Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Effects of polymorphism in FABP2 Ala54Thr on serum lipids and glycemic control in low glycemic index diets are associated with gender among Han Chinese with type 2 diabetes mellitus
Authors Liu PJ, Liu YP , Qin HK, Xing T, Li SS , Bao YY
Received 2 December 2018
Accepted for publication 4 February 2019
Published 27 March 2019 Volume 2019:12 Pages 413—421
DOI https://doi.org/10.2147/DMSO.S196738
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Peng Ju Liu,1,* Yan Ping Liu,1,* Hui Kun Qin,2 Tong Xing,2 Shan Shan Li,1 Yuan Yuan Bao1
1Departments of Clinical Nutrition, Peking Union Medical College Hospital, China Academic Medical Science and Peking Union Medical College, Beijing, People’s Republic of China; 2Department of Nutrition, Pinggu Hospital of Traditional Chinese Medicine, Beijing, People’s Republic of China
*These authors contributed equally to this work
Background/aims: Low glycemic index (GI) diets may have beneficial effects on glycemic control and serum lipid levels in patients with type 2 diabetes, but whether its effect is affected by polymorphisms of genes associated with lipid metabolism remains unclear. This study investigated whether the effects of a low-GI diet on serum lipids and glycemic control in patients with diabetes are associated with polymorphisms of FABP2 Ala54Thr (rs1799883).
Methods: A retrospective study was conducted involving 165 patients with type 2 diabetes mellitus (T2DM) who participated in two completed trials. Parameters reflecting the glycemic control, inflammatory factors, and fasting plasma lipids before and after intervention were measured, and the polymorphism of rs1799883 for each participant was genotyped using a MassARRAY. Differences between the genotypes of rs1799883 before or after the intervention were compared, and changes in the lipid profiles, glycemic control, inflammatory profiles, and dietary intake from baseline were analyzed using an analysis of covariance (generalized linear model).
Results: When the data were analyzed as a whole, after 4–5 weeks of similar low-GI diet intervention, we found that the decrease of triglycerides (TG) in the homozygous Ala54 carriers was more significant than that in the Thr54 allele carriers ([−0.58±1.24] vs [−0.14±1.08], P=0.015) with the adjustment for potential confounding factors; furthermore, compared with the Thr54 carriers, there was a significant trend in the decrease of total cholesterol (TC) in the homozygous Ala54 carriers (P=0.057). Subgroup analysis revealed that in women the homozygous Ala54 carriers exhibited a significant decrease of serum TG, TC, fasting blood glucose, and glycated albumin in women, but this was not noted in men.
Conclusion: The effect of FABP2 Ala54Thr polymorphism on response to blood lipids and glycemic control in low-GI diets is associated with gender among Han Chinese patients with T2DM.
Keywords: type 2 diabetes mellitus, plasma lipids, fatty acid-binding protein 2, single-nucleotide polymorphism, low glycemic index diet
Introduction
Plasma lipids, including low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), triglycerides (TG), and total cholesterol (TC), are heritable and modifiable risk factors for coronary artery disease,1 which is a leading cause of death.2 To manage and prevent heart disease, numerous human genetic studies of lipid levels have been performed to identify targets for therapies that treat dyslipidemia,1 with hundreds of genes proposed as candidates. Among them, the fatty acid-binding protein 2 (FABP2) gene, a member of the FABP superfamily, is located on the long arm of chromosome 4; it encodes for the intestinal fatty acid-binding protein, thus it plays a key role in the absorption and intracellular transport of long-chain fatty acids in the diet.3,4 An in vivo study proposed that, in the absence of confounding factors such as environmental and genetic variables, FABP2 polymorphism specifically influences small-intestinal lipid absorption without modifying glucose uptake or metabolism.5 The association between Ala54Thr polymorphism of FABP2 and several chronic diseases has been extensively studied. Thr54 carriers of FABP2 have been shown to be associated with higher body mass index (BMI), insulin resistance, metabolic syndrome, type 2 diabetes mellitus (T2DM), and both fasting and postprandial hyperlipidemia.4,6–11 Several studies have suggested that this variant of FABP2 increases the lipemic response to food ingestion and chronically exposes its carriers to postprandial hyperlipidemia.8,13 Furthermore, it was reported that Thr54 carriers have higher lipid oxidation rates than Ala54-homozygous persons do.13 All of the aforementioned factors may contribute to impaired insulin action, cause glucose intolerance, and increase diabetes risk.8,13,14 Individuals with T2DM, in addition to frequently exhibiting increased serum fasting TG and decreased HDL-c, may also exhibit elevated postprandial serum TG.15 Several studies have suggested the association of Ala54Thr polymorphism with increased serum fasting and postprandial TG,8,13 and Almeida et al12 found that the Thr54Thr genotype of FABP2 in patients with T2DM increased fatty acids absorption, and they suggested that this might increase susceptibility to the effects of dietary lipids.13 In addition, Ala54Thr FABP2 polymorphism has been reported to play a distinct role in T2DM among different populations and sexes.10,16 However, data on fat metabolism and glycemic response associated with this polymorphism in Han Chinese patients with T2DM are scarce.
