Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 11
Effects of oral supplementation with FOS and GOS prebiotics in women with adult acne: the “S.O. Sweet” study: a proof-of-concept pilot trial
Authors Dall'Oglio F, Milani M, Micali G
Received 9 July 2018
Accepted for publication 6 September 2018
Published 8 October 2018 Volume 2018:11 Pages 445—449
DOI https://doi.org/10.2147/CCID.S179627
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Federica Dall’Oglio,1 Massimo Milani,2 Giuseppe Micali1
1Dermatology Clinic, University of Catania, Catania, Italy; 2Medical Department, Cantabria Labs Difa Cooper, Caronno Pertusella (VA), Italy
Background: We evaluated the effects of 3-month prebiotic oral supplementation with fructo-oligosaccharides (FOS) and galacto-oligosaccharides (GOS) on glucose and lipid metabolic parameters in women with adult acne (female adult acne).
Methods: Twelve women, mean age 35 years, with mild to moderate acne were enrolled. Exclusion criteria were severe acne, body mass index (BMI) >25, history of diabetes mellitus, polycystic ovary syndrome, regular intake of prebiotics or probiotics, and history of inflammatory intestinal diseases. At baseline visit (T0), at month 1 (T1), and at month 3 (T2) fasting glucose, blood insulin, glycated hemoglobin (HbA1c), C-peptide, triglycerides, total cholesterol levels, and BMI were measured. Subjects were treated with a food supplement containing FOS (100 mg) and GOS (500 mg), one sachet daily, for 3 months. Subjects were instructed to follow their regular diet, and no dietary restrictions were suggested.
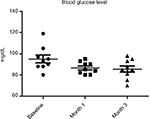
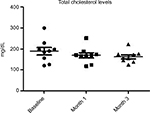
Results: At baseline, the BMI, mean ± SD, was 23±0.7. No modification of BMI was observed during the study. At baseline, fasting blood glucose levels were 92±7 mg/dL. A significant (P=0.02) reduction was observed at month 1 (86±5 mg/dL) and at month 3 (85±7 mg/dL) (–10%). Total cholesterol levels were reduced significantly (P=0.018) from 184±19 to 161±10 mg/dL (–13%) at the end of the study. Triglycerides at baseline were 51 mg/dL and were reduced to 46 mg/dL (P=0.05). Insulin and C-peptide plasma levels showed a nonsignificant reduction trend from baseline to the end of the study. In subjects with baseline insulin level >6 µUI/mL (n=6) the FOS/GOS supplementation induced a significant (P=0.03) reduction from 7.8 to 4.3 µUI/mL at day 90 (–45%). C-peptide was reduced from 2.1 to 1.6 ng/mL (month 3). HbA1c at baseline was 35 mg/dL and 32 mg/dL at the end of the study (NS).
Conclusion: In adult female acne, supplementation with prebiotic FOS and GOS was associated with positive effects on glycemic and lipid metabolic parameters.
Keywords: adult female acne, glycemic index, diet, prebiotics, fructo-oligosaccharides, galacto-oligosaccharides
Introduction
Adult female acne (AFA) is a skin disease of increasing incidence1 that may affect up to 50% of women above 25 years of age.2 Its pathogenesis is unknown. Recent epidemiologic data demonstrated that AFA may be associated with metabolic syndrome and high glycemic index3 caused by elevated blood level of IGF-1, which may induce comedogenesis and inflammation of sebaceous glands.4 Some epidemiological data have shown that a high-carbohydrate (>55% of energy from carbohydrate) and high-fat diet worsens acne.5 Aizawa and Niimura6 demonstrated that adult women with acne presented mild insulin resistance during oral glucose tolerance test. Chronic and acute hyperinsulinemia upregulates the production of IGF-1, which is a potent mitogen stimulating cellular growth at the follicle level as well.7 In animal models, overexpression of IGF-1 induces hyperkeratosis and epidermal hyperplasia.8 Furthermore, IGF-1 increases the production of sebum.9 IGF-1 serum levels are elevated in women with postadolescent acne.10 Both insulin and IGF-1 stimulate the production of androgens in ovarian and testicular tissues.11,12 These data support the hypothesis that the endocrine cascade induced by hyperinsulinemia enhances sebum production and acne development. Modification of intestinal microbiota has been implicated in insulin resistance.13 Larsen et al14 have shown that gut microbiota in type 2 diabetic subjects differs from nondiabetic adults. In obese subjects, therapeutic modification of intestinal microbiota is associated with an improvement of insulin sensitivity.15 Fructo-oligosaccharides (FOS) and galacto-oligosaccharides (GOS) are prebiotic substances able to improve intestinal microbiota and oral supplementation with both increases the amount of intestinal Bifidumbacterium spp. and Lactobacillus spp.16 Experimental data show that oral supplementation with FOS and GOS could have positive effect on glucose metabolism in healthy nondiabetic individuals, thereby reducing the glycemic diet load effects.17 So far, no data regarding the effects of FOS/GOS dietary supplementation on glycemic parameters in subjects with AFA are available.
