Back to Journals » Chronic Wound Care Management and Research » Volume 7
Effects of Lucilia sericata Maggot Therapy in Chronic Wound Treatment: A Randomized Clinical Trial
Authors Nezakati E, Hasani MH, Zolfaghari P, Rashidan M , Sohrabi MB
Received 3 February 2020
Accepted for publication 5 May 2020
Published 22 May 2020 Volume 2020:7 Pages 11—17
DOI https://doi.org/10.2147/CWCMR.S248149
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Marco Romanelli
Ehsan Nezakati,1 Mohammad Hossain Hasani,2 Pouneh Zolfaghari,3 Marjan Rashidan,4 Mohammad Bagher Sohrabi5
1Department of Infectious Diseases, Imam Hossain Center for Education, Research and Treatment, Shahroud University of Medical Sciences, Shahroud, Iran; 2Department of Internal Medicine, Islamic Azad University, Shahroud Branch, Shahroud, Iran; 3Vice-Chancellery of Health, Shahroud University of Medical Sciences, Shahroud, Iran; 4Department of Microbiology, School of Medicine, Shahroud University of Medical Sciences, Shahroud, Iran; 5Education Unit, School of Medicine, Shahroud University of Medical Sciences, Shahroud, Iran
Correspondence: Mohammad Bagher Sohrabi Tel +982 332395054
Fax +982 332394800
Email [email protected]
Background: Chronic ulcers are one of the challenges of treatment today and cost a lot to the health system. The present study was conducted to investigate the effect of Lucilia sericata maggot therapy in chronic wound treatment.
Materials and Methods: This study was conducted as a clinical trial study with 90 eligible patients. Patients were randomly assigned to two equal intervention and control groups. Both groups received routine treatments for chronic ulcers. Patients in the intervention group received maggot therapy with larvae of Lucilia sericata. For all patients, a smear and culture of wound discharges were acquired. The condition of wound healing, the type of infection, and the reduction of microorganisms were compared between the two groups.
Results: Staphylococcus aureus was present in 68.9% of the patients and was the most abundant infection among all patients. Results of culturing after larval treatment at different times revealed a decrease in the number of all bacteria, especially Pseudomonas aeruginosa, Escherichia coli, and S. aureus, but the response rate for Enterococcus was the lowest. Also, the wound healing rate and reduction in necrotic tissue at the end of the second week (p=0.041) and the third week (p=0.012) was significantly higher in the intervention group.
Conclusion: Larvae of L. sericata have the highest effects on P. aeruginosa and had the least effect on the growth of Enterococcus. Also, our results showed larvae of L. sericata therapy can significantly improve wound healing rate.
Keywords: chronic ulcers, maggot therapy, larvae of Lucilia sericata, wound healing
Introduction
As populations become older, the number of patients suffering from ulcers due to chronic diseases, such as pressure ulcers, diabetic and vascular ulcers, is increasing.1 Chronic wound is a wound whose recovery requires a long time or it happens commonly and recurring which normally reduces the quality of life.2 Wound infection is usually caused by exogenous microorganisms in the air and polymicrobial flora, including aerobics, facultative anaerobes, and obligates anaerobes, Staphylococcus, Enterococcus, Corynebacterium, and Enterobacteriaceae species. Escherichia coli is one of the common causes of chronic wound infections. Various studies have shown the production of beta-lactamase enzymes by significant number of Enterobacteriaceae family, increasing methicillin-resistant Staphylococcus aureus (MRSA) infections, and bacterial biofilm formation. Thus, it is necessary to find alternative therapies for antibiotics or assisting in treatment, antibiotics.1,2 One of the most important and effective factors in wound healing is the provision of the substrate and the conditions for recovery. These conditions include debridement of dead tissues, prevention, and control of infections and inflammation, and equilibrium in the amount of moisture in the wound.3 Wound debridement is one of the most important stages in the treatment of ulcers, especially resisting ulcers.2,3 Debridement includes the removal of necrotic tissues and secretions, foreign matter, and bacteria.1 There are several methods for debridement, which include: surgical, mechanical, chemical, autolysis, and biological methods.4 Different studies have been done on different methods of debridement, but no study has demonstrated the superiority of a method to other methods.5 Debridement by larva as a biological method for the first time in 1550 by Dr. William Baer was used to improve the wounds of the army soldiers and later used for sterilization in controlled conditions.6
Along with increasing resistance to penicillin and other antibiotic groups and the failure of routine therapies, the use of larval care has been considered.7 Larva therapy is used in open wounds and wounds containing gangrene and necrotic tissues, with or without infection.8,9 Larva therapy stimulates wound healing and reduces bacterial load.10 So far, various studies have examined the clinical effects of larval treatment but they have reported different results.11,12 Given the above, the purpose of this study was to investigate the effect of Maggot of Lucilia sericata therapy in chronic wound treatment.
