Back to Journals » Journal of Inflammation Research » Volume 16
Effects of Neuropeptides on Dendritic Cells in the Pathogenesis of Psoriasis
Authors Zhang J, Zhao S, Xing X, Shang L, Cao J , He Y
Received 21 November 2022
Accepted for publication 24 December 2022
Published 6 January 2023 Volume 2023:16 Pages 35—43
DOI https://doi.org/10.2147/JIR.S397079
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Monika Sharma
Jingya Zhang,1 Siqi Zhao,1 Xinzhu Xing,1 Lin Shang,1 Jiali Cao,1 Yanling He1,2
1Department of Dermatology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, People’s Republic of China; 2National Clinical Research Center for Skin and Immune Diseases, Branch in Beijing Chaoyang Hospital, Beijing, People’s Republic of China
Correspondence: Yanling He, Department of Dermatology, Beijing Chaoyang Hospital, Capital Medical University, 8 Gongti South Road, Chaoyang District, Beijing, 100020, People’s Republic of China, Tel/Fax +86-10-85231889, Email [email protected]
Abstract: Psoriasis is an autoimmune disease that is characterized by discolored, scaled patches of skin. Clinically, it is found that psychological factors often induce or aggravate the disease. Current research suggests that the pathogenesis of psoriasis involves the nervous and immune systems. This article reviews how neuropeptides secreted by nerve fibers affect dendritic cells in psoriasis. In this review, we describe that the neuropeptides calcitonin gene-related peptide, substance P, and vasoactive intestinal peptide can act on dendritic cells and participate in the pathogenesis of psoriasis. These neuropeptides can affect the secretion of interleukin (IL)-12 and IL-23 by dendritic cells, which stimulate T helper (Th)1, Th17, and Th22 cells to produce immune responses and cause the manifestation of psoriasis. The application of neuropeptide inhibitors can improve the skin lesions of psoriasis, which has been confirmed in clinical trials. Therefore, neuroimmune response may be a new direction to develop new drug treatments and perspectives in the development of psoriasis.
Keywords: psoriasis, dendritic cell, calcitonin gene-related peptide, substance P, vasoactive intestinal peptide
Introduction
Psoriasis
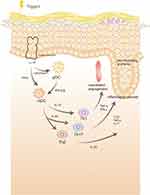
Psoriasis is a chronic, systemic immune-mediated disease that cannot be completely cured. The typical skin lesions are composed of scaly erythema or plaque. The causes are thought to be multifactorial but are not yet well understood. Immunological studies identified the IL-23/Th17 axis plays a key role in psoriasis pathogenesis (Figure 1). Recently, there is some evidence that dendritic cells (DCs) play a major role in the development of this disease.1 Many comorbidities are associated with psoriasis, such as psoriatic arthritis, metabolic syndrome (MS), cardiac metabolic disease (CVD), Crohn’s disease, and uveitis.2 Psychiatric/psychological comorbidities have been increasingly recognized, but pathogenesis remains unknown. Clinically, patients with psoriasis often have anxiety, sleep disturbance, depression, and other psychosis,3 and bidirectional interactions have been identified between psoriasis and psychiatric disorders.4 Psychosocial stress also plays a role in the exacerbation of psoriasis.5 Other clinical correlates include the regression of psoriasis plaque secondary to denervated areas after cutaneous dysfunction.6 Neuropeptides regulate various psychological and physiological functions, including learning, memory, sleep disorders and pain. Neuropeptides can also act as neurotransmitters, inhibit pain, evoke comfort, and modulate immune responses. A high level of Substance P (SP) is also observed in depression, which indicates that the pathological mechanism of depression may be related to an SP/neurokinin-1 (NK-1) mediated reaction dysfunction.7 The calcitonin gene-related peptide (CGRP) and vasoactive intestinal peptide (VIP) levels in the participants in the “high stress” group were significantly higher than the levels of participants in the “low stress” group.4 Psychological abnormalities can lead to the emergence of psoriasis, in which the pathophysiological mechanism is jointly participated by neuropeptides and immune cells. The immune system and nervous system are closely connected, and each play a role in the pathogenesis of psoriasis (Table 1).