Study aim
To evaluate, in a pilot study, the effects of prebiotic oral supplementation with FOS and GOS on glucose and lipid metabolic parameters in women with adult acne.
Subjects and methods
Study design
The study (“S.O. Sweet trial”) was designed as open prospective proof-of-concept trial. The clinical setting was the acne outpatient service at the Dermatology Clinic of Catania University. The study took place from July 2017 to December 2017. The institutional review board of the Catania University approved the study protocol. The trial was conducted according to Good Clinical Practice Guidelines and Helsinki Declaration (update 2014).18
Subjects
Eligible subjects included women aged ≥25 years with mild to moderate acne. A total of 12 women, mean age 35 years, were included in the study after their written informed consent. Exclusion criteria were severe acne, body mass index (BMI) >25, smoking habits, use of oral contraceptives, history of diabetes mellitus, regular intake of prebiotics or probiotics, and history of inflammatory intestinal diseases.
Study outcomes
At baseline visit (T0), at day 30 (month 1), and at day 90 (month 2), fasting glucose, blood insulin, glycated hemoglobin (HbA1c), C-peptide, triglycerides, total cholesterol levels, and BMI were measured. HbA1c levels were measured by low-pressure liquid chromatography. Serum C-peptide levels were measured by radioimmunoassay using commercial kits (Diagnostic Systems Laboratories Inc, Webster, TX, USA).
Intervention
Subjects were treated with a food supplement containing FOS (100 mg) and GOS (500 mg), one sachet daily (Sebogard oral, Cantabria Labs Difa Cooper, Caronno Pertusella, Italy), for 3 consecutive months. Subjects were instructed to follow their regular diet, and no dietary restrictions were suggested.
Statistical analysis
Statistical analysis was performed using GraphPad Statistical Software (GraphPad Software, Inc., La Jolla, CA, USA). Continuous variables were expressed as mean ± SD. The primary outcomes of the study were to evaluate the evolution of glycemic and lipidic parameters. For inferential statistical analysis, we used the ANOVA multiple comparison test and the paired Student’s t-test. In view of the proof-of-concept nature of the present trial, a formal sample size calculation was not performed. We decided to enroll at least ten evaluable subjects.
Results
All enrolled women completed the 3-month study period. At baseline the BMI, mean ± SD, was 23±0.7. No modification of BMI was observed during the study duration (BMI 23±6 at month 3). At baseline, fasting blood glucose levels were 92±7 mg/dL. A progressive and significant (P=0.02; ANOVA) reduction was observed at month 1 (86±5 mg/dL) and at month 3 (85±7 mg/dL) (–10%) (Figure 1). Total cholesterol levels were reduced significantly (P=0.05) from 184±19 to 161±10 mg/dL (–13%) at the end of the study (Figure 2). Triglycerides at baseline were 51 mg/dL and were reduced to 46 mg/dL (P=0.05). Insulin and C-peptide plasma levels showed a nonsignificant reduction trend from baseline to the end of the study. In subjects with baseline insulin level >6 µUI/mL (n=6) the FOS/GOS supplementation induced a significant (P=0.03) reduction from 7.8 to 4.3 µUI/mL at day 90 (–45%) (Figure 3). C-peptide was reduced from 2.1 to 1.6 ng/mL at day 90. HbA1c at baseline was 35 mg/dL, and it was 32 mg/dL (difference not significant) at the end of the study. The product was very well tolerated. No serious adverse events were reported during the entire study duration. Even if it was not an endpoint of this trial, a general improvement of acne lesion count was also observed in comparison with baseline condition.