Materials and Methods
This triple-blind clinical trial was performed on 90 patients with chronic ulcers. The study was conducted on referred patients of two educational hospitals (Imam Hossain and Khatam-AL-Anbia hospitals) in Shahroud, Iran, from June 2018 to August 2018. The enrolled patients were randomly allocated to intervention and control groups.
Inclusion criteria: Having a chronic ulcer over 3 months on the outside of the body; no use of antibiotics in the last 3 weeks; no history of surgery in the desired position; no need for anticoagulants and steroids drugs; no addiction to alcohol or drugs; satisfaction to participate in study.
Exclusion criteria: Use of radiation and phototherapy in a recent month; suffering to severe diseases such as heart, liver, and kidney disease; having plenty of pain during treatment; having an uncontrollable fever during treatment. All patients were examined for necrotic tissue before splitting into the intervention and control groups and patients with deep and thick (over 70% of wound bed) necrotic tissue requiring surgery to clear necrotic tissue were excluded. So, all patients were vascular examined by Doppler ultrasound and those with severe vascular failure were excluded.
Blinding description: In this study, patients, and the person responsible for wound healing assessment and analyzer were blinded.
The assignment of patients into two groups of intervention and control was done by a qualified nurse according to the list of assignment order which was developed by statistic consultant. Assessment of wounds was done by a qualified nurse without any knowledge of the type of intervention.
For all of the wounds in both groups, a smear sample and culture were prepared, and the type of infection was determined. Both groups received routine treatment of chronic ulcers. Routine treatment includes topical and systemic treatment, including regular wound healing, debridement, wet dressing, nutritional support, and antibiotic treatment. Before intervention phase, primary wound evaluation was done and damaged tissues were removed from wounds. Patients in the intervention group received larva therapy in addition to routine therapies. However, patients in the control group were treated with routine methods only. The number of larvae per wound varies depending on the extent and depth of the wound and the degree of infection in the various parts of the damaged site at the site of the wound, but usually, be laid 8 to 10 larvae per cm2 of ulcer. Larvae should usually be removed within 48–72 hours after prescription.
Measured patient-related variables included age, sex, smoking, substance abuse and the duration of the ulcer. The variables related to the ulcer included type of ulcer, site of ulcer, wound depth (Grade II (i.e. dermal and epidermis) and Grade III involvement (i.e. joint involvement and tendon)), septic arthritis, and type of bacteria. Two times a week, the site was replaced by new larvae and the rate of necrotic tissue and new granulation tissue was evaluated and measured. In this study, the main outcome variables were removing necrotic tissues and appearing of granulated tissues. All treatments were performed for patients in both groups for at least three weeks and maximum until complete healing of the wounds. After larval treatment, the wounds were re-cultured by standard methods. The flow chart of the study is shown in Figure 1.
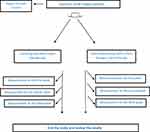 |
Figure 1 Flow diagram of study. |
Data analysis was performed based on intention to treat analysis by statistical software SPSS version 16. For describing the findings, descriptive statistics such as mean± standard deviation and frequency were used. Due to the quantitative and qualitative variables, independent T and Chi-square tests were used in the significance level of P <0.05.
The protocol of this study has been approved by the ethics committee of Islamic Azad University, Shahrood Branch (IR.IAU.SHAHROOD.REC.1396.53). This study has been registered in Iranian of clinical trials registration system (IRCT20100102002954N13). The essential information and the objectives of the study were explained to the patients, and a written informed consent was obtained for participation in the study.
Results
From all patients, 67 patients (74.9%) were male and the rest were female. The mean age of all patients was 55.4± 11.6 years (45–66 years). The mean duration of the disease was 3.7 months for chronic ulcers. Eighty-three percent of patients had lower limb ulcer and 17% had wound in the sacrum area. The demographic and clinical history data of patients in the two groups are presented in Table 1. So during the study, none of the patients needed urgent amputations and there was no case of sepsis during treatment; therefore, none of the patients were excluded from the study.
