Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 9
Effects of long-term exercise training on adipose tissue expression of fractalkine and MCP-1 in patients with type 2 diabetes and stable coronary artery disease: a substudy of a randomized controlled trial
Authors Njerve I, Byrkjeland R, Arnesen H, Åkra S, Solheim S, Seljeflot I
Received 13 September 2015
Accepted for publication 23 November 2015
Published 14 March 2016 Volume 2016:9 Pages 55—62
DOI https://doi.org/10.2147/DMSO.S96299
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Ida Unhammer Njerve,1–3 Rune Byrkjeland,1–3 Harald Arnesen,1–3 Sissel Åkra,1,2 Svein Solheim,1,2 Ingebjørg Seljeflot1–3
1Department of Cardiology, Center for Clinical Heart Research, Oslo University Hospital, Ullevål, 2Center for Heart Failure Research, Oslo University Hospital, 3Faculty of Medicine, University of Oslo, Oslo, Norway
Purpose: Adipose tissue inflammation plays a role in atherosclerosis and type 2 diabetes (T2DM). We aimed to investigate whether 12 months of exercise training in patients with both T2DM and coronary artery disease (CAD) reduced the genetic expression of the proinflammatory markers fractalkine (CX3CL1) and its receptor (CX3CR1) and monocyte chemoattractant protein-1 (MCP-1) in the subcutaneous adipose tissue. Expression of the genes in the circulating leukocytes and circulating levels of the markers were also investigated.
Patients and methods: A total of 137 patients with T2DM and CAD were included to study the effects of exercise on atherosclerosis progression and glucose control. Patients were randomized to exercise training (combined aerobic and strength training) or control. At inclusion and after 12 months, fasting blood samples and a subcutaneous adipose tissue sample were taken. RNA was extracted from the adipose tissue and circulating leukocytes, and the expression levels were examined by reverse transcription-polymerase chain reaction. Circulating fractalkine and MCP-1 were determined by enzyme-linked immunosorbent assay.
Results: The analyses were performed in 114 patients who completed the study and adhered to the intervention principle. At baseline, gene expression of fractalkine and CX3CR1 in the adipose tissue was similar in the two groups. There were no change within either group and no between-group differences in changes from baseline. Circulating fractalkine increased after 12 months in the exercise group (P=0.044), significantly more compared to controls (P=0.042), however only in the patients with advanced vascular disease. Neither the expression levels of MCP-1 nor the circulating levels changed significantly in either group. At baseline, CX3CR1 expression in the adipose tissue was associated with body mass index (P<0.001).
Conclusion: No significant effects of long-term exercise training on adipose tissue expression of fractalkine, CX3CR1, or MCP-1 were found in our patients with combined CAD and T2DM. However, a slight increase in circulating fractalkine after the intervention was recorded. The association of CX3CR1 expression with body mass index might indicate increased immune activation in the adipose tissue.
Keywords: diabetes, coronary artery disease, subcutaneous adipose tissue, exercise, MCP-1, fractalkine (CX3CL1)
Introduction
The adipose tissue secretes several inflammatory cytokines to the circulation, and the inflammatory activity increases with the increasing adipose tissue mass.1 The adipose tissue contains adipocytes and a stromal vascular fraction (SVF), consisting of endothelial cells, fibroblasts, leukocytes, macrophages, nerve terminals, and other cells, capable of producing inflammatory proteins, which can be secreted to the systemic circulation. The adipose tissue in association with inflammation has been mostly studied in the visceral adipose tissue; however, studies on the subcutaneous adipose tissue have also shown inflammatory activity.2
Inflammation is important in insulin resistance and also a possible causal link between atherosclerosis and type 2 diabetes (T2DM), obesity, and metabolic syndrome.3 In a model of cardiometabolic inflammation in healthy humans, a state of insulin resistance was rendered by the infusion of low-dose lipopolysaccharide intravenously. In gluteal subcutanous adipose tissue from these subjects, mRNA expression of the chemokines monocyte chemoattractant protein-1 (MCP-1) and fractalkine (CX3CL1) was significantly increased the first hours after lipopolysaccharide infusion,4 indicating that these chemokines in the adipose tissue play a role in insulin resistance. A recent study investigating the effects of exercise on the subcutaneous adipose tissue in mice showed that exercise induced metabolically beneficial changes in the subcutaneous adipose tissue resulting in reduced insulin resistance.5
Fractalkine6 and MCP-17–9 are produced and secreted both by adipocytes and by the SVF of the adipose tissue. In one in vitro study of human adipose tissue, it was shown that MCP-1 was released at higher levels by the nonadipocyte fraction compared to the adipocytes.10
The elevated circulating levels of fractalkine have been reported in patients with T2DM compared to healthy controls6 and also in patients with coronary artery disease (CAD).11 However, we did not find higher levels in patients with both stable CAD and T2DM compared to patients with stable CAD alone.12 As the circulating levels have been found elevated in both diseases, but not additive when the diseases are coexisting, it may indicate similar regulatory pathways, by sharing risk factors, eg, hypertension, obesity, insulin resistance, low physical activity level, and other lifestyle factors.