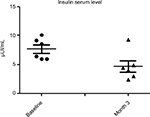  | Figure 3 Evolution of insulin serum level (subset of subjects with baseline insulin levels >6 µUI/mL) (P=0.03; paired t-test). |
Discussion
Acne is a common and complex skin disease that affects individuals of all ages.19 Acne is most common in teenagers, but acne can be observed in 54% of adult females and 40% of adult males, and its prevalence does not decrease substantially with age.20 AFA is a skin disease of increasing incidence that may affect up to 50% of women above 25 years of age.1,2 AFA is classically distinguished into three subtypes:21 “persistent acne” (adolescent acne that is persistent after adolescence), “late onset acne” (characteristically starts after 25 years of age with no previous history of acne), and “recurrent acne” (distinguished by the disappearance of acne for years and by its recurrence). Factors involved in the pathogenesis of adult acne include genetic factors, hypercolonization of resistant strains of Propionibacterium acnes, hyperandrogenism with or without polycystic ovarian syndrome,1 increased serum levels of insulin and IGF-1,22 and the use of comedogenic substances.23 Also, stress and smoking24 seem to be associated with AFA, even if some epidemiological data do not support this link.25 Recent evidences show that glycemic load and glycemic index of whole diet may participate in the pathogenesis of AFA by increasing serum levels of insulin that in turn may increase the blood levels of IGF-1 and adiponectin responsible for comedogenesis and inflammation.26 In a trial conducted by Smith et al,27 a low glycemic load diet significantly reduced acne lesion count improving insulin sensitivity. However, in this trial there was a concomitant weight loss in the group assigned to low glycemic load diet, which could have interfered with the net clinical outcome. An alteration of intestinal microbiota has also been claimed in one study, with 66% of 57 patients affected by AFA showing positive reactivity to stool-isolated coliforms compared to none of the control patients without active skin disease.28 Finally, high fat and sugar diet with low fibers may lead to gut motility alterations, loss of normal microbial biofilm (Bifidobacterium in particular), and increased intestinal permeability (loss of intestinal barrier).29 The latter may cause efflux of lipopolysaccharide endotoxins into systemic circulation, promoting low-grade inflammation, oxidative stress, release of substance P, and increased permeability of IGF-1, which in turn cause insulin resistance, hyperinsulinemia, type 2 diabetes, and likely onset of acne.30 Finally, oral nutritional supplementation with prebiotics like FOS and GOS has been demonstrated to increase stool colony counts of Bifidobacteria and Lactobacilli, contributing to maintain efficient intestinal mucosal barrier.31 Prebiotics could improve insulin sensitivity, thus controlling the role of high glycemic diets in acne exacerbation.32 The results of our study support the hypothesis that dietary supplementation with some prebiotics, in particular FOS and GOS, may improve some blood parameters of sugar and lipid metabolism in women with AFA. Some limitations should be taken in account while evaluating our results. This was an open, uncontrolled trial performed in 12 subjects. The sample size was small. However, we wanted to enroll subjects with specific inclusion and exclusion criteria to reduce confounding factors (no diabetes, no polycystic ovarian syndrome, etc), and these aspects have substantially reduced the eligible population. Therefore, our study should be considered as a pilot trial.
Conclusion
In AFA, oral supplementation with prebiotic FOS and GOS was associated with positive effects on glycemic and lipid metabolism parameters. Further controlled comparative studies are warranted to better evaluate these effects in a larger population of AFA subjects.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
FDo and GM have disclosed that they have no significant relationships with or financial interests in any commercial companies related to this study or article. MM is an employee of Cantabria Labs Difa Cooper. MM reports grants from Difa Cooper, during the conduct of the study, and grants from Difa Cooper, outside the submitted work.