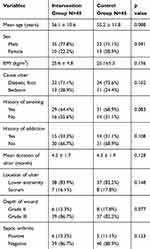 |
Table 1 Comparison of Demographic Data and Clinical Variables in Intervention and Control Groups |
The results of culture before treatment in patients with chronic ulcers are shown in Table 2. As can be seen, S. aurous with 62 cases (68.9%) was the most abundant infection among all patients.
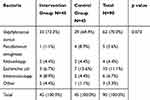 |
Table 2 Culture Characteristics Before Treatment in Intervention and Control Groups |
Results of culturing after larval treatment at different times revealed a decrease in the number of all bacteria, but the response rate for enterococcus was the lowest, which could indicate the resistance of this bacterium to treatment. The complete results of wound culture at different times are shown in Table 3.
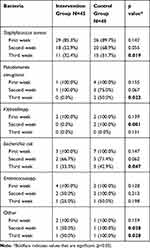 |
Table 3 Culture Characteristics After Treatment at Different Times in Intervention and Control Groups |
The wound healing rate and removing necrotic tissues was not significantly different between the two groups at the end of the first week of study (p=0.103). The results of study showed significant differences in wound healing rate and reduction of necrotic tissues between two groups at the second and third weeks after intervention. The wound healing rates after treatment at different times in intervention and control groups are shown in Table 4.
 |
Table 4 The Wound Healing Rate After Treatment at Different Times in Intervention and Control Groups |
Discussion
The results of this study demonstrate the use of larva in the treatment of chronic wounds could significantly reduce bacterial load/bioburden and improve wound healing. In the third week after treatment, decrees of bacteria were significantly higher compared to the control group. Also, the results of this study showed that the effect of larva therapy on some microbial strains such as Pseudomonas aeruginosa and E. coli is very good and on some microbial strains such as Enterobacter is relatively weak. Our findings are consistent with the results of studies by Kerridge et al and Renner et al.11,12
The treatment of wounds or chronic injuries is usually slow and hard, especially if routine treatments are used. These wounds are seen in most people with poor health status.13 The use of larval treatment has been reported more than 100 years ago. Previous studies have emphasized the importance of using Larva therapy as an effective way to treat chronic ulcers. Larval treatment reduces the risk of amputation, reduces the use of antibiotics, prevents long-term hospitalization, and reduces the incidence of outpatient visits.14 Larval treatment can be used as the first-line treatment in patients with antibiotic resistance, infectious ulcers, immunosuppression, and diabetic disease.13,14 Several studies, either in the form of a clinical trial or as a systematic review, have examined the effect of larval treatment on different patients group and different aspects.15–17
Valachová et al, in their study, showed that larval treatment is more effective than conventional treatments in debridement of chronic ulcers that is consistent with the results of our study.18
Lepage et al, in their clinical trial study, investigated the effect of larval treatment in comparison with hydrogel in wound healing and increasing of quality-adjusted life years (QALYs), which did not show any difference between the two groups. No antibiotic was used in this study. Only changes in the wound culture were investigated. The difference between the results of this study and the present study can be attributed to the difference in the technique of the two studies and the antibiotics used in this study that probably using antibiotics will increase the speed of recovery and also eliminate the necrotic parts faster.19
Morgan and Nigam evaluated the effect of larval treatment on bacterial load and resistance to S. aurous and observed that larval treatment is not more effective than hydrogel on reducing bacterial load but is more effective in eradicating MRSA. The results of this study are largely consistent with our findings and the reason for the minor differences between the two studies can be attributed to the difference in the sample size of the two studies.20
In terms of the depth of involvement in our study, 85% of patients had deep ulcer (Grade III) and only 10% of patients had Grade II ulcers. In our study, 59.9% of patients had septic arthritis and 90% had infectious ulcers while in Telford et al, 56.4% had deep ulcers (Grade III), 43.6% had Grade II ulcers and 11.1% of patients had septic arthritis. The comparison of results showed that septic arthritis was more prevalent in our study. The difference between the results of this study and the present study may be due to the choice of the type of chronic ulcer and the mean age difference between the two groups.21
In the study of Blueman and Bousfield, the frequency of smoking was 23% in control group and 14% in larval treatment group, while in our study 63.3% of patients were smokers. Using cigarettes and opium can slowdown wound healing and prolong the course of treatment. Therefore, in the treatment of chronic ulcers, the condition of the patient and especially the use of cigarette as a confounding factor for wound healing should always be considered.22
The important point in our study was the examination of ulcers caused by cultures and smears that have not been addressed in other studies. The results of this study showed that after treatment with larvae, there was a significant decrease in bacterial growth rate in consecutive weeks after intervention and that the highest decreasing rate of bacterial growth in smear and culture was observed in the third week after intervention (P. aeruginosa completely decreased and E. coli decreased up to 50% ). Our study results showed a maximum capacity for larval treatment in preventing the growth of bacteria which is consistent with the results of the Gilead et al study.23
Larvae had a lower effect on reducing the growth of enterococci, in spite of the decline in the growth rate of all bacterial species in the second week, enterococci resistance showed the larvae in the second week. So in the third week, there was only a 25% drop in growth, so that growth rate of enterococci from 75% in the first week to 25% in the third week.24
Limitations
One of the important limitations of this research was the failure of some patients to use larvae in their treatment, which was justified by patients. Also the relatively long period of treatment and high cost were other limitations that were solved with the help of the research deputy of Islamic Azad University of Shahrood.