 |
Table 1 Effect of Neuropeptides on Dendritic Cell |
The Role of Dendritic Cells in Psoriasis
DCs are a type of immune cell that are created in bone marrow-derived cells. First discovered by Steinman in the spleen of a mouse, DCs are star-shaped and named after the Greek word for tree “dendron.” Afterward, DCs were found to stimulate T cells and immune response.8 Nowadays, human DCs are divided into various subtypes by gene expression, including myeloid/conventional DC1s (cDC1s), myeloid/conventional DC2s (cDC2s), plasmacytoid DCs (pDCs), Langerhans cells (LCs), precursor DCs (pre-DCs), monocyte-derived DCs (mo-DCs), and untyped DCs.9 Additionally, DCs constitute an important bridge between the activation of innate and adaptive immunity. In innate immunity, they recognize and respond to pathogens and danger signal molecules, creating an acute inflammatory response. Meanwhile, their role in acquired immunity is to process extracellular and intracellular antigens and activate naive T cells through major histocompatibility complex (MHC) molecules to initiate the T cell immune process.8 In addition to playing a role in resisting the invasion of external microorganisms, DCs play an important role in the development of tumors10 and the generation of immune diseases. Furthermore, DCs play a central role in the pathogenesis of psoriasis through the IL-23-mediated T helper (Th) 17 pathway, which is considered the main activation pathway for this disease.11 Keratinocytes, cells of the epidermis, stimulate DCs, and in turn, DCs secrete cytokines to initiate the immune response process of T cells.
Under the combined action of genes, environmental stimuli, and mechanical stimuli, damaged keratinocytes release self-nucleotides and form antibacterial peptide LL-37-self-nucleotide complexes. These complexes directly stimulate pDCs to produce large amounts of type I interferons or myeloid DCs, leading to the maturation and activation of myeloid DCs. Activated DCs can produce interleukin (IL)-12 and IL-23 and activate and stimulate Th1, Th17, and Th22 cells to produce cytokines such as interferon-γ (IFN-γ), IL-22, tumor necrosis factor-α (TNF-α), and IL-17.1 In turn, IL-17 can act on keratinocytes, leading to disorders of amine metabolism,12 resulting in a loop of inflammation, an inflammatory cytokine storm, keratinocyte proliferation, infiltrative erythema, and vascular proliferation and dilation. Additionally, these inflammatory factors can act on vascular endothelial cells13 and joint synovial cells,14 causing the comorbidity of psoriasis. LCs switch from a pro-inflammatory to an immunomodulant pattern during psoriasis.15,16 LC function deteriorates in patients with psoriasis, which may play a decisive role in the pathogenesis of the disease.17 Through single-cell flow cytometry, scientists have discovered many new DC subtypes associated with the pathogenesis of psoriasis, such as CD14+ DC3s, which are increased in psoriatic lesions and produce IL-1B and IL-23A.18 However, research on DCs in psoriasis is ongoing.
Effect of Neuropeptides on DCs
Calcitonin Gene-Related Peptide
CGRP, a peptide consisting of 37 amino acids, is a neuromodulator that exists in C and Aδ sensory nerve fibers.19,20 CGRP belongs to the calcitonin/CGRP family, which also includes calcitonin, adrenomedullin, amylin, and adrenomedullin 2/intermediate protein.21 CGRP exists in two forms in humans: αCGRP and βCGRP. αCGRP is highly expressed in sensory neurons and βCGRP is highly expressed in the enteric nervous system.22 Their receptors are composed of one of two G protein coupled receptors (GPCRs), calcitonin receptors (CTR) or calcitonin receptor-like receptors (CLR). Further diversity comes from the heterodimers of these GPCRs utilizing one of the three receptor activity modifying proteins (RAMPs). This produces CGRP receptors (CLR/RAMP1), AM1 and AM2 receptors (CLR/RAMP2 or RAMP3), and AMY1, AMY2 and AMY3 receptors (CTR/RAMPs1-3 complexes, respectively).21 CGRP was first found to be related to sympathetic regulation and was later found to play an important role in vasodilation regulation and neurogenic inflammation.23
