Back to Journals » Journal of Pain Research » Volume 12
Effects of low-dose ketamine infusion on remifentanil-induced acute opioid tolerance and the inflammatory response in patients undergoing orthognathic surgery
Authors Kido K , Toda S, Shindo Y, Miyashita H, Sugino S , Masaki E
Received 19 June 2018
Accepted for publication 17 December 2018
Published 17 January 2019 Volume 2019:12 Pages 377—385
DOI https://doi.org/10.2147/JPR.S177098
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Michael Schatman
Kanta Kido,1 Shinichi Toda,1 Yuki Shindo,1 Hitoshi Miyashita,2 Shigekazu Sugino,3 Eiji Masaki1
1Department of Dental Anesthesiology and Pain Management, Tohoku University Hospital, Sendai, Miyagi, Japan; 2Department of Oral Medicine and Surgery, Division of Oral and Maxillofacial Surgery, Graduate School of Dentistry, Tohoku University, Sendai, Miyagi, Japan; 3Department of Anesthesiology and Perioperative Medicine, Tohoku University Hospital, Sendai, Miyagi, Japan
Purpose: Remifentanil is associated with acute opioid tolerance that can lead to increased postoperative consumption of opioid analgesics. The purpose of this study was to determine whether a low dose of ketamine prevents remifentanil-induced acute opioid tolerance and affects the neutrophil–lymphocyte ratio (NLR), a newly recognized biomarker of inflammation.
Materials and methods: Forty patients undergoing orthognathic surgery were enrolled in this prospective, randomized, double-blind study and randomly assigned to intraoperative administration of one of the following anesthetic regimens: high-dose remifentanil (0.6 µg/kg/minute); low-dose remifentanil (0.2 µg/kg/minute); or high-dose remifentanil with ketamine (remifentanil 0.6 µg/kg/minute with 0.5 mg/kg ketamine just after induction followed by an intraoperative infusion of ketamine 5 µg/kg/minute until wound closure). Fentanyl by intravenous patient-controlled analgesia was used for postoperative pain control. Visual Analog Scale pain scores and fentanyl consumption were recorded in the first 24 hours postoperatively. Perioperative serum C-reactive protein level and NLR were also determined.
Results: Baseline characteristics were similar in the three study groups. There were no between-group differences in Visual Analog Scale pain scores during the study period. The high-dose remifentanil group had a significantly higher requirement for fentanyl than the other two groups. Addition of ketamine did not affect the C-reactive protein level but increased the NLR; this increase was associated with decreased fentanyl consumption.
Conclusion: High-dose intraoperative remifentanil induced postoperative acute opioid tolerance that was prevented by infusion of low-dose ketamine. Ketamine increased the postoperative NLR associated with decreased fentanyl requirement for postoperative pain control.
Keywords: acute tolerance, central sensitization, NMDA receptor, neutrophil–lymphocyte ratio, remifentanil
Introduction
Perioperative pain management in patients who cannot undergo neuraxial regional block or peripheral nerve block continues to be challenging. Therefore, the options for pain control remain limited in many patients undergoing head and neck surgery under general anesthesia.
Opioids are the most common form of pain management both intraoperatively and postoperatively. Remifentanil is an ultra-short-acting μ-opioid receptor agonist that is often used for intraoperative analgesia because it has a rapid onset and offset, thereby allowing easily controllable analgesia.1,2 Moreover, remifentanil can be used at a high dose and for a long duration to prevent intraoperative pain without delaying postoperative recovery or causing respiratory depression. However, exposure to high-dose remifentanil may paradoxically induce hyperalgesia and acute opioid tolerance, manifesting as more severe postoperative pain and greater analgesic requirements.3–7 A review by Angst8 found that postoperative acute opioid tolerance and/or hyperalgesia and increased opioid consumption were consistently reported when patients received a cumulative remifentanil dose >50 µg/kg intraoperatively.