References
Dréno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27(9):1063–1070. | ||
Zeichner JA, Baldwin HE, Cook-Bolden FE, Eichenfield LF, Fallon-Friedlander S, Rodriguez DA. Emerging Issues in Adult Female Acne. J Clin Aesthet Dermatol. 2017;10(1):37–46. | ||
Kaymak Y, Adisen E, Ilter N, Bideci A, Gurler D, Celik B. Dietary glycemic index and glucose, insulin, insulin-like growth factor-I, insulin-like growth factor binding protein 3, and leptin levels in patients with acne. J Am Acad Dermatol. 2007;57(5):819–823. | ||
Kurokawa I, Danby FW, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18(10):821–832. | ||
Bowe WP, Joshi SS, Shalita AR. Diet and acne. J Am Acad Dermatol. 2010;63(1):124–141. | ||
Aizawa H, Niimura M. Mild insulin resistance during oral glucose tolerance test (OGTT) in women with acne. J Dermatol. 1996;23(8):526–529. | ||
Deplewski D, Rosenfield RL. Growth hormone and insulin-like growth factors have different effects on sebaceous cell growth and differentiation. Endocrinology. 1999;140(9):4089–4094. | ||
Bol DK, Kiguchi K, Gimenez-Conti I, Rupp T, Digiovanni J. Overexpression of insulin-like growth factor-1 induces hyperplasia, dermal abnormalities, and spontaneous tumor formation in transgenic mice. Oncogene. 1997;14(14):1725–1734. | ||
Smith TM, Gilliland K, Clawson GA, Thiboutot D. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128(5):1286–1293. | ||
Balta I, Ekiz O, Ozuguz P, et al. Insulin resistance in patients with post-adolescent acne. Int J Dermatol. 2015;54(6):662–666. | ||
Barbieri RL, Smith S, Ryan KJ. The role of hyperinsulinemia in the pathogenesis of ovarian hyperandrogenism. Fertil Steril. 1988;50(2):197–212. | ||
Bebakar WM, Honour JW, Foster D, Liu YL, Jacobs HS. Regulation of testicular function by insulin and transforming growth factor-beta. Steroids. 1990;55(6):266–270. | ||
Dumas ME, Barton RH, Toye A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103(33):12511–12516. | ||
Larsen N, Vogensen FK, van den Berg FW, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5(2):e9085. | ||
Vrieze A, van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–916. | ||
Collins MD, Gibson GR. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999;69(5):1052s–1057. | ||
Costa GT, Guimarães SB, Sampaio HA. Fructo-oligosaccharide effects on blood glucose: an overview. Acta Cir Bras. 2012;27(3):279–282. | ||
General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81(3):14. | ||
Williams HC, Dellavalle RP, Garner S. Acne vulgaris. The Lancet. 2012;379(9813):361–372. | ||
Bergfeld WF. A lifetime of healthy skin: implications for women. Int J Fertil Womens Med. 1999;44(2):83–95. | ||
Ramos-E-Silva M, Ramos-E-Silva S, Carneiro S. Acne in women. Br J Dermatol. 2015;172 Suppl 1:20–26. | ||
Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18(10):833–841. | ||
White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2 Pt 3): S34–S37. | ||
Yang YS, Lim HK, Hong KK, et al. Cigarette smoke-induced interleukin-1 alpha may be involved in the pathogenesis of adult acne. Ann Dermatol. 2014;26(1):11–16. | ||
Youn SW. The role of facial sebum secretion in acne pathogenesis: facts and controversies. Clin Dermatol. 2010;28(1):8–11. | ||
Cappel M, Mauger D, Thiboutot D. Correlation between serum levels of insulin-like growth factor 1, dehydroepiandrosterone sulfate, and dihydrotestosterone and acne lesion counts in adult women. Arch Dermatol. 2005;141(3):333–338. | ||
Smith RN, Mann NJ, Braue A, Mäkeläinen H, Varigos GA. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86(1):107–115. | ||
Bowe WP, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis – back to the future? Gut Pathog. 2011;3(1):1. | ||
Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121(6):2126–2132. | ||
Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015; 8:371. | ||
Haarman M, Knol J. Quantitative real-time PCR assays to identify and quantify fecal Bifidobacterium species in infants receiving a prebiotic infant formula. Appl Environ Microbiol. 2005;71(5):2318–2324. | ||
Kumar S, Mahajan BB, Kamra N. Future perspective of probiotics in dermatology: an old wine in new bottle. Dermatol Online J. 2014;20(9):13030/qt8br333fc. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.