The glycemic index (GI) is a classification of carbohydrate-containing foods according to the glycemic response that they evoke,17 and a low-GI diet is effective in glycemic control and in improving various markers of cardiovascular risk in people with diabetes.18 The utility of low-GI diets in the management of T2DM has been demonstrated by two systematic reviews, in which low-GI diets led to a 5% reduction in hemoglobin A1c (HbA1c).19,20 The effect of low-GI diets on plasma lipid profiles has also been the focus of considerable attention. However, the results of studies on the subject have shown inconsistent findings.18,21,22 Whether the effects of low-GI diets on serum lipid levels, glycemic control, and inflammatory factors are affected by polymorphisms of genes related to lipid metabolism is not yet clear; therefore, this study investigated whether the effects of low-GI diets on blood lipids, glycemic control, and inflammatory factors in patients with diabetes are affected by polymorphisms of FABP2 Ala54Thr (rs1799883).
Materials and methods
This was a retrospective study involving 165 individuals who had previously been recruited in our two completed trials, in which 114 people received intervention diets (daily substitution of either extruded adzuki bean convenience food or black-grained wheat for a partial staple food) and 51 people (as a co-control group of the two trials) received a traditional diabetic diet, with both diets being low-GI diets.23,24 The participants (aged between 30 and 80 years) were recruited from Pinggu Hospital of Traditional Chinese Medicine (Beijing, People’s Republic of China) in 2016, and they were all diagnosed with T2DM according to the American Diabetes Association Diagnosis Criteria.25 They all presented with at least one of the following: 1) latest lab-testing fasting glucose ≥6.1 mmol or 2) HbA1c >6.5%, as in the previous studies (Trial registration: NCT02999867 at www.ClinicalTrials.gov).23,24 The study protocol was approved by the Ethics Committee of Peking Union Medical College Hospital of the Chinese Academy of Medical Science (Unique Protocol ID: PUMCH-ZS-1048). This study was conducted in accordance with both the Declaration of Helsinki of 1975, as revised in 1983, and the guidelines of the center’s institutional review board. All the participants were informed of the details of the study, and every participant provided written informed consent. All participants received intensive nutritional education, mainly related to dietary nutrition and T2DM management every week during follow-up in the test period. Systematic dietary programs were designed according to the Dietary Guidelines for the Chinese Residents 2013 and the China Medical Nutrition Therapy Guidelines for Diabetes 2010.23,24 The participant’s medications and/or other clinical interventions needed to remain unchanged during the intervention period.
Dietary assessment
The dietary intake of each participant was assessed through 3-day food records that included 2 days from Monday to Friday and 1 day on the weekend. Before the intervention, the participants received a dietitian’s instructions regarding how to record their own diet diary, and they were given templates for diaries. After reviewing the diet records, the data were analyzed by using the Nutrition Clinic Consultation Management System (Zhending Health Technology Co. Ltd., Shanghai, People’s Republic of China).
Anthropometric measurement
Anthropometric measurements of individuals wearing light clothing and without shoes were conducted by well-trained examiners. Height was measured to the nearest 0.1 cm with a portable stadiometer. Weight was measured in an upright position to the nearest 0.1 kg with a calibrated scale. BMI was calculated according to the formula BMI = weight (kg)/height2 (m2), and waist circumference measurements were taken at the end of normal expiration to the nearest 0.1 cm, measuring from midway between the lower borders of the rib cage and the iliac crest.