The mechanism by which postoperative hyperalgesia/acute opioid tolerance induced is still not fully understood; so, there is no effective prevention or treatment strategy for hyperalgesia after remifentanil-based anesthesia. Several studies have identified central sensitization via the glutaminergic system and activation of the N-methyl-d-aspartate (NMDA) receptor to have significant roles in this mechanism.9–12
Ketamine is the most widely used NMDA receptor antagonist. It is thought to assist in the regulation of various pain states by desensitizing excitatory spinal cord NMDA receptors, thereby inhibiting pain transmission.13 It is known that use of high-dose ketamine for anesthesia has a dose-related stimulatory effect on the cardiovascular system, can cause psychiatric disturbance, and increases plasma cortisol concentrations.14,15 It has also been reported that intraoperative administration of low-dose ketamine can inhibit central sensitization and prevent opioid-induced hyperalgesia, thereby reducing opioid use and postoperative pain16–19 without undesirable effects. However, other studies20,21 could not confirm any effect of intraoperative ketamine on postoperative pain scores or opioid consumption, and two meta-analyses even concluded that ketamine could increase opioid-induced hyperalgesia.22,23 The effect of ketamine coadministration on remifentanil-induced hyperalgesia during craniofacial surgery has not been clarified.
Ketamine can also decrease the secretion of several proinflammatory mediators of inflammation and the immune response, including interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-alpha, as well as production of C-reactive protein (CRP) and natural killer cell activity.24–27 Cytokine levels have been associated with development of hyperalgesia and nociceptive sensitivity.28–32 Furthermore, the neutrophil–lymphocyte ratio (NLR) has recently been proposed to be a sensitive biomarker of systemic inflammation.33,34 Measurement of the NLR might be a useful way for assessing the relationship between the inflammatory response and postoperative pain. However, few studies have examined the relationship of combined use of remifentanil and ketamine with the NLR and postoperative pain.
In this prospective randomized controlled study, we tested the following hypothesis: high-dose remifentanil induces acute opioid tolerance, whereas ketamine coadministration prevents it and reduces postoperative opioid consumption. In addition, ketamine modifies the postoperative side effect profile in orthognathic surgery. A secondary hypothesis was that ketamine administration affects immunoreactivity, which is associated with postoperative pain. The aims of this study were to determine whether intraoperative coadministration of ketamine prevents remifentanil-induced acute opioid tolerance postoperatively and to investigate the relevance of ketamine to the inflammatory response and postoperative opioid consumption in patients undergoing orthognathic surgery.
Materials and methods
Patients
This prospective randomized controlled study was approved by the Tohoku University Hospital Ethics Committee (approval number 26-46). Forty patients with an American Society of Anesthesiologists (ASA) physical status of I–II who were scheduled for mandibular orthognathic surgery were enrolled. The exclusion criteria were chronic use of analgesia, a psychiatric disorder, obesity (body mass index >30), a chronic inflammatory disease, and acute cardiovascular disease. Written informed consent was obtained from all patients and the study was conducted in accordance with the Declaration of Helsinki.
Procedure
All patients underwent the same surgical procedure, bilateral sagittal split ramus osteotomy for mandibular prognathism performed by two experienced surgeons. Before surgery, all patients were shown how to use a patient-controlled analgesia (PCA) pump (CADD-Legacy PCA, model 6300; Smiths Medical Japan, Tokyo, Japan) and how to rate their pain using a 100-mm Visual Analog Scale (VAS: 0, no pain; 100, worst pain).
Before induction of anesthesia, 6.6 mg of dexamethasone and 1.25 mg of droperidol were administered for prevention of postoperative nausea and vomiting (PONV). After a 3-minute infusion of remifentanil 0.4 µg/kg/minute, anesthesia was induced by propofol administered via a syringe pump (TE-371, Terumo Inc., Tokyo, Japan) with a built-in target-controlled infusion system (Diprifusor™, AstraZeneca, London, UK). Rocuronium 0.8 mg/kg was then administered to facilitate nasotracheal intubation. All patients were mechanically ventilated with 40% oxygen after intubation to maintain end-tidal CO2 of 35–40 mmHg. Anesthesia was maintained using remifentanil at the doses described below and by propofol administered as a target-controlled infusion to maintain a bispectral index of 40–60.
Patients were randomly assigned, in a double-blinded fashion, to one of three groups (12 patients/group).14 Before the study started, a random-number table was generated, specifying the group to which each patient would be assigned upon entry into the trial. For each patient, an envelope containing the group assignment was prepared, sealed, and sequentially numbered.