In a recent systematic review, it was concluded that exercise training in patients with cardiovascular risk can reduce the circulating levels of cytokines such as tumor necrosis factor-alpha, but with less evidence of reduction in the circulating MCP-1 levels.13 While there are some previous studies investigating MCP-1, with either beneficial or neutral effect of physical exercise on circulating levels, as well as gene expression levels in the adipose tissue,14–17 to the best of our knowledge, the effect of regular exercise on the fractalkine levels in the subcutaneous adipose tissue has not been reported on.
In this substudy of a randomized clinical trial investigating the effects of 12 months regular physical exercise on measures of atherosclerosis and glucose control, we have examined the expression of fractalkine (CX3CL1) and its receptor (CX3CR1) and MCP-1 in the subcutaneous adipose tissue of patients with both T2DM and stable CAD.
We investigated whether 1 year of regular exercise training had an impact on the expression levels as well as on the circulating levels and gene expression levels in circulating leukocytes of these biomarkers.
As both markers have been found associated with insulin resistance, our hypothesis was that regular exercise training would reduce the levels, thus a beneficial impact on inflammation in these patients. We further wanted to explore any associations between the inflammatory markers and measures of glucometabolic state in this population with the combined disease state.
Materials and methods
Study population
Patients with known T2DM and angiographically verified CAD (n=137) were included in a randomized, controlled clinical trial investigating the effects of exercise training on the progression of atherosclerosis and glucose control at the Department of Cardiology, Oslo University Hospital, Ullevål, Oslo, Norway, between August 2010 and March 2012 .
Exclusion criteria included severe diabetic complications, cancer, stroke, or acute coronary syndrome within the last 3 months prior to inclusion, unstable angina, uncompensated heart failure, serious arrhythmia, serious valvular disease, serious rheumatological diseases, chronic obstructive pulmonary disease stadium Global initiative for chronic Obstructive Lung Disease (GOLD) IV, thromboembolic disease, ongoing infections, severe musculoskeletal disorders, and other illnesses seriously limiting the ability for participating in physical activity.
Patients with previous acute myocardial infarction and/or microvascular complications of diabetes were defined as a subgroup with advanced vascular disease (n=68).18
All participants gave written informed consent to participate in the study. The study was approved by the Regional Ethics Committee and was conducted in accordance with the Declaration of Helsinki and is registered at clinicaltrials.gov (NCT01232608). At the time of inclusion, the patients were randomized 1:1 to the exercise group or to the control group with normal follow-up by their general practitioner, not discouraged from performing physical exercise.
Exercise training intervention
The study participants who were randomized to the exercise group underwent a 12-month combined aerobic and resistance exercise program, planned and conducted in collaboration with the Norwegian School of Sport Sciences, Oslo, Norway. Details on the exercise program have been described previously.18 Briefly, the exercise program consisted of two group-based exercise sessions of 60 minutes duration under the supervision of qualified instructors and a third weekly home-based individual exercise session. The total exercise volume was 150 minutes per week, approximately two-thirds was aerobic and one-third resistance exercise. The attendance to the supervised sessions and individual exercise diaries were registered. A total adherence to the intervention was determined for each participant by a percentage of adherence (0%–100%).18
Laboratory methods
Blood samples, including PAXgene blood RNA tubes (PreAnalytix Qiagen GmBH, Hombrechtikon, Germany), were drawn by standard venipuncture between 8 am and 10 am after an overnight fast and before the intake of morning medication at inclusion and after 12 months intervention. Fasting glucose, glycosylated hemoglobin (HbA1c), C-peptide, and insulin were determined by conventional laboratory methods. Insulin resistance was assessed by the updated homeostatic model assessment 2 of insulin resistance.19,20 Serum was prepared by centrifugation within 1 hour at 2,500× g for 10 minutes for the determination of fractalkine. Citrated plasma was obtained by centrifugation within 1 hour at 4°C at 3,000× g for 20 minutes for the determination of MCP-1. The samples were stored at −80°C until analyses. The subcutaneous adipose tissue samples were taken from the gluteal region the same morning and immediately frozen and stored at −80°C until RNA extraction.
