 200 μmol/L). There was no significant difference in adiponectin level between wild-type PPARγ2 and PPARγ2 Pro12Ala-expressing DHA-untreated cells. Additionally, PPARγ2 Pro12Ala variant showed no effect on TNF-α secretion in adipocytes, compared to that of wild-type PPARγ2.
In summary, our study showed that overexpression of PPARγ2 gene in preadipocytes resulted in increased lipid droplet accumulation and adipocyte differentiation, and exhibited anti-inflammatory effects by reducing proinflammatory cytokine (TNF-α and resistin) secretion and increasing anti-inflammatory cytokine (adiponectin) production. Most of these effects, except for TNF-α secretion, were attenuated owing to the overexpression of PPARG2 Pro12Ala variant. Moreover, the effects of PPARγ2 Pro12Ala variant varied at different concentrations of DHA treatment. The effects of PPARγ2 Pro12Ala variant on adiponectin secretion were dependent on the presence or absence of DHA treatment. Additionally, the effect of PPARγ2 Pro12Ala variant on lipid accumulation disappeared with the increase in DHA concentration. Thus, our results showed that the effects of PPARγ2 Pro12Ala variant were dependent on gene-environment interactions. Several clinical studies also found that the regulation of body fat by PPARγ2 Pro12Ala variant was affected by dietary conditions.21,22 However, further studies are needed to clarify the exact effects of PPARγ2 Pro12Ala variant under different dietary conditions.
Abbreviations
ATCC, American type culture collection; DHA, docosahexaenoic acid; DMEM, Dulbecco’s modified Eagle’s medium; PBS, phosphate-buffered saline; PPAR, peroxisome proliferator-activated receptor; SNK, Student-Newman-Keuls; TNF-α, tumor necrosis factor-α.
Author Contributions
JL and LYZ designed the study. RHW, ZPD and SX performed the experiments. RHW analyzed the data and wrote the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Knouff C, Auwerx J. Peroxisome proliferator-activated receptor-gamma calls for activation in moderation: lessons from genetics and pharmacology. Endocr Rev. 2004;25:899–918. doi:10.1210/er.2003-0036
2. Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi:10.1016/j.cell.2005.11.026
3. Kamble PG, Gustafsson S, Pereira MJ, et al. Genotype-based recall to study metabolic effects of genetic variation: a pilot study of PPARG Pro12Ala carriers. Ups J Med Sci. 2017;122:234–242. doi:10.1080/03009734.2017.1405127
4. Deeb SS, Fajas L, Nemoto M, et al. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet. 1998;20:284–287. doi:10.1038/3099
5. Gouda HN, Sagoo GS, Harding AH, Yates J, Sandhu MS, Higgins JP. The association between the peroxisome proliferator-activated receptor-gamma2 (PPARG2) Pro12Ala gene variant and type 2 diabetes mellitus: a HuGE review and meta-analysis. Am J Epidemiol. 2010;171:645–655. doi:10.1093/aje/kwp450
6. Masud S, Ye S, Group SAS. Effect of the peroxisome proliferator activated receptor-gamma gene Pro12Ala variant on body mass index: a meta-analysis. J Med Genet. 2003;40:773–780. doi:10.1136/jmg.40.10.773
7. Heikkinen S, Argmann C, Feige JN, et al. The Pro12Ala PPARgamma2 variant determines metabolism at the gene-environment interface. Cell Metab. 2009;9:88–98. doi:10.1016/j.cmet.2008.11.007
8. Luan J, Browne PO, Harding AH, et al. Evidence for gene-nutrient interaction at the PPARgamma locus. Diabetes. 2001;50:686–689. doi:10.2337/diabetes.50.3.686
9. Pihlajamaki J, Schwab U, Kaminska D, et al. Dietary polyunsaturated fatty acids and the Pro12Ala polymorphisms of PPARG regulate serum lipids through divergent pathways: a randomized crossover clinical trial. Genes Nutr. 2015;10:43. doi:10.1007/s12263-015-0493-z
10. Samane S, Christon R, Dombrowski L, et al. Fish oil and argan oil intake differently modulate insulin resistance and glucose intolerance in a rat model of dietary-induced obesity. Metabolism. 2009;58:909–919. doi:10.1016/j.metabol.2009.02.013
11. de Luis D, Domingo JC, Izaola O, Casanueva FF, Bellido D, Sajoux I. Effect of DHA supplementation in a very low-calorie ketogenic diet in the treatment of obesity: a randomized clinical trial. Endocrine. 2016;54:111–122. doi:10.1007/s12020-016-0964-z
12. Munro IA, Garg ML. Prior supplementation with long chain omega-3 polyunsaturated fatty acids promotes weight loss in obese adults: a double-blinded randomised controlled trial. Food Funct. 2013;4:650–658. doi:10.1039/c3fo60038f
13. Hegele RA, Cao H, Harris SB, Zinman B, Hanley AJ, Anderson CM. Peroxisome proliferator-activated receptor-gamma2 P12A and type 2 diabetes in Canadian Oji-Cree. J Clin Endocrinol Metab. 2000;85:2014–2019. doi:10.1210/jcem.85.5.6610
14. Li S, Chen W, Srinivasan SR, Boerwinkle E, Berenson GS; S. Bogalusa Heart. The peroxisome proliferator-activated receptor-gamma2 gene polymorphism (Pro12Ala) beneficially influences insulin resistance and its tracking from childhood to adulthood: the Bogalusa Heart Study. Diabetes. 2003;52:1265–1269. doi:10.2337/diabetes.52.5.1265
15. Ludovico O, Pellegrini F, Di Paola R, et al. Heterogeneous effect of peroxisome proliferator-activated receptor gamma2 Ala12 variant on type 2 diabetes risk. Obesity (Silver Spring). 2007;15:1076–1081. doi:10.1038/oby.2007.617
16. Florez JC, Jablonski KA, Sun MW, et al; Diabetes Prevention Program Research. Effects of the type 2 diabetes-associated PPARG P12A polymorphism on progression to diabetes and response to troglitazone. J Clin Endocrinol Metab. 2007;92:1502–1509. doi:10.1210/jc.2006-2275
17. Patel SD, Rajala MW, Rossetti L, Scherer PE, Shapiro L. Disulfide-dependent multimeric assembly of resistin family hormones. Science. 2004;304:1154–1158. doi:10.1126/science.1093466
18. Rajala MW, Qi Y, Patel HR, et al. Regulation of resistin expression and circulating levels in obesity, diabetes, and fasting. Diabetes. 2004;53:1671–1679. doi:10.2337/diabetes.53.7.1671
19. Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi:10.1146/annurev.biochem.77.061307.091829
20. Astapova O, Leff T. Adiponectin and PPARgamma: cooperative and interdependent actions of two key regulators of metabolism. Vitam Horm. 2012;90:143–162. doi:10.1016/B978-0-12-398313-8.00006-3
21. Aguayo-Armendariz J, Montalvo-Corral M, Gonzalez-Martinez KA, et al. Central obesity and body fat, but not body mass index, are associated with the Pro12Ala polymorphism in the peroxisome proliferator-activated receptor gamma gene in a population with a high consumption of saturated and trans-fatty acids. Nutr Res. 2018;57:28–35. doi:10.1016/j.nutres.2018.05.003
22. Rodrigues APS, Rosa LPS, Silveira EA. PPARG2 Pro12Ala polymorphism influences body composition changes in severely obese patients consuming extra virgin olive oil: a randomized clinical trial. Nutr Metab (Lond). 2018;15:52. doi:10.1186/s12986-018-0289-4
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.