On the morning of surgery, an anesthesiologist not involved in evaluation of the patient opened the patient’s allocated envelope and prepared the assigned syringes containing remifentanil, ketamine, and/or saline solution. All the other study investigators were blinded to group allocation. The three treatment groups are described below.
High-dose remifentanil group
A higher dose of remifentanil than usual is often used for craniofacial surgery to control pain and hemodynamics. In this study, patients in the high-dose remifentanil group received an intraoperative infusion of remifentanil 0.6 µg/kg/minute. According to a review article on remifentanil-induced hyperalgesia,8 postoperative acute opioid tolerance, hyperalgesia, and increased opioid consumption are more likely to occur if the cumulative intraoperative remifentanil dose is >50 µg/kg. In this trial, a high dose of remifentanil was calculated to be 0.6 µg/kg/minute on the basis of the findings of a pilot study in which we took into account patient body weight and duration of surgery. The cumulative dose of remifentanil that would induce hyperalgesia was calculated to be 50 µg/kg ×55 kg =2,750 µg. In this protocol, the total dose of remifentanil in the high-dose group was expected to be 0.6 µg/kg/minute ×55 kg ×90 minutes=2,970 µg.
Low-dose remifentanil group
These patients received an intraoperative infusion of remifentanil 0.2 µg/kg/minute.
High-dose remifentanil with ketamine group
These patients received an intraoperative infusion of remifentanil 0.6 µg/kg/minute with ketamine 0.5 mg/kg administered immediately after induction of anesthesia as a loading dose followed by a maintenance infusion of 5 µg/kg/minute until completion of surgery.14,21
Inadequate anesthesia was defined as a heart rate that exceeded the baseline value by 15% and/or a systolic blood pressure that exceeded the baseline value by 20% for 1 minute or longer. The remifentanil concentration was increased stepwise by 0.05 µg/kg/minute when inadequate anesthesia was suspected. When persistent hypotension (defined as a systolic blood pressure <80 mmHg or a mean arterial pressure <60 mmHg) was observed, an intravenous bolus dose of ephedrine was administered. Bradycardia (defined as a heart rate <50 beats/minute) was treated with atropine. Fentanyl 4 µg/kg and flurbiprofen axetil 1 mg/kg were administered intravenously at the end of surgery. After cessation of propofol and remifentanil, the trachea was extubated when the patient could respond to verbal commands, such as gripping the hand of the anesthesiologist, and had a spontaneous respiratory rate >10 breaths/minute, a tidal volume >8 mL/kg, and an end-tidal CO2 partial pressure of <50 mmHg. Postoperative analgesia consisted of fentanyl delivered via a PCA pump containing 500–700 µg fentanyl in a total volume of 50–70 mL saline (giving a final fentanyl concentration of 10 µg/mL). The PCA device was set to deliver fentanyl 0.5 µg/kg as a bolus dose with a 6-minute-lockout interval. Continuous infusion was not used. This PCA regimen was continued for 24 hours after surgery. Flurbiprofen axetil 50 mg could be administered as rescue analgesia if requested by the patient.
Measurements
Primary outcome measures were pain severity, fentanyl consumption, and the number of PCA demands in the first 24 hours after surgery. The secondary outcome measures were the CRP level, neutrophil and lymphocyte counts, and the NLR, all of which are indicators of the inflammatory response.34 The total doses of remifentanil and propofol administered during surgery were recorded. Pain intensity was assessed using the VAS scores recorded before leaving the operating room and at 1, 3, 6, 12, and 24 hours after surgery. Anesthesia-related complications, including PONV, respiratory depression (<10 breaths/minute or SpO2 <92%), hallucinations, and diplopia, were recorded. PONV was treated by an intravenous bolus of metoclopramide 10 mg. The CRP level and NLR were determined from blood samples taken preoperatively and on days 1 and 7 postoperatively. The NLR was determined by the absolute neutrophil count divided by the absolute lymphocyte count. Body temperature was recorded at each assessment point. Surgical-site infections that occurred in the first postoperative week were also recorded.