Fractalkine and MCP-1 were determined by commercially available enzyme-linked immunosorbent assay kits from R&D Systems, Inc. (Minneapolis, MN, USA). Interassay coefficients of variation were 7.8% and 9.0%, respectively.
Total RNA from the PAX gene tubes was extracted by the use of a PAXgene® Blood RNA Kit (PreAnalytix Qiagen GmBH), with an extra cleaning step (RNeasy® MinElute® Cleanup Kit; Qiagen NV, Venlo, the Netherlands). Total RNA from the adipose tissue was isolated, including disruption and homogenization in Tissue lyser (Qiagen NV), by the use of the High Pure RNA Tissue Kit (Hoffman-La Roche Ltd., Basel, Switzerland), according to a combination of the kit protocol and previous experience in our laboratory. RNA concentration (ng/μL) and quality were measured by the NanoDrop™1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
cDNA was synthesized using the qScript™ cDNA SuperMix (Quanta BioSciences, Gaithersburg, MD, USA), a predefined RNA concentration of 5 ng/μL, and the polymerase chain reaction (PCR) device Mastercycler Personal (Eppendorf AG, Hamburg, Germany). Real-time PCR was performed on ViiA™ 7 (Thermo Fisher Scientific) with TaqMan® CX3CL1, CX3CR1, and MCP-1 Gene Expression Assays (Hs00171086_m1, Hs01922583_s1 and Hs 00234140_m1, respectively), normalized to β2-microglobulin (Hs99999907_m1) and the TaqMan® Universal PCR Master Mix (P/N 4324018). The RNA levels were determined by relative quantification using the ΔΔCT method.21
Statistical analysis
Statistical calculations were performed using SPSS version 22.0. P-values <0.05 were defined as statistically significant. Demographic data are given as proportions, mean (± standard deviation), or median (25th and 75th percentiles) for skewed data. Differences between groups were analyzed by Student’s t-test, Mann–Whitney U test, or chi-square test as appropriate. Within group changes were calculated using Wilcoxon signed rank test. The differences in change between the groups were performed by Mann–Whitney U test. Baseline associations were studied by the use of Spearman’s rho correlations.
Results
Baseline characteristics of the population are shown in Table 1 and have previously been published.22 Patients were using medication for T2DM and CAD according to the current guidelines. Of the 137 who were included, 123 completed the study. The participants with the lowest adherence to the intervention principle (n=9) were excluded18; thus, 114 patients were analyzed for the intervention effect. The nine patients who were excluded from the analyses had <40% adherence to the intervention. The amount of exercise needed for beneficial effects in patients with these combined chronic diseases is not known. Therefore, we chose to accept patients with >40% adherence, reflecting >60 minutes exercise per week for 12 months.18
The baseline characteristics of the 114 patients were similar to the total population, and there were no significant differences between the randomized groups in the variables at baseline.
mRNA extraction from the subcutaneous adipose tissue was successful in 113 samples at baseline and 73 at follow-up, due to unsuccessful sampling or patients declining the procedure.
As previously reported, there were no significant differences between the groups in changes in weight, waist circumference, or diabetes medication during the study period.18
Subcutaneous adipose tissue
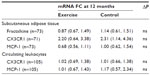
In the adipose tissue, the gene expression levels of CX3CR1, fractalkine, and MCP-1 did not differ between the groups neither at baseline nor in changes from baseline to 12 months. When analyzing according to the subgroups with and without advanced vascular disease, there were also no differences in changes. Table 2 shows the changes in the biomarkers as related to baseline levels. In the exercise group, the expression levels of both fractalkine and MCP-1 were reduced after 12 months, however not statistically significant. CX3CR1 expression was more highly expressed by approximately twofold in both groups after 12 months, but is not statistically significant.