Conclusion
Larvae therapy has a variety of effects on different bacterial species that have the highest effects on P. aeruginosa, E. coli, and S. aureus and had the least effect on the growth of Enterococcus. Therefore, it is suggested that more extensive studies on the number of patients should be done in multicenter studies on the effects of larval treatment on bacterial growth in chronic ulcers.
Abbreviations
QALYs, Quality-adjusted life years; MRSA, Methicillin-resistant Staphylococcus aureus; E. coli, Escherichia coli; S. aureus, Staphylococcus aureus; P. aeruginosa, Pseudomonas aeruginosa; L. sericata, Lucilia sericata.
Data Sharing Statement
The dataset used and/or analyzed during the present study is available from the corresponding author upon reasonable request.
Ethics, Consent, and Registration
This study has an ethics code number (IR.IAU.SHAHROOD.REC.1396.53) from the ethics committee of Islamic Azad University of Shahrood Branch and has been registered in Iranian clinical trials system with code IRCT20100102002954N13. The essential information and the objectives of the study were explained to the patients, and written informed consent was obtained for all participants for participation in the study.
Acknowledgment
The present study was supported by Islamic Azad University of Shahrood Branch. We hereby acknowledge the research deputy. We would also like to thank all patients.
Author Contributions
All authors of this article have played an active role in all aspects of the preparation of the article, including: design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Polakovičova S, Polák Š, Kuniaková M, et al. The effect of salivary gland extracts of Lucilia sericata maggots on human dermal fibroblast proliferation within collagen/hyaluronan membrane in vitro: transmission electron microscopy study. Adv Skin Wound Care. 2015;28(5):221–226. doi:10.1097/01.ASW.0000461260.03630.a0
2. Horobin AJ, Shakesheff KM, Woodrow S, Robinson C, Pritchard DI. Maggots and wound healing: an investigation of the effects of secretions from Lucilia sericata larvae upon interactions between human dermal fibroblasts and extracellular matrix components. Br J Dermatol. 2003;148(5):923–933. doi:10.1046/j.1365-2133.2003.05314.x
3. Shakil S, Khan AU. Infected foot ulcers in male and female diabetic patients: a clinicobioinformative study. Ann Clin Microbiol Antimicrob. 2010;9:2–6. doi:10.1186/1476-0711-9-2
4. Cabeza-De-Vaca F, Macias AE, Alvarez JA, et al. Diabetic foot microbiology through biopsy cultures. Rev Invest Clin. 2009;61(4):281–285.