The release of CGRP is regulated by cation channels expressed on sensory nerve endings and transient receptor potential (TRP) channels, including TRPV1 and TRPA1.24 When the ion channel is activated, the influx of Ca2+ is activated, and the neuropeptide CGRP stored in the neuron is released, thereby inducing neurogenic inflammation.25 TRPV1 mainly transmits pain and can be activated by high temperatures (> 43°C), low pH (pH ≤ 5.9), and other chemical stimuli. Moreover, 70% of CGRP-immunoreactive neurons co-localize with TRPV1 channels.25,26 Additionally, TRPV1 can interact with skin sensory nerve fibers, mast cells, epidermal cells, dermal blood vessels, hair follicles, sebocytes, and sweat gland cells27 to mediate immune inflammatory responses. In contrast, TRPA1 was first identified as a non-selective cation channel responsive to cold sensation (< 15°C) and exogenous stimulatory compounds and endogenous compounds produced during tissue injury or neurogenic inflammation.28,29 Many TRPA1-positive neurons co-express TRPV1 channels and can be sensitized or activated by an increase in intracellular calcium. Some experimental evidence suggests that TRPV1 and TRPA1 receptor channels exhibit a synergistic effect.24 Furthermore, TRPA1 communicates with endothelial cells, keratinocytes, LCs, and fibroblasts. By releasing CGRP, neurons activate skin cells, which in turn release histamine or pro-inflammatory cytokines to activate sensory nerve endings, creating a bidirectional positive feedback loop that leads to increased inflammation.25
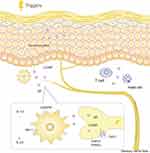
Many studies have observed a close relationship between CGRP and the pathogenesis of psoriasis.30–41 The number of nerve fibers secreting CGRP in the skin lesions of patients with psoriasis was significantly higher than in those of patients without psoriasis.30 Additionally, the content of CGRP in the plasma of patients with psoriasis was higher than that of those without the disease.31 When TRPV1 and TRPA1 ion channels are activated with external pressure, a large amount of CGRP is synthesized in the neuronal soma and released along the axon. CGRP activates CGRP receptors in DCs, causing DCs to secrete IL-23, which may lead to immune disorders.32 As early as 1993, scientists found that CGRP expresses in epidermal LCs and inhibits LC antigen presentation.33 In addition, several studies have found that CGRP can act on keratinocytes, phagocytes, T cells, mast cells, fibroblasts, and vascular endothelial cells.34–37 Moreover, the compound resolvin D3 inhibits capsaicin-induced TRPV1 currents in dorsal root ganglion (DRG) neurons through N-formyl peptide receptor 2 to reverse pruritus after imiquimod (IMQ) administration, and repeated administration largely prevents the development of psoriatic pruritus and skin inflammation while reducing the expression of CGRP in DRG neurons.38 Additionally, epidural injection of lidocaine to block CGRP release in patients with psoriasis can alleviate skin lesions. Lidocaine significantly reduces IL-23 production by DCs co-cultured in vitro with DRG neurons.39 The expression of the CGRP receptor genes RAMP1 and CALCRL was significantly increased in DCs (especially cDC1 and cDC2) in the skin of patients with psoriasis. Therefore, the CGRP receptor-dominant dermal DC may be a downstream response of CGRP neurogenic regulation. Botulinum toxin is a commonly used nerve blocker in dermatology. It can not only paralyze muscles, but also change the level of nerve factors secreted in the nervous system.40 A study has shown that the injection of botulinum toxin B (BTX-B) can improve psoriasis-like skin lesions in IMQ-treated mice. In their experiment, the researchers found that in mice injected with BTX-B, neuron release of CGRP decreased, and the amount of CD11c+ DCs entering the skin and that of secreted IL-23 was significantly reduced.41 Furthermore, pre-clinical studies have shown that sensory denervation induced by TRPV1 agonist (resiniferatoxin) treatment can lead to a significant decrease in IL-23 expression, while IMQ induces a transient increase in CGRP expression in the DRG.36 CGRP upregulated the expression of IL-17A and IL-22 in a LC/γδ -T cell co-culture model through the paracrine effect of LCs.42 CGRP has also been found to stimulate CD301b+ dermal DCs to produce IL-23 and stimulate γδ-T cells to produce immune responses.43 Mature and immature DCs have also been reported to express type 1 CGRP receptors, and the signals of these receptors may inhibit T cell proliferation driven by mature DCs by downregulating CD86 and HLA-DR.44 Nevertheless, the precise action mechanism of CGRP on DCs in psoriasis has not been elucidated, and it requires further research.