Circulating levels
The circulating levels of MCP-1 and fractalkine did not differ between the groups at baseline (Table 3). In the exercise group, the levels of fractalkine were increased significantly after 12 months compared to baseline (P=0.044), different from the changes in the control group (P=0.042). When analyzing the advanced vascular disease groups separately, there was no difference in change in those without advanced disease. The increase in the exercise group was present only in patients with advanced disease (n=28) (P=0.048), although not statistically different from controls (P=0.078). There was no significant change in the MCP-1 level within or between the groups during the intervention.
Circulating leukocytes
There were no significant between-group differences in the expression levels of MCP-1 and CX3CR1 in circulating leukocytes at baseline, although CX3CR1 in the exercise group was 1.28-fold higher expressed compared to the control group (P=0.079). The level of CX3CR1 was reduced in the exercise group (P=0.043); however, the change was not significantly different from controls when the baseline levels were taken into account. Neither significant changes from baseline to 12 months were found for MCP-1 within any of the groups nor was there any between-group difference in changes. There were also no differences in changes regarding the advanced vascular disease groups.
We did not analyze fractalkine expression, as it has previously been shown not to be expressed in circulating leukocytes.23
Correlations
At baseline, serum fractalkine levels correlated inversely to HbA1c (P=0.013) (Table 4); otherwise, no significant correlations were observed between the glucometabolic variables and fractalkine or MCP-1 in the circulation. In the adipose tissue, the expression level of CX3CR1, but not fractalkine or MCP-1, was significantly correlated with body mass index (BMI) (P<0.001), whereas there was an inverse correlation between leukocyte expression of MCP-1 and BMI (P=0.020) (Table 4). No significant correlations were observed between the circulating levels of the markers and the expression of corresponding genes in leukocytes or the adipose tissue (data not shown).
Discussion
The gene expression levels of fractalkine, CX3CR1, or MCP-1 in the subcutaneous adipose tissue in patients with T2DM and stable CAD did not change significantly after the exercise intervention. However, we could show a slight increase in the circulating levels of fractalkine after 12 months exercise compared to controls, however, only in the patients with advanced vascular disease. Whether the slight increase in the circulating levels of fractalkine in the exercise group with advanced vascular disease should be judged as a deleterious effect of exercise in these patients is obviously debatable and should be confirmed in other studies.
The gene expression of CX3CR1 in leukocytes was significantly reduced after 12 months intervention, however, not different from controls when the baseline levels were taken into account. Leukocyte MCP-1 expression did not change during the intervention.
The “inflammatory state” of the adipose tissue could be influenced by the size of the adipose depot, the content of immune cells, the diet, the fitness, the comorbidity, and the use of medication. The majority of the research on inflammation in the adipose tissue is performed in obese humans or animals. In the present study, the intervention did not change the expression levels of the measured biomarkers in the subcutaneous adipose tissue. The population was classified as overweight, but not as obese, and there might be a gradual increase in inflammation from lean to obese state. The participants had unchanged body weight after 12 months of intervention18 and a reduction in the markers after an intervention has been shown to be accompanied by weight loss by others.17 The fact that our patients have both CAD and T2DM might also influence the results as both diseases are related to inflammation. Also, the accomplished exercise levels might not have been sufficiently intense to induce a reduction in the investigated markers. The lack of improvement after the intervention might also be related to disease severity, which could be discussed along with the finding of neutral effect on circulating fractalkine levels in the group without advanced vascular disease, whereas a tendency to an increase was obtained in the group with advanced disease.
The use of medication and adherence to the intervention may also have influenced the results. Most patients included in our study were using antiplatelet therapy (94%) and statins (93%), both known to have anti-inflammatory effects.24,25
In a study of healthy individuals, statins were shown to reduce the circulating levels of fractalkine26 and the same was shown for high-dose atorvastatin in patients with CAD by Damås et al.11 We have previously reported that there were no significant changes in diabetes medication use during the study period.18 However, we can only speculate that the use of medications could mask a possible beneficial additional effect of exercise training.
Both fractalkine and MCP-1 are expressed in many of the cell types that comprise the subcutaneous adipose tissue (eg, adipocytes and macrophages) and are released from both adipocytes and the SVF.27 In a previous study of human subcutaneous adipose tissue, it was shown that CX3CR1 mRNA was only present in the SVF and not in adipocytes6; therefore, it could be likely that the CX3CR1 determined in the present work derives from the SVF. With the method used, homogenizing the tissue for total gene expression, it cannot be disregarded that any effect on the stromal fraction may have been masked.