Statistical analyses
Our sample size estimation was based on the differences in 24-hour fentanyl consumption expected between the high-dose and low-dose remifentanil groups. In our pilot study of five patients, the fentanyl consumption was approximately 400 µg (SD 200 µg) in the first 24 hours postoperatively in the high-dose remifentanil group. Using G*power software version 3.0.10 for Mac (Heinrich-Heine-Universität, Düsseldorf, Germany) with an α error of 0.05 for detecting a 50% difference in fentanyl consumption, we estimated that a sample size of 12 patients per treatment group would be required. Post hoc calculation of the achieved power was 0.8. Allowing for a dropout rate of 10%, the total number of patients needed was set at 40. The Kolmogorov–Smirnov test was used to determine normality. Fentanyl consumption and the number of PCA demands were compared between the three groups by one-way analysis of variance and the total doses of remifentanil and propofol were compared using the Kruskal–Wallis test. The VAS score, CRP value, neutrophil and lymphocyte count, and NLR at each time point were compared among the three groups using two-way analysis of variance for repeated measures. The Tukey’s test was used as needed for post hoc comparisons. The Chi-squared test was used for categorical variables (sex, ASA physical status, and the number of patients who needed the drugs). Correlations between fentanyl consumption and the NLR on postoperative day 1 were calculated using the Spearman’s rank correlation test. The statistical analyses were performed using GraphPad Prism 6 for Mac (GraphPad Software Inc., San Diego, CA, USA). A P-value <0.05 was considered statistically significant.
Results
Forty patients were enrolled in this study. One patient was excluded because of a psychiatric disorder and three patients were withdrawn because of the need for an additional surgical procedure (n=1; high-dose remifentanil group), postoperative bleeding requiring repeat surgery (n=1; low-dose remifentanil group), and refusal to use PCA (n=1; high-dose remifentanil with ketamine group). Patient characteristics (age, height, weight, body mass index, sex, and ASA status) were not significantly different between the three study groups (Table 1). The duration of surgery and anesthesia; total doses of propofol administered; total amount of bleeding; number of patients who needed atropine, ephedrine, and postoperative rescue analgesia (flurbiprofen axetil); and incidence of PONV were comparable between the groups (Table 2). The dose of remifentanil administered in the two high-dose remifentanil groups was as planned (ie, >50 µg/kg). There were no postoperative complications in any of the study groups.
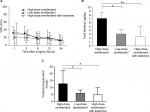
VAS pain scores did not differ significantly between the three groups at any of the assessment time points in the 24 hours postoperatively (Figure 1A), indicating that the PCA pump was appropriate for postoperative pain management. Fentanyl consumption was significantly greater in the first 24 hours postoperatively in the high-dose remifentanil group (4.65±3.34 µg) than in the low-dose remifentanil group (2.41±1.06 µg; P<0.05) and the high-dose remifentanil with ketamine group (1.65±1.62 µg; P<0.01; Figure 1B). Similarly, the number of PCA demands was significantly greater in the high-dose remifentanil group (12.8±9.3) than in the low-dose remifentanil group (5.9±2.3; P<0.05) and the high-dose remifentanil with ketamine group (4.8±5.0; P<0.05) (Figure 1C).
Perioperative inflammatory response was compared between the study groups using the CRP level, neutrophil and lymphocyte counts, and the NLR as inflammatory biomarkers (Table 3). There was no significant difference in the CRP level or neutrophil count preoperatively or on postoperative days 1 and 7 between the groups. The lymphocyte count was significantly lower in the high-dose remifentanil with ketamine group than in the high-dose remifentanil group on postoperative day 1 (P<0.05; 0.8±0.3×103/µL vs 1.2±0.3×103/µL). On postoperative day 1, NLR in the high-dose remifentanil with ketamine group was significantly higher than that in the high-dose remifentanil group and also higher than that in the low-dose remifentanil group (P<0.001 vs the high-dose remifentanil group, P<0.05 vs the low-dose remifentanil group). To investigate the clinical significance of the NLR changes, we tested correlations between postoperative fentanyl consumption and pre- and postoperative NLR levels in all patients using Spearman’s rank-order correlation (Table 4); no significant correlation was found and the correlation coefficients were 0.07 and –0.06, respectively. There was no significant difference in body temperature between the study groups (data not shown), and there were no surgical-site infections. In addition, no side effects induced by ketamine including hallucination or diplopia were observed in the ketamine group.