5. Andersen A, Hill KE, Stephens P, Thomas DW, Jorgensen B, Krogfelt KA. Bacterial profiling using skin grafting, standard culture and molecular bacteriological methods. J Wound Care. 2007;16(4):171–175. doi:10.12968/jowc.2007.16.4.27025
6. Bexfield A, Bond AE, Roberts EC, et al. The antibacterial activity against MRSA strains and other bacteria of a <500Da fraction from maggot excretions/secretions of Lucilia sericata (Diptera: calliphoridae). Microbes Infect. 2008;10(4):325–333. doi:10.1016/j.micinf.2007.12.011
7. Bowling FL, Salgami EV, Boulton AJ. Larval therapy: a novel treatment in eliminating methicillin-resistant Staphylococcus aureus from diabetic foot ulcers. Diabetes Care. 2007;30(2):370–371. doi:10.2337/dc06-2348
8. Cazander G, van Veen KE, Bernards AT, Jukema GN. Do maggots have an influence on bacterial growth? A study on the susceptibility of strains of six different bacterial species to maggots of Lucilia sericata and their excretions/secretions. J Tissue Viability. 2009;18(3):80–87. doi:10.1016/j.jtv.2009.02.005
9. Howell-Jones RS, Wilson MJ, Hill KE, Howard AJ, Price PE, Thomas DW. A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J Antimicrob Chemother. 2005;55(2):143–149. doi:10.1093/jac/dkh513
10. Jaklic D, Lapanje A, Zupancic K, Smrke D, Gunde-Cimerman N. Selective antimicrobial activity of maggots against pathogenic bacteria. J Med Microbiol. 2008;57(Pt 5):617–625. doi:10.1099/jmm.0.47515-0
11. Kerridge A, Lappin-Scott H, Stevens JR. Antibacterial properties of larval secretions of the blowfly, Lucilia sericata. Med Vet Entomol. 2005;19(3):333–337. doi:10.1111/j.1365-2915.2005.00577.x
12. Renner R, Treudler R, Simon JC. Maggots do not survive in pyoderma gangrenosum. Dermatology. 2008;217(3):241–243. doi:10.1159/000148251
13. Steenvoorde P, Jukema GN. The antimicrobial activity of maggots: in-vivo results. J Tissue Viability. 2004;14(3):97–101. doi:10.1016/S0965-206X(04)43005-8
14. Thomas S, Andrews AM, Hay NP, Bourgoise S. The anti-microbial activity of maggot secretions: results of a preliminary study. J Tissue Viability. 1999;9(4):127–132. doi:10.1016/S0965-206X(99)80032-1
15. Wilasrusmee C, Marjareonrungrung M, Eamkong S, et al. Maggot therapy for chronic ulcer: a retrospective cohort and a meta-analysis. Asian J Surg. 2013;
16. Gao B, Zhu S. An insect defensin-derived β-hairpin peptide with enhanced antibacterial activity. ACS Chem Biol. 2013.
17. Téllez GA, Acero MA, Pineda LA, Castaño JC. Larvaterapia aplicada a heridas con poca carga de tejido necrótico y caracterización enzimática de la excreción, secreción y hemolinfa de larvas [Effect of maggot therapy on minimally necrotic tissues: characterization of larval enzymatic excretion/secretion]. Biomedica. 2012;32(3):312–320. Spanish.
18. Valachová I, Bohová J, Pálošová Z, et al. Expression of lucifensin in Lucilia sericata medicinal maggots in infected environments. Cell Tissue Res. 2013;353(1):165–171. doi:10.1007/s00441-013-1626-6
19. Lepage OM, Doumbia A, Perron-Lepage MF, Gangl M. The use of maggot debridement therapy in 41 equids. Equine Vet J Suppl. 2012;43:120–125. doi:10.1111/j.2042-3306.2012.00609.x
20. Morgan C, Nigam Y. Naturally derived factors and their role in the promotion of angiogenesis for the healing of chronic wounds. Angiogenesis. 2013;16(3):493–502. doi:10.1007/s10456-013-9341-1
21. Telford G, Brown AP, Rich A, English JS, Pritchard DI. Wound debridement potential of glycosidases of the wound-healing maggot, Lucilia sericata. Med Vet Entomol. 2012;26(3):291–299. doi:10.1111/j.1365-2915.2011.01000.x
22. Blueman D, Bousfield C. The use of larval therapy to reduce the bacterial load in chronic wounds. J Wound Care. 2012;21(5):244–253. doi:10.12968/jowc.2012.21.5.244
23. Gilead L, Mumcuoglu KY, Ingber A. The use of maggot debridement therapy in the treatment of chronic wounds in hospitalised and ambulatory patients. J Wound Care. 2012;21(2):78, 80, 82–85. doi:10.12968/jowc.2012.21.2.78
24. Zarchi K, Jemec GB. The efficacy of maggot debridement therapy–a review of comparative clinical trials. Int Wound J. 2012;9(5):469–477. doi:10.1111/j.1742-481X.2011.00919.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