Measurements of blood sample
All participants underwent venous blood collection in the morning after fasting for at least 8 hours to determine levels of fasting blood glucose (FBG), glycosylated HbA1c, glycated albumin (GA), tumor necrosis factor alpha (TNF-α), and IL-6 before and after intervention. In addition, concentrations of TC, TG, HDL-c, and LDL-c were measured from previously collected blood samples. FBG, TC, TG, HDL-c, and LDL-c concentrations were measured by an automatic analyzer (Olympus AU5400; Olympus, Tokyo, Japan) using commercial kits from Wako Pure Chemical Industries Ltd. (Tokyo, Japan). The level of HbA1c was analyzed using a glycosylated glucose meter (Bio-Rad Laboratories Inc., Hercules, CA, USA) based on HPLC. GA was tested through enzymology using the Japan Lucina GA-L kit from Asahi Kasei Corporation (Tokyo, Japan). Detection of hs-CRP using the immune transmission ratio was conducted using a Beckman Coulter LX-20 automatic biochemical analyzer (Beckman Coulter Inc., Brea, CA, USA). TNF-α and IL-6 levels were detected by ELISA using a Siemens Immulite 1000 (Siemens AG, Munich, Germany).
DNA extraction
DNA was extracted from the blood leukocytes using a TIANamp Blood DNA Maxi Kit DP349-02 (Tiangen Biotech Co, Ltd., Beijing, People’s Republic of China) according to the instruction manual. The polymorphisms of rs1799883 were genotyped using MassARRAY in this study. Extension probes were designed so that 25 different SNP assays could be amplified and analyzed in a PCR cocktail. The assay mix including forward, reverse, and extension primers and 10–20 ng of genomic DNA was heated for 2 minutes at 50°C, denatured at 95°C for 5 minutes, and cycled at 95°C for 15 seconds and 60°C for 1 minute for a total of 30 cycles. After purification, objective fragments were detected using a matrix-assisted laser desorption/ionization–time-of-flight mass spectrometer (Agena Bioscience, San Diego, CA, USA) and analyzed using the manufacturer’s software.
Statistical analysis
Statistical analysis was conducted using SPSS 16.0 software. The genotype distribution was assessed for the Hardy–Weinberg equilibrium and subsequently analyzed using the chi-squared test. First, regardless of gender, the participants were divided into two groups according to their genotypes, including the homozygous Ala54 group and the Thr54 group (Ala54/Thr54 and Thr54/Thr54). Then, considering possible gender differences in the effect of FABP2 on fat metabolism,9 the data were analyzed separately by gender. Categorical variables were represented by frequency or percentage and examined, whereas quantitative variables were presented as mean ± SD. Differences in age, course of disease, BMI, waist circumference, ratio of men, types of low-GI diet, and use of lipid-lowering agents between the groups at baseline were analyzed using chi-square or Student’s t-tests when appropriate. An analysis of covariance (generalized linear model) was performed to assess the differences in lipid profiles, inflammatory factors, glycemic control variables, nutrient intakes before and after intervention, as well as changes in lipid profiles, glycemic control, inflammatory profiles, and dietary intakes from baseline; P<0.05 was considered statistically significant.
Results
Basic characteristics of the study population
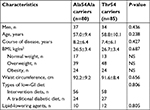
In our study, 165 individuals with T2DM were evaluated. A total of 80 (48.5%) had the homozygous Ala54 genotype, and 85 (51.5%) had the variant genotype (18 Thr54Thr and 67 Ala54Thr). According to the Hardy–Weinberg equilibrium, no significant difference was found between the observed value and expected value of genotype distribution (P>0.05). No significant differences were noted in age, course of disease, BMI, waist circumference, ratio of men, types of low-GI diet, and use of lipid-lowering agents between the two groups (Table 1).
  | Table 1 Basic characteristics of the two groups Abbreviations: BMI, body mass index; GI, glycemic index; NS, not significant. |
Dietary intake
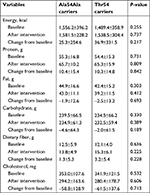
The dietary intake of each participant was assessed according to the dietary diaries recorded by the participant by using the Nutrition Clinic Consultation Management System (Zhending Health Technology Co. Ltd.), and the nutrient intakes were calculated automatically based on the Chinese Food Composition Table (2009).26 With the adjustment for age, sex, BMI, and course of disease, no significant differences (P>0.05 for all) were revealed in energy and nutrient intake between the Ala54Ala and Thr54 carriers before and after intervention (Table 2) or in both sex groups (data not shown).