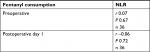  | Table 4 Correlation between fentanyl consumption and NLR Note: Spearman’s rank correlation test. Abbreviations: NLR, neutrophil–lymphocyte ratio; r, correlation coefficient; n, number of subjects. |
Discussion
The findings of this study indicate that intraoperative coadministration of ketamine decreases the likelihood of remifentanil-induced postoperative acute opioid tolerance without adverse effects and significantly increases the NLR in 24 hours following the orthognathic surgery. Therefore, low-dose ketamine could be useful for prevention of the acute postoperative opioid tolerance associated with remifentanil-based anesthesia in patients undergoing orthognathic surgery.
In this study, intraoperative coadministration of ketamine decreased the risk of development of remifentanil-induced acute postoperative opioid tolerance without adverse effects in patients undergoing orthognathic surgery. It is widely known that exposure to high-dose remifentanil is associated with postoperative hyperalgesia and acute opioid tolerance that manifests as increased postoperative pain intensity and greater analgesic requirements.3–7 However, distinguishing between postoperative hyperalgesia and acute tolerance in the clinical setting is challenging.8 In this study, we considered postoperative fentanyl consumption as indicative of the development of acute tolerance,4 because von Frey filaments could not effectively be applied to the intraoral incision and surrounding area to assess the pain threshold and extent.27 Although the detailed mechanism underlying the hyperalgesia (or opioid tolerance) is not fully understood, the most effective strategies for prevention of remifentanil-induced hyperalgesia in animal models have focused on central sensitization via the glutaminergic system and activation of the NMDA receptor.9,11,12,35 Coadministration of morphine and MK-801, a noncompetitive NMDA receptor antagonist, or LY274614, a competitive NMDA receptor antagonist, prevented hyperalgesia or opioid tolerance in the isolated rat spinal cord36 and other animals.37,38 Indeed, administration of an NMDA antagonist, such as ketamine or magnesium, has been shown to attenuate remifentanil-induced hyperalgesia in several human studies.5,8,16,19,39–41 A meta-analysis of 14 randomized controlled trials that included 729 patients concluded that perioperative administration of an NMDA receptor antagonist attenuated the increase in postoperative pain intensity and opioid consumption and increased patient satisfaction without increasing adverse effects.23 Although these clinical studies did not include patients undergoing intraoral or maxillofacial surgery, in whom the options for pain control are limited, our findings confirm that low-dose ketamine is effective for decreasing acute postoperative opioid tolerance even in patients undergoing orthognathic surgery without undesirable complications. Leal et al21 recently reported that low-dose ketamine did not prevent remifentanil-induced hyperalgesia after laparoscopic cholecystectomy. However, in their study, the intraoperative dose of remifentanil was <50 µg/kg,8 lower than that used in our study. Therefore, there was a possibility that remifentanil did not adequately induce postoperative hyperalgesia in their study. Another study reported that intraoperative low-dose ketamine did not prevent remifentanil-induced acute opioid tolerance after scoliosis surgery.20 However, the participants in that study were adolescents aged 12–18 years, in whom the pharmacokinetics of ketamine may be different from those in the adults who participated in our study. Overall, our findings suggest that an infusion of ketamine could prevent acute postoperative opioid tolerance in adult patients undergoing orthognathic surgery. Therefore, coadministration of ketamine could be considered if administration of high-dose remifentanil (≥50 µg/kg) is part of the anesthetic plan.