Substance P
SP is an undecanone widely distributed in afferent sensory fibers, belonging to the tachykinin family, along with neurokinin A and neurokinin B. SP transmits pain and an itching sensation from the periphery to the central nervous system.45,46 SP is expressed by a variety of cells including neurons, astrocytes, microglia, epithelial cells, and endothelial cells. Additionally, immune cells, such as T cells, macrophages, dendritic cells, or eosinophils secrete SP.47 SP is similar to CGRP in the nervous system and can be released from C and Aδ sensory nerve fibers.29 SP is also an important mediator of inflammation in various tissues, including the skin, and nociceptive stimuli-induced hyperalgesia results in the release of SP. In 1989, fibers expressing SP were found to be more abundant in the skin lesions of patients with psoriasis than in the skin of those without psoriasis. These fibers were mainly distributed in the papillary dermis and rarely in the epidermis.48 Later, it was found that SP could act on NK-1 receptors located on mast cells, causing the degranulation of mast cells to release histamine,49 which results in itching. Further, SP can directly act on blood vessels, causing cutaneous vasodilation. Further studies found that NK-1 receptors also exist on human CD11c+ and mouse CD11c+/CD11c+ CD11b+ DCs.50 In patients with psoriasis, SP can induce the in vitro maturation and activation of CD11c+ DCs, as well as the release of IL-12p70 and IL-23,51 which are involved in the pathological progression of the disease; meanwhile NK-1 receptor antagonists can eliminate this effect.52 In addition, SP-treated DCs efficiently enter lymph nodes and induce inflammatory DCs to produce IL-12, thereby promoting type 1 polarized immunity, and may be involved in the incidence of psoriasis.53 Specifically, SP promotes the transcriptional activator of DC inflammatory cytokines by inducing the rapid enhanced activation of NF-κB54 (Figure 2).
Vasoactive Intestinal Peptide
VIP is a hormone peptide consisting of 28 amino acids. It was discovered because of its strong vasodilator activity and was isolated from intestinal extracts, causing it to originally be considered as a gastrointestinal hormone. Later discovered in the brain and other neural tissues, VIP is now considered to be a neuropeptide, widely distributed in the central and peripheral nervous systems, with a wide range of biological effects, acting as a neurotransmitter or neuromodulator.55,56 VIP typically signals through the adenylate cyclase pathway and mediates neurogenic inflammation, keratinocyte proliferation and formation, and vascular growth.57 The VIP has receptors on keratinocytes, mast cells, and DCs.25,58 In the local skin anatomy, nerves that secrete VIP were also found to be highly connected with highly connected with the autonomic nerve fibers and the fibers at the junction of the dermis and epidermis.6
A clinical experimental study observed that the plasma VIP level of the psoriasis group was significantly higher than that of the control group.59 Immunohistochemical analysis showed that the number of VIP positive nerve fibers was significantly higher than that of psoriasis non lesional skin and healthy control skin.4 VIP has different effects on DCs in various differentiation and stimulation states. For example, immature DCs treated with VIP exhibit increased CD86 expression and induced CD4+ T cell proliferation. Moreover, VIP-treated immature DC activated CD4+ T cells in vitro and in vivo exhibit a Th2 phenotype. However, the addition of VIP in the early stages of DC differentiation leads to the failure of DCs to mature after inflammatory stimulation.60 In vitro cell experiments showed that VIP treatment significantly enhanced the ability of granulocyte-macrophage colony-stimulating factor and IL-14-induced DCs to stimulate alloreactive T cells and produce IL-12p70. IL-12 can promote the differentiation of T cells into the Th1 subtype, which is related to the occurrence of psoriasis.10,61 Moreover, for activated DCs, VIP can inhibit the expression of B7.1/B7.2, thus inhibiting its stimulatory activity against antigen-specific T cells.62 We speculate that the dysregulation of VIP expression may affect the development of psoriasis through this pathway.
Discussion
In summary, the nervous system is involved in the pathogenesis of psoriasis, not only through the psychological factors63 but also by participating in the production of psoriatic skin lesions and comorbidities. Moreover, neuropeptides affect the main immune response process involving DCs and T lymphocytes. For example, CGRP and SP directly stimulate DCs to secrete IL-23 to initiate the immune process of psoriasis.57 Thus, neuropeptides and immune cells exhibit a close relationship. The content involved in this review is only a part of neuroimmunity. In addition to the neuropeptides mentioned in the article, hypothalamus-pituitary-adrenal axis, neurotrophic factors and so on also play a role in the psoriasis pathogenesis. The nervous system role in the disease needs to be further investigated. Since our ultimate goal is to treat diseases.