Differences in variables between the two groups at baseline and after intervention and changes in variables from baseline
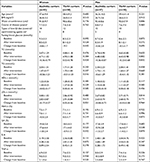
When the participants were divided into two groups according to the genotype rs1799883, TG levels were greater in the homozygous Ala54 carriers than they were in the variant genotype carriers at baseline ([2.17±1.87] vs [1.80±1.29], P=0.001), and no significant differences existed in FBG, HbA1c, GA, inflammatory factors, TC, HDL-c, and LDL-c at baseline and after intervention between the two groups (P>0.05 for all). When comparing changes of the aforementioned variables from baseline in the two groups with adjustment for age, sex, BMI, and disease course, we found that the decrease of TG in the homozygous Ala54 carriers was more significant than that in the variant genotype carriers ([−0.58±1.24] vs [−0.14±1.08], P=0.015). Furthermore, compared with the variant genotype carriers, a significant trend was found in the decrease of TC in the homozygous Ala54 carriers (P=0.057), and no significant differences were seen in the changes of FBG, HbA1c, GA, inflammatory factors, HDL-c, and LDL-c from baseline in the two groups (Table 3).
Differences between carriers of different genotypes in both sex groups at baseline and after intervention and changes in variables from baseline
To determine possible gender differences in the effects of FABP2 on metabolism, the data were analyzed separately by gender. Overall, no significant differences were found in age, BMI, waist circumference, and course of disease as well as the parameters of glycemic control, inflammation, and lipid profiles between the carriers of different genotypes in both sex groups at baseline and after intervention (P>0.05 for all). When comparing the changes of the aforementioned parameters from baseline in both sex groups with adjustments for age, BMI, and course of disease, we found that the decreases of FBG, TC, TG, and GA in the female homozygous Ala54 carriers were more significant than those in the variant genotype carriers. Moreover, in women, compared with variant genotype carriers, a significant trend was revealed in the decrease of LDL-c in the homozygous Ala54 carriers (P=0.051). In men, no significant differences were noted between carriers of different genotypes in the changes of parameters regarding glycemic control, inflammation, and lipid profiles (Table 4).
Discussion
The present study was a retrospective analysis of data from 165 patients with T2DM from our two previous completed trials. We observed the association between polymorphism of FABP2 Ala54Thr (rs1799883) with the response of glycemic control, lipid profiles, and inflammatory markers in an intervention featuring a low-GI diet for 4–5 weeks among Han Chinese patients with T2DM. In general, polymorphism of FABP2 Ala54Thr may affect the response of serum lipids to a low-GI diet. However, our subgroup analysis indicated that the effect of polymorphism of FABP2 Ala54Thr on glycemic control and serum lipids is more significant in women than in men.