In addition, a low dose of ketamine significantly increased the NLR on day 1 after surgery in this study. It is well known that ketamine decreases inflammation and the immune response, including secretion of several proinflammatory mediators.24–26,42,43 Moreover, attenuation of cytokine secretion has been shown to decrease the intensity of hyperalgesia, nociceptive sensitivity, and postoperative pain.28–30,32,44 Therefore, we used the NLR, a newly identified and sensitive biomarker of inflammation, to assess whether coadministration of ketamine is associated with inflammation, the immune response, and postoperative pain. Our results suggest that ketamine has immunosuppressive effects, as indicated by a decrease in the number of lymphocytes; however, the composition of lymphocytes was not examined in this study. Ketamine at a low concentration has been shown in vitro to trigger apoptosis in T lymphocytes via the mitochondrial pathway45 and has also been reported to decrease the count and activity of natural killer cells after exposure in vivo.46,47 These studies might be able to explain at least in part the postoperative reduction in the lymphocyte count in this study. Only one study has investigated the relationship between the preoperative NLR and postoperative pain in patients undergoing laparoscopic cholecystectomy.48 That study showed that a higher preoperative NLR was associated with lower analgesic requirements. Our finding that a high ketamine-induced postoperative NLR was associated with decreased postoperative opioid consumption might also help to shed light on the mechanism by which ketamine prevents remifentanil-induced acute opioid tolerance.
However, we found no significant difference in the CRP level between the three study groups. This result is consistent with a meta-analysis that found administration of ketamine and production of cytokines did not cause changes in the CRP level.26 Ketamine may produce an immunosuppressive response rather than an anti-inflammatory response in patients undergoing remifentanil-based anesthesia.
Limitations
This study has several limitations. First, we did not include a control group receiving normal saline to confirm whether low-dose remifentanil induces postoperative acute opioid tolerance. However, it would be unethical to withhold opioids in patients undergoing this type of surgery. Second, only the NLR was used to assess the inflammatory response. Therefore, a further study is needed to determine the relationship between the NLR and other markers of inflammation. Also, we examined the NLR at only three time points: preoperatively, POD1, and POD7. It is also necessary to examine NLR at other time points, such as intraoperatively or at POD3, to evaluate the correlation between ketamine administration and NLR, and the associated clinical significance. Further study is thus needed. Third, we used dexamethasone and flurbiprofen axetil to control postoperative pain and PONV in the immediate postoperative period in all patients. Both these drugs have an anti-inflammatory effect, so the possibility that they could have affected the NLR cannot be excluded. Fourth, the high-dose remifentanil group might have undergone more invasive surgeries than the high-dose remifentanil with ketamine group based on the longer operative time and higher bleeding, although there were no statistically significant differences in both groups. Also, 8% of participants in the low-dose remifentanil group were males vs 33% in each of the two other groups. These factors might partly explain the differences in fentanyl requirement among the three groups.
Conclusion
Administration of low-dose ketamine (0.5 mg/kg+5 µg/kg/minute) during remifentanil-based anesthesia decreased acute opioid tolerance in the 24 hours following orthognathic surgery. There was also an association between decreased postoperative fentanyl consumption after administration of ketamine and an increase in the NLR. Further studies are needed to determine the clinical significance of the changes in NLR brought about by ketamine.
Acknowledgment
The authors would like to thank Drs. Atsuko Matoba and Makoto Yasuda for useful discussions and data collection.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Minto CF, Schnider TW, Egan TD, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology. 1997;86(1):10–23. | ||