The effect of standard treatment on the levels of neuropeptides in patients with psoriasis is imperfect and needs to be explored. One study examined the change of serum VIP level after treatment for immune mediated inflammatory diseases (including psoriasis and psoriatic arthritis) by biological agents and showed that TNF-α treatment can increase the level of serum VIP, while anti IL-12/23 treatment can reduce circulating VIP.59 Another study found that narrowband ultraviolet B (NB-UVB) treatment had no significant effect on serum neuropeptide including SP, CGRP, brain-derived neurotrophic factor (BDNF), or corticotropin-releasing factor (CRF) levels.64
As mentioned earlier, psoriasis symptoms can reappear. Therefore, researchers have developed many new drugs, such as biological agents or small-molecule targeted drugs, to treat refractory psoriasis.65 However, with the long-term use of such biological agents, some people will gradually develop resistance to these drugs, requiring the discovery of new therapeutic targets. As a result, neuropeptide therapies have been applied to some patients. For example, blocking the release of CGRP with CGRP antibody or other drugs has been shown to improve the symptoms of patients with psoriasis.36 Local application of capsaicin, a SP depleting agent, can effectively treat pruritic psoriasis.66 Patients with plaque psoriasis also experienced relief after receiving a botulinum toxin A injection through a single target plaque. After 10 weeks, the skin lesions were obviously improved. The level of SP and CGRP was decreased by fluorescence labeling in local lesions.67 These results provide a promising avenue for the treatment of psoriasis in the future. Moreover, subcutaneous injection of SP in mice reduced cytokines secreted by DCs, and improved skin lesions through the antagonist and inhibitor terrestrosin D. In addition, VIP, nerve growth factor, and pituitary adenylate cyclase activating polypeptide also play an important role in the pathogenesis of psoriasis and may become a new direction of drug research and development.
Acknowledgments
We would like to thank Professor Liwei Ran for her contribution to our study. And we would like to thank Editage for English language editing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by National Natural Science Foundation of China (81773314), and by Beijing Natural Science Foundation (7172082).
Disclosure
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Kim TG, Kim SH, Lee MG. The origin of skin dendritic cell network and its role in psoriasis. Int J Mol Sci. 2017;19(1):42. doi:10.3390/ijms19010042
2. Caputo V, Strafella C, Cosio T, et al. Pharmacogenomics: an update on biologics and small-molecule drugs in the treatment of psoriasis. Genes. 2021;12(9):1398. doi:10.3390/genes12091398
3. Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29(7):805–813. doi:10.1515/revneuro-2017-0108
4. Kwon CW, Fried RG, Nousari Y, Ritchlin C, Tausk F. Psoriasis: psychosomatic, somatopsychic, or both? Clin Dermatol. 2018;36(6):698–703. doi:10.1016/j.clindermatol.2018.08.009
5. Hunter HJ, Griffiths CE, Kleyn CE. Does psychosocial stress play a role in the exacerbation of psoriasis? Br J Dermatol. 2013;169(5):965–974. doi:10.1111/bjd.12478
6. Saraceno R, Kleyn CE, Terenghi G, Griffiths CE. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155(5):876–882. doi:10.1111/j.1365-2133.2006.07518.x
7. Marek-Jozefowicz L, Czajkowski R, Borkowska A, et al. The brain-skin axis in psoriasis: psychological, psychiatric, hormonal, and dermatological aspects. Int J Mol Sci. 2022;23(2):669. doi:10.3390/ijms23020669
8. Cabeza-Cabrerizo M, Cardoso A, Minutti CM, Pereira da Costa M, Reis ESC. Dendritic cells revisited. Annu Rev Immunol. 2021;39:131–166. doi:10.1146/annurev-immunol-061020-053707
9. Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology. 2018;154(1):3–20. doi:10.1111/imm.12888
10. Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12(4):265–277. doi:10.1038/nrc3258
11. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–1960. doi:10.1001/jama.2020.4006
12. Lou F, Sun Y, Xu Z, et al. Excessive polyamine generation in keratinocytes promotes self-RNA sensing by dendritic cells in psoriasis. Immunity. 2020;53(1):204–16 e10. doi:10.1016/j.immuni.2020.06.004
13. Garshick MS, Ward NL, Krueger JG, Berger JS. Cardiovascular risk in patients with psoriasis: JACC review topic of the week. J Am Coll Cardiol. 2021;77(13):1670–1680. doi:10.1016/j.jacc.2021.02.009
14. Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet. 2018;391(10136):2273–2284. doi:10.1016/s0140-6736(18)30830-4
15. Glitzner E, Korosec A, Brunner PM, et al. Specific roles for dendritic cell subsets during initiation and progression of psoriasis. EMBO Mol Med. 2014;6(10):1312–1327. doi:10.15252/emmm.201404114
16. Eidsmo L, Martini E. Human Langerhans cells with pro-inflammatory features relocate within psoriasis lesions. Front Immunol. 2018;9:300. doi:10.3389/fimmu.2018.00300
17. Cumberbatch M, Singh M, Dearman RJ, Young HS, Kimber I, Griffiths CE. Impaired Langerhans cell migration in psoriasis. J Exp Med. 2006;203(4):953–960. doi:10.1084/jem.20052367
18. Nakamizo S, Dutertre CA, Khalilnezhad A, et al. Single-cell analysis of human skin identifies CD14+ type 3 dendritic cells co-producing IL1B and IL23A in psoriasis. J Exp Med. 2021;218(9):e20202345. doi:10.1084/jem.20202345
19. Iyengar S, Ossipov MH, Johnson KW. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain. 2017;158(4):543–559. doi:10.1097/j.pain.0000000000000831
20. Charles A, Pozo-Rosich P. Targeting calcitonin gene-related peptide: a new era in migraine therapy. Lancet. 2019;394(10210):1765–1774. doi:10.1016/s0140-6736(19)32504-8
21. Hay DL, Garelja ML, Poyner DR, Walker CS. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR review 25. Br J Pharmacol. 2018;175(1):3–17. doi:10.1111/bph.14075
22. Hargreaves R, Olesen J. Calcitonin gene-related peptide modulators: the history and renaissance of a new migraine drug class. Headache. 2019;59(6):951–970. doi:10.1111/head.13510
23. Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94(4):1099–1142. doi:10.1152/physrev.00034.2013
24. Kleeberg-Hartmann J, Vogler B, Messlinger K. Petasin and isopetasin reduce CGRP release from trigeminal afferents indicating an inhibitory effect on TRPA1 and TRPV1 receptor channels. J Headache Pain. 2021;22(1):23. doi:10.1186/s10194-021-01235-5
25. Choi JE, Di Nardo A. Skin neurogenic inflammation. Semin Immunopathol. 2018;40(3):249–259. doi:10.1007/s00281-018-0675-z
26. Benitez-Angeles M, Morales-Lazaro SL, Juarez-Gonzalez E, Rosenbaum T. TRPV1: structure, endogenous agonists, and mechanisms. Int J Mol Sci. 2020;21(10):3421. doi:10.3390/ijms21103421
27. Stander S, Moormann C, Schumacher M, et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol. 2004;13(3):129–139. doi:10.1111/j.0906-6705.2004.0178.x
28. Nassini R, Materazzi S, Benemei S, Geppetti P. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev Physiol Biochem Pharmacol. 2014;167:1–43. doi:10.1007/112_2014_18
29. Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi:10.1146/annurev-cellbio-101011-155833
30. Jiang WY, Raychaudhuri SP, Farber EM. Double-labeled immunofluorescence study of cutaneous nerves in psoriasis. Int J Dermatol. 1998;37(8):572–574. doi:10.1046/j.1365-4362.1998.00533.x
31. Reich A, Orda A, Wisnicka B, Szepietowski JC. Plasma concentration of selected neuropeptides in patients suffering from psoriasis. Exp Dermatol. 2007;16(5):421–428. doi:10.1111/j.1600-0625.2007.00544.x