The correlation between CX3CR1 and BMI, which in average fit people can reflect the amount of adipose tissue, might be because the adipose tissue mass itself is larger and therefore contains more macrophages in the SVF.28
Monocytes express relatively more CX3CR1 compared to mature macrophages; thus, our correlation between CX3CR1 and BMI could indicate a “younger” population of macrophages or a higher turnover of macrophages in the tissue.29 It could also be discussed as a self-energizing effect of the low-grade inflammation, trying to recruit more monocytes expressing CX3CR1 into the adipose tissue.
At baseline, there was significant correlation between the gene expression levels of CX3CR1 in the adipose tissue and BMI as discussed, whereas other glucometabolic variables did not associate with the gene expression of the variables. Circulating fractalkine levels were inversely correlated with HbA1c, which is not easily explainable, but it can be discussed along with our previous finding of no increased levels of fractalkine in patients with both CAD and diabetes compared to having either diagnosis alone.12
The lack of correlations between circulating MCP-1 and insulin and homeostatic model assessment 2 of insulin resistance score is in accordance with a previous report,30 although in this study, patients did not have diabetes. Different from our result of no correlation between MCP-1 and BMI, another study in patients without diabetes showed a significant correlation between circulating MCP-1 levels and BMI.10 However, this was in a small study of eight healthy persons with BMI classified as normal to obese, and not taking medications.
We observed no correlation between MCP-1 mRNA in circulating leukocytes and HbA1c. MCP-1 gene expression in the leukocytes showed, however, an inverse relation to BMI. This was somewhat surprising but might represent a compensatory mechanism to counteract negative inflammation.
The expression levels of the biomarkers do not seem to be reflected in the circulating protein levels as there were no significant correlations between them.
The source for the circulating markers we have chosen to study can also be discussed. In addition to the adipose tissue and leukocytes, skeletal muscle has been shown to be a source of the proteins. A secretome analysis of muscle biopsies before and after acute and chronic exercise showed increased gene expression levels of MCP-1 and fractalkine, which were reflected in the circulating levels.31
The choice of subcutaneous adipose tissue sampling from the gluteal as opposed to the abdominal region could be discussed. It has been suggested that the gluteal adipose tissue might be considered beneficial or protective. However, we have previously shown an unbeneficial inflammatory state in the adipose tissue from the gluteal region of patients with cardiovascular disease and the metabolic syndrome.2 It should also be emphasized that investigating the genes expressed in the adipose tissue does not necessarily reflect the tissue protein content as not all mRNA transcripts are translated to proteins.
Limitations to this study include the fact that it is a substudy; thus, the power calculations prior to the study were not performed for the investigations of these markers, but for HbA1c-levels. In addition, the patients were well treated with regard to their diabetes with HbA1c-levels of 7.4 (6.8, 8.3)% (57 [51, 67] mmol/mol). Nevertheless, positive effects of the exercise training were observed in HbA1c of patients without complicated cardiovascular disease states.18 Also, for the purpose of readable adipose tissue samples, the number of samples is relatively small. The challenges of performing an exercise intervention in this patient group have been discussed elsewhere.18
Conclusion
Taken together, no significant effects of long-term exercise training on the adipose tissue expression of CX3CL1, CX3CR1, or MCP-1 were found in our population of patients with combined T2DM and CAD. A slight increase in the circulating fractalkine after the intervention was recorded, however, only in patients with advanced vascular disease. The variables, either circulating or genetically expressed, seem to be limitedly influenced by glucose control. The association between CX3CR1 and BMI might be indicative of increased immune activation in the adipose tissue.
Acknowledgments
The work was supported by Stein Erik Hagen Foundation for Clinical Heart Research, and Ada og Hagbart Waages Humanitære og Veldedige Stiftelse, Oslo, Norway.
Disclosure
The authors report no conflicts of interest in this work.
References
McArdle MA, Finucane OM, Connaughton RM, et al. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endocrinol (Lausanne). 2013;4:52. | |
Weiss TW, Seljeflot I, Hjerkinn EM, et al. Adipose tissue pro-inflammatory gene expression