Scott LJ, Perry CM. Remifentanil: a review of its use during the induction and maintenance of general anaesthesia. Drugs. 2005;65(13):1793–1823. | ||
Guignard B, Bossard AE, Coste C, et al. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93(2):409–417. | ||
Kim SH, Lee MH, Seo H, Lee IG, Hong JY, Hwang JH. Intraoperative infusion of 0.6-0.9 µg·kg(-1)·min(-1) remifentanil induces acute tolerance in young children after laparoscopic ureteroneocystostomy. Anesthesiology. 2013;118(2):337–343. | ||
Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106(1–2):49–57. | ||
van Gulik L, Ahlers SJ, van de Garde EM, Knibbe CA. Remifentanil during cardiac surgery is associated with chronic thoracic pain 1 yr after sternotomy. Br J Anaesth. 2012;109(4):616–622. | ||
Fletcher D, Martinez V. Opioid-induced hyperalgesia in patients after surgery: a systematic review and a meta-analysis. Br J Anaesth. 2014;112(6):991–1004. | ||
Angst MS. Intraoperative use of remifentanil for TIVA: postoperative pain, acute tolerance, and opioid-induced hyperalgesia. J Cardiothorac Vasc Anesth. 2015;29(Suppl 1):S16–S22. | ||
Rivat C, Laulin JP, Corcuff JB, Célèrier E, Pain L, Simonnet G. Fentanyl enhancement of carrageenan-induced long-lasting hyperalgesia in rats: prevention by the N-methyl-D-aspartate receptor antagonist ketamine. Anesthesiology. 2002;96(2):381–391. | ||
Stubhaug A, Breivik H, Eide PK, Kreunen M, Foss A. Mapping of punctuate hyperalgesia around a surgical incision demonstrates that ketamine is a powerful suppressor of central sensitization to pain following surgery. Acta Anaesthesiol Scand. 1997;41(9):1124–1132. | ||
Célèrier E, Rivat C, Jun Y, et al. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology. 2000;92(2):465–472. | ||
Pasternak GW, Kolesnikov YA, Babey AM. Perspectives on the N-methyl-D-aspartate/nitric oxide cascade and opioid tolerance. Neuropsychopharmacology. 1995;13(4):309–313. | ||
Kohrs R, Durieux ME. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998;87(5):1186–1193. | ||
Roytblat L, Korotkoruchko A, Katz J, Glazer M, Greemberg L, Fisher A. Postoperative pain: the effect of low-dose ketamine in addition to general anesthesia. Anesth Analg. 1993;77(6):1161–1165. | ||
Schmid RL, Sandler AN, Katz J. Use and efficacy of low-dose ketamine in the management of acute postoperative pain: a review of current techniques and outcomes. Pain. 1999;82(2):111–125. | ||
Joly V, Richebe P, Guignard B, et al. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology. 2005;103(1):147–155. | ||
Zhao M, Joo DT. Enhancement of spinal N-methyl-D-aspartate receptor function by remifentanil action at delta-opioid receptors as a mechanism for acute opioid-induced hyperalgesia or tolerance. Anesthesiology. 2008;109(2):308–317. | ||
Hang LH, Shao DH, Gu YP. The ED50 and ED95 of ketamine for prevention of postoperative hyperalgesia after remifentanil-based anaesthesia in patients undergoing laparoscopic cholecystectomy. Swiss Med Wkly. 2011;141:w13195. | ||
Koppert W, Sittl R, Scheuber K, Alsheimer M, Schmelz M, Schüttler J. Differential modulation of remifentanil-induced analgesia and postinfusion hyperalgesia by S-ketamine and clonidine in humans. Anesthesiology. 2003;99(1):152–159. | ||
Engelhardt T, Zaarour C, Naser B, et al. Intraoperative low-dose ketamine does not prevent a remifentanil-induced increase in morphine requirement after pediatric scoliosis surgery. Anesth Analg. 2008;107(4):1170–1175. | ||
Leal PC, Salomão R, Brunialti MK, Sakata RK. Evaluation of the effect of ketamine on remifentanil-induced hyperalgesia: a double-blind, randomized study. J Clin Anesth. 2015;27(4):331–337. | ||
Liu Y, Zheng Y, Gu X, Ma Z. The efficacy of NMDA receptor antagonists for preventing remifentanil-induced increase in postoperative pain and analgesic requirement: a meta-analysis. Minerva Anestesiol. 2012;78(6):653–667. | ||
Wu L, Huang X, Sun L. The efficacy of N-methyl-D-aspartate receptor antagonists on improving the postoperative pain intensity and satisfaction after remifentanil-based anesthesia in adults: a meta-analysis. J Clin Anesth. 2015;27(4):311–324. | ||
Zhang RX, Li A, Liu B, et al. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain. 2008;135(3):232–239. | ||