32. Dainichi T, Kitoh A, Otsuka A, et al. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat Immunol. 2018;19(12):1286–1298. doi:10.1038/s41590-018-0256-2
33. Hosoi J, Murphy GF, Egan CL, et al. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 1993;363(6425):159–163. doi:10.1038/363159a0
34. Gerbaud P, Guibourdenche J, Jarray R, et al. APN/CD13 is over-expressed by psoriatic fibroblasts and is modulated by CGRP and IL-4 but not by retinoic acid treatment. J Cell Physiol. 2018;233(2):958–967. doi:10.1002/jcp.25941
35. Yu XJ, Li CY, Xu YH, Chen LM, Zhou CL. Calcitonin gene-related peptide increases proliferation of human HaCaT keratinocytes by activation of MAP kinases. Cell Biol Int. 2009;33(11):1144–1148. doi:10.1016/j.cellbi.2009.07.003
36. Zhang X, Cao J, Zhao S, et al. Nociceptive sensory fibers drive interleukin-23 production in a murine model of psoriasis via calcitonin gene-related peptide. Front Immunol. 2021;12:743675. doi:10.3389/fimmu.2021.743675
37. Naukkarinen A, Jarvikallio A, Lakkakorpi J, Harvima IT, Harvima RJ, Horsmanheimo M. Quantitative histochemical analysis of mast cells and sensory nerves in psoriatic skin. J Pathol. 1996;180(2):200–205. doi:10.1002/(SICI)1096-9896(199610)180:2<200::AID-PATH632>3.0.CO;2-Z
38. Lee SH, Tonello R, Im ST, et al. Resolvin D3 controls mouse and human TRPV1-positive neurons and preclinical progression of psoriasis. Theranostics. 2020;10(26):12111–12126. doi:10.7150/thno.52135
39. Yin Q, Sun L, Cai X, et al. Lidocaine ameliorates psoriasis by obstructing pathogenic CGRP signaling-mediated sensory neuron-dendritic cell communication. J Invest Dermatol. 2022;142:2173–2183.e6. doi:10.1016/j.jid.2022.01.002
40. Cosio T, Campione E. A new hypothesis in botulin therapy for depression: insula cortex modification. Dermatol Ther. 2019;32(5):e13008. doi:10.1111/dth.13008
41. Amalia SN, Uchiyama A, Baral H, et al. Suppression of neuropeptide by botulinum toxin improves imiquimod-induced psoriasis-like dermatitis via the regulation of neuroimmune system. J Dermatol Sci. 2021;101(1):58–68. doi:10.1016/j.jdermsci.2020.11.003
42. Peng F, Zhao S, Zhang X, Long S, He Y. Calcitonin gene-related peptide upregulates IL-17A and IL-22 in gammadelta-T cells through the paracrine effect of Langerhans cells on LC/gammadelta-T co-culture model. J Neuroimmunol. 2022;364:577792. doi:10.1016/j.jneuroim.2021.577792
43. Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH. Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity. 2015;43(3):515–526. doi:10.1016/j.immuni.2015.08.016
44. Carucci JA, Ignatius R, Wei Y, et al. Calcitonin gene-related peptide decreases expression of HLA-DR and CD86 by human dendritic cells and dampens dendritic cell-driven T cell-proliferative responses via the type I calcitonin gene-related peptide receptor. J Immunol. 2000;164(7):3494–3499. doi:10.4049/jimmunol.164.7.3494
45. Bernstein JE. Capsaicin and substance P. Clin Dermatol. 1991;9(4):497–503. doi:10.1016/0738-081x(91)90078-y
46. Mehta D, Granstein RD. Immunoregulatory effects of neuropeptides on endothelial cells: relevance to dermatological disorders. Dermatology. 2019;235(3):175–186. doi:10.1159/000496538
47. Mashaghi A, Marmalidou A, Tehrani M, Grace PM, Pothoulakis C, Dana R. Neuropeptide substance P and the immune response. Cell Mol Life Sci. 2016;73(22):4249–4264. doi:10.1007/s00018-016-2293-z
48. Naukkarinen A, Nickoloff BJ, Farber EM. Quantification of cutaneous sensory nerves and their substance P content in psoriasis. J Invest Dermatol. 1989;92(1):126–129. doi:10.1111/1523-1747.ep13071340