Often, patients with T2DM exhibit increased serum fasting TG as well as elevated postprandial serum TG.15 For better glycemic control, a low-GI diet is often recommended to patients with T2DM due to its role in reducing HbA1c demonstrated by two systematic reviews.19,20 However, the effects of a low-GI diet on serum lipids have shown inconsistent findings.18,21,22 Fleming and Godwin reported that a low-GI diet may help lower total and LDL-c and had no significant effects on HDL-c and TG in their systematic review and meta-analysis.21 However, in the same year, another systematic review showed that low-GI diets were effective in increasing HDL-c but had no significant effects on LDL-c and TG in the management of individuals with T2DM.18 Notably, a more recent systematic review using the Cochrane Database concluded that no convincing evidence exists that low-GI diets have a clear beneficial effect on plasma lipids.22 Because plasma lipid levels are determined by a multifactorial process that involves both environmental and genetic factors, these inconsistent results are possibly associated with variables such as polymorphisms of genes related to lipid metabolism, different study populations, and differences in definitions of low-GI diets. Therefore, in addition to environmental factors, numerous studies have explored the effects of polymorphisms of genes associated with plasma lipid levels. Recently, genome-wide association studies revealed more than a 100 single-nucleotide polymorphisms that are associated with lipid levels and explain 35%–40% of the genetic variability in plasma lipid phenotypes.1,27,28 In addition to environmental factors including high-fat or high-carbohydrate diets, association of the Ala54Thr polymorphism with increased fasting and postprandial TG has been suggested,8,13 and the potential mechanism might be that the Thr54 genotype of FABP2 in patients with diabetes increases fatty acids absorption, which leads to an increased conversion to triacylglycerols,8 and increases susceptibility to the effects of dietary lipids.13 Furthermore, Nakanishi et al reported that the effects of FABP2 polymorphism on TG and LDL-c were associated with long-term gender differences.10 Therefore, we analyzed not only the data of the participants as a whole but also the data of men and women separately. In our study, according to the previous dietary records of the participants, no significant differences were found in energy and nutrient intakes between the homozygous Ala54 carriers and variant genotype carriers. After 4–5 weeks of the low-GI diet interventions, the decrease of TG levels from baseline in the homozygous Ala54 carriers was generally more significant than that in the variant genotype carriers, although the TG level in the homozygous Ala54 carriers was higher than in the variant carriers at baseline. In addition, compared with the variant genotype group, a significant trend was found in the decrease of TC in the homozygous Ala54 group. Furthermore, we separately analyzed the data for men and women. Compared with Thr54 carriers, we found that the homozygous Ala54 carriers exhibited significant decreases in TG and TC as well as a decrease of LDL-c in women, but not in men. Based on the findings of our present study, an association exists between polymorphisms of FABP2 Ala54Thr (rs1799883) and the effects of low-GI diets on plasma lipid levels in female Han Chinese individuals with T2DM, but not in men. However, the reason for this finding remains unclear.
In addition to abnormal lipid levels, elevation of inflammatory factors is considered a risk factor for cardiovascular disease. Only a few studies have examined the relationship between genetic polymorphisms of rs1799883 and inflammatory factors. A study from de Luis et al found that nondiabetic obese individuals carrying the Thr/Ala54 or Thr/Thr54 phenotype had higher levels of C-reactive protein and IL-6 compared with Ala54/Ala54 carriers.29 However, we found no significant differences in IL-6 or in TNF-α at baseline and after intervention between Ala54 homozygotes carriers and Thr54 carriers. Moreover, we noted no significant differences in changes of inflammatory factors from baseline between the two groups in both sexes. Similarly, another study reported that both Ala54Ala carriers and Thr54 carriers exhibited no statistically significant differences in changes of TNF-α and IL-6 after 3 months of a lifestyle modification program.30
The effects of Thr54 polymorphism in the FABP2 protein on glycemic control response to low-GI diets were also observed in our study. Previously, several studies have showed that Thr54 carriers of FABP2 are associated with higher BMIs, insulin resistance, and metabolic syndrome,6–8,31 and a review suggested that FABP2 (rs1799883) Ala54Thr polymorphisms are associated with increased susceptibility to T2DM among Asians.4 In addition, Weiss et al reported that FABP2 Thr54 carriers had lower glucose tolerances and lower levels of insulin action than do Ala54-homozygous carriers in sedentary nondiabetic individuals following a low-fat diet, and they also suggested that the increased lipid oxidation rates in FABP2 Thr54 carriers might be due to glucoregulatory dysfunction.14 Interestingly, although we found no significant effect of FABP2 (rs1799883) Ala54Thr polymorphisms on glycemic control overall in patients with diabetes receiving low-GI diets, through subgroup analysis, we found that Ala54Ala carriers had a significant decrease of FBG and GA 4–5 weeks after low-GI diet intervention in women but not in men. Because we did not measure immediate postprandial lipid changes and fat oxidation rates, we could not reasonably explain these results in the present study. Therefore, further prospective studies are necessary.