Beilin B, Rusabrov Y, Shapira Y, et al. Low-dose ketamine affects immune responses in humans during the early postoperative period. Br J Anaesth. 2007;99(4):522–527. | ||
Dale O, Somogyi AA, Li Y, Sullivan T, Shavit Y. Does intraoperative ketamine attenuate inflammatory reactivity following surgery? A systematic review and meta-analysis. Anesth Analg. 2012;115(4):934–943. | ||
Senapathi TG, Widnyana IM, Wiryana M, et al. Effectiveness of low-dose intravenous ketamine to attenuate stress response in patients undergoing emergency cesarean section with spinal anesthesia. J Pain Res. 2016;9:689–692. | ||
Gebhard F, Pfetsch H, Steinbach G, Strecker W, Kinzl L, Brückner UB. Is interleukin 6 an early marker of injury severity following major trauma in humans? Arch Surg. 2000;135(3):291–295. | ||
Hong JY, Lim KT. Effect of preemptive epidural analgesia on cytokine response and postoperative pain in laparoscopic radical hysterectomy for cervical cancer. Reg Anesth Pain Med. 2008;33(1):44–51. | ||
Johnston IN, Milligan ED, Wieseler-Frank J, et al. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24(33):7353–7365. | ||
Liang D, Shi X, Qiao Y, Angst MS, Yeomans DC, Clark JD. Chronic morphine administration enhances nociceptive sensitivity and local cytokine production after incision. Mol Pain. 2008;4:7. | ||
Watkins LR, Maier SF, Goehler LE. Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain. 1995;63(3):289–302. | ||
Kutlucan L, Kutlucan A, Basaran B, et al. The predictive effect of initial complete blood count of intensive care unit patients on mortality, length of hospitalization, and nosocomial infections. Eur Rev Med Pharmacol Sci. 2016;20(8):1467–1473. | ||
Qin B, Ma N, Tang Q, et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol. 2016;26(3):372–376. | ||
Sun J, Lin H, He G, Lin W, Yang J. Magnesium sulphate attenuate remifentanil-induced postoperative hyperalgesia via regulating tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord. BMC Anesthesiol. 2017;17:30. | ||
Feng J, Kendig JJ. N-methyl-D-aspartate receptors are implicated in hyperresponsiveness following naloxone reversal of alfentanil in isolated rat spinal cord. Neurosci Lett. 1995;189(2):128–130. | ||
Tiseo PJ, Inturrisi CE. Attenuation and reversal of morphine tolerance by the competitive N-methyl-D-aspartate receptor antagonist, LY274614. J Pharmacol Exp Ther. 1993;264(3):1090–1096. | ||
Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62(3):259–274. | ||
Song JW, Lee YW, Yoon KB, Park SJ, Shim YH. Magnesium sulfate prevents remifentanil-induced postoperative hyperalgesia in patients undergoing thyroidectomy. Anesth Analg. 2011;113(2):390–397. | ||
Lee C, Song YK, Jeong HM, Park SN. The effects of magnesium sulfate infiltration on perioperative opioid consumption and opioid-induced hyperalgesia in patients undergoing robot-assisted laparoscopic prostatectomy with remifentanil-based anesthesia. Korean J Anesthesiol. 2011;61(3):244–250. | ||
Richebé P, Capdevila X, Rivat C. Persistent postsurgical pain: pathophysiology and preventative pharmacologic considerations. Anesthesiology. 2018;129(3):590–607. | ||
Eldufani J, Nekoui A, Blaise G. Non-anesthetic effects of ketamine: a review article. Am J Med. 2018;131(12):1418–1424. | ||
Liu FL, Chen TL, Chen RM. Mechanisms of ketamine-induced immunosuppression. Acta Anaesthesiol Taiwan. 2012;50(4):172–177. | ||
Kartalov A, Trajkov D, Spiroski M, et al. The effect of a small dose of ketamine on postoperative analgesia and cytokine changes after laparoscopic cholecystectomy. Prilozi. 2012;33(1):217–229. | ||
Braun S, Gaza N, Werdehausen R, et al. Ketamine induces apoptosis via the mitochondrial pathway in human lymphocytes and neuronal cells. Br J Anaesth. 2010;105(3):347–354. | ||
Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg. 2003;97(5):1331–1339. | ||
Forget P, Collet V, Lavand’homme P, De Kock M. Does analgesia and condition influence immunity after surgery? Effects of fentanyl, ketamine and clonidine on natural killer activity at different ages. Eur J Anaesthesiol. 2010;27(3):233–240. | ||
Daoudia M, Decruynaere C, Le Polain de Waroux B, Thonnard JL, Plaghki L, Forget P. Biological inflammatory markers mediate the effect of preoperative pain-related behaviours on postoperative analgesics requirements. BMC Anesthesiol. 2015;15:183. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.