49. Remrod C, Lonne-Rahm S, Nordlind K. Study of substance P and its receptor neurokinin-1 in psoriasis and their relation to chronic stress and pruritus. Arch Dermatol Res. 2007;299(2):85–91. doi:10.1007/s00403-007-0745-x
50. Marriott I, Bost KL. Expression of authentic substance P receptors in murine and human dendritic cells. J Neuroimmunol. 2001;114(1–2):131–141. doi:10.1016/s0165-5728(00)00466-5
51. Guo J, Qi C, Liu Y, et al. Terrestrosin D ameliorates skin lesions in an imiquimod-induced psoriasis-like murine model by inhibiting the interaction between Substance P and Dendritic cells. Phytomedicine. 2022;95:153864. doi:10.1016/j.phymed.2021.153864
52. Wang Y, Li P, Zhang L, et al. Stress aggravates and prolongs imiquimod-induced psoriasis-like epidermal hyperplasis and IL-1β/IL-23p40 production. J Leukoc Biol. 2020;108(1):267–281. doi:10.1002/jlb.3ma0320-363rr
53. Takashima A. Harnessing DCs by substance P. Blood. 2013;121(15):2815–2816. doi:10.1182/blood-2013-02-483354
54. Marriott I, Mason MJ, Elhofy A, Bost KL. Substance P activates NF-kappaB independent of elevations in intracellular calcium in murine macrophages and dendritic cells. J Neuroimmunol. 2000;102(2):163–171. doi:10.1016/s0165-5728(99)00182-4
55. Said SI. Vasoactive intestinal peptide. J Endocrinol Invest. 1986;9(2):191–200. doi:10.1007/bf03348097
56. Tepper SJ. History and review of anti-calcitonin gene-related peptide (CGRP) therapies: from translational research to treatment. Headache. 2018;58(Suppl 3):238–275. doi:10.1111/head.13379
57. Ayasse MT, Buddenkotte J, Alam M, Steinhoff M. Role of neuroimmune circuits and pruritus in psoriasis. Exp Dermatol. 2020;29(4):414–426. doi:10.1111/exd.14071
58. Kakurai M, Fujita N, Murata S, Furukawa Y, Demitsu T, Nakagawa H. Vasoactive intestinal peptide regulates its receptor expression and functions of human keratinocytes via type I vasoactive intestinal peptide receptors. J Invest Dermatol. 2001;116(5):743–749. doi:10.1046/j.1523-1747.2001.01306.x
59. Lamana A, Castro-Vázquez D, de la Fuente H, et al. VIP/VPAC axis expression in immune-mediated inflammatory disorders: associated miRNA signatures. Int J Mol Sci. 2022;23(15):8578. doi:10.3390/ijms23158578
60. Chorny A, Gonzalez-Rey E, Delgado M. Regulation of dendritic cell differentiation by vasoactive intestinal peptide: therapeutic applications on autoimmunity and transplantation. Ann N Y Acad Sci. 2006;1088:187–194. doi:10.1196/annals.1366.004
61. Lu J, Zheng MH, Yan J, Chen YP, Pan JP. Effects of vasoactive intestinal peptide on phenotypic and functional maturation of dendritic cells. Int Immunopharmacol. 2008;8(10):1449–1454. doi:10.1016/j.intimp.2008.06.002
62. Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004;56(2):249–290. doi:10.1124/pr.56.2.7
63. Komiya E, Tominaga M, Kamata Y, Suga Y, Takamori K. Molecular and cellular mechanisms of itch in psoriasis. Int J Mol Sci. 2020;21(21):8406. doi:10.3390/ijms21218406
64. Narbutt J, Olejniczak I, Sobolewska-Sztychny D, et al. Narrow band ultraviolet B irradiations cause alteration in interleukin-31 serum level in psoriatic patients. Arch Dermatol Res. 2013;305(3):191–195. doi:10.1007/s00403-012-1293-6
65. Nogueira M, Puig L, Torres T. JAK Inhibitors for treatment of psoriasis: focus on selective TYK2 inhibitors. Drugs. 2020;80(4):341–352. doi:10.1007/s40265-020-01261-8
66. Ellis CN, Berberian B, Sulica VI, et al. A double-blind evaluation of topical capsaicin in pruritic psoriasis. J Am Acad Dermatol. 1993;29(3):438–442. doi:10.1016/0190-9622(93)70208-b
67. Aschenbeck KA, Hordinsky MK, Kennedy WR, et al. Neuromodulatory treatment of recalcitrant plaque psoriasis with onabotulinumtoxin A. J Am Acad Dermatol. 2018;79(6):1156–1159. doi:10.1016/j.jaad.2018.07.058
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.