To the best of our knowledge, this is the only study that analyzes the effects of Thr54 polymorphism in the FABP2 protein on plasma lipids, glycemic control, and inflammatory factors among patients with T2DM on similar low-GI diets. All participants received intensive nutritional education, mainly related to dietary nutrition and T2DM management every week during follow-up in the test period, and the dietary assessment was based on an average of three food records. Moreover, unlike other studies, both groups in our study received similar low-GI diets, which minimized the impact of environmental factors and more clearly illustrated the effect of the genetic polymorphisms of rs1799883 on plasma lipid levels. However, our study had several limitations that should be noted. First, we only made short-term retrospective observations on the effects of the FABP2 Ala54Thr genotype on metabolic and inflammatory changes to low-GI diets, but we did not examine the immediate and long-term effects that may help us to further understand the consequences for patients with T2DM. Second, similar to previous studies, the sample size of this study was relatively small, and our study was a single-center study, which may limit the generalizability of the findings. Third, in our study, we did not measure the concentrations of adiponectin and leptin whose imbalances may be important mediators of the elevated risk of developing T2DM and cardiovascular disease linked closely to dyslipidemia.32 Furthermore, we did not investigate the relationship between polymorphism of FABP2 Ala54Thr and adiponectin and leptin as well as the associations between lipid response to low-GI diets and adiponectin and leptin. These are all areas we can further explore through future studies.
Conclusion
From the results of the present study, we conclude that the effects of polymorphism in FABP2 Ala54Thr on serum lipids and glycemic control are associated with gender among patients with T2DM receiving a short-term low-GI diet. The immediate effects of a low-GI diet on postprandial plasma lipids and its long-term regulatory effect on plasma lipids must be further explored in studies with higher population sizes to improve interpretations of the function of the FABP2 protein.
Data sharing statements
We do not intend to share any further data at present.
Acknowledgment
We express our sincere thanks to Beijing Qingwutong Health Technology Co., Ltd., for its free genetic measurements as well as to Dr Zhang Ying for his technical support.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to report in this work. The authors alone are responsible for the content and writing of this paper.
References
Willer CJ, Schmidt EM, Sengupta S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45(11):1274–1283. | ||
Lloyd-Jones D, Adams RJ, Brown TM, et al; Writing Group Members. Heart disease and stroke statistics – 2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. | ||
Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7(6):489–503. | ||
Qiu CJ, Ye XZ, Yu XJ, Peng XR, Li TH. Association between FABP2 Ala54Thr polymorphisms and type 2 diabetes mellitus risk: a huge review and meta-analysis. J Cell Mol Med. 2014;18(12):2530–2535. | ||
Levy E, Ménard D, Delvin E, et al. The polymorphism at codon 54 of the FABP2 gene increases fat absorption in human intestinal explants. J Biol Chem. 2001;276(43):39679–39684. | ||
Tavridou A, Arvanitidis KI, Tiptiri-Kourpeti A, et al. Thr54 allele of fatty-acid binding protein 2 gene is associated with obesity but not type 2 diabetes mellitus in a Caucasian population. Diabetes Res Clin Pract. 2009;84(2):132–137. | ||
Vimaleswaran KS, Radha V, Mohan V. Thr54 allele carriers of the Ala54Thr variant of FABP2 gene have associations with metabolic syndrome and hypertriglyceridemia in urban South Indians. Metabolism. 2006;55(9):1222–1226. | ||
Baier LJ, Sacchettini JC, Knowler WC, et al. An amino acid substitution in the human intestinal fatty acid binding protein is associated with increased fatty acid binding, increased fat oxidation, and insulin resistance. J Clin Invest. 1995;95(3):1281–1287. | ||
Galluzzi JR, Cupples LA, Otvos JD, Wilson PW, Schaefer EJ, Ordovas JM. Association of the A/T54 polymorphism in the intestinal fatty acid binding protein with variations in plasma lipids in the Framingham offspring study. Atherosclerosis. 2001;159(2):417–424. | ||
Nakanishi S, Yamane K, Kamei N, Okubo M, Kohno N. The effect of polymorphism in the intestinal fatty acid-binding protein 2 gene on fat metabolism is associated with gender and obesity amongst non-diabetic Japanese-Americans. Diabetes Obes Metab. 2004;6(1):45–49. | ||
Georgopoulos A, Aras O, Tsai MY. Codon-54 polymorphism of the fatty acid-binding protein 2 gene is associated with elevation of fasting and postprandial triglyceride in type 2 diabetes. J Clin Endocrinol Metab. 2000;85(9):3155–3160. | ||
Almeida JC, Gross JL, Canani LH, Zelmanovitz T, Perassolo MS, Azevedo MJ. The Ala54Thr polymorphism of the FABP2 gene influences the postprandial fatty acids in patients with type 2 diabetes. J Clin Endocrinol Metab. 2010;95(8):3909–3917. | ||
Weiss EP, Brown MD, Shuldiner AR, Hagberg JM. Fatty acid binding protein-2 gene variants and insulin resistance: gene and gene-environment interaction effects. Physiol Genomics. 2002;10(3):145–157. | ||
Weiss EP, Brandauer J, Kulaputana O, et al. FABP2 Ala54Thr genotype is associated with glucoregulatory function and lipid oxidation after a high-fat meal in sedentary nondiabetic men and women. Am J Clin Nutr. 2007;85(1):102–108. | ||
Mero N, Syvänne M, Taskinen MR. Postprandial lipid metabolism in diabetes. Atherosclerosis. 1998;141(Suppl 1):S53–S55. | ||
Bu L, Salto LM, De Leon KJ, De Leon M. Polymorphisms in fatty acid binding protein 5 show association with type 2 diabetes. Diabetes Res Clin Pract. 2011;92(1):82–91. | ||
Jenkins DJ, Wolever TM, Taylor RH, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34(3):362–366. | ||
Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505–516. | ||
Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003;26(8):2261–2267. | ||
Thomas D, Elliott EJ. Low glycemic index, or low glycemic load, diets for diabetes mellitus. Cochrane Database Syst Rev. 2009;21(1): CD006296. | ||
Fleming P, Godwin M. Low-glycaemic index diets in the management of blood lipids: a systematic review and meta-analysis. Fam Pract. 2013;30(5):485–491. | ||
Clar C, Al-Khudairy L, Loveman E, et al. Low glycaemic index diets for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2017;7:CD004467. | ||
Liu Y, Qiu J, Yue Y, Li K, Ren G. Dietary black-grained wheat intake improves glycemic control and inflammatory profile in patients with type 2 diabetes: a randomized controlled trial. Ther Clin Risk Manag. 2018;14:247–256. | ||
Liu Y, Wang Q, Li S, Yue Y, Ma Y, Ren G. Convenient food made of extruded adzuki bean attenuates inflammation and improves glycemic control in patients with type 2 diabetes: a randomized controlled trial. Ther Clin Risk Manag. 2018;14:871–884. | ||
American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13–S27. | ||
Yang YX, Wang GX, Pan XC. China Food Composition. 2nd ed. Beijing: Peking University Medical Press; 2009. | ||
Asselbergs FW, Guo Y, van Iperen EP, et al. Large-scale gene-centric meta-analysis across 32 studies identifies multiple lipid loci. Am J Hum Genet. 2012;91(5):823–838. | ||
Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–713. | ||
de Luis DA, Sagrado MG, Aller R, Izaola O, Conde R. Influence of ALA54THR polymorphism of fatty acid-binding protein 2 on obesity and cardiovascular risk factors. Horm Metab Res. 2007;39(11):830–834. | ||
de Luis DA, Aller R, Izaola O, Sagrado MG, Conde R. Influence of ALA54THR polymorphism of fatty acid binding protein 2 on lifestyle modification response in obese subjects. Ann Nutr Metab. 2006;50(4):354–360. | ||
De Luis DA, Sagrado GM, Aller R, Izaola O, Conde R, De La Fuente B. Influence of Ala54Thr polymorphism of fatty acid-binding protein 2 on insulin resistance and adipocytokines in patients with diabetes mellitus type 2. Eur Rev Med Pharmacol Sci. 2010;14(2):89–95. | ||
López-Jaramillo P, Gómez-Arbeláez D, López-López J, et al. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm Mol Biol Clin Investig. 2014;18(1):37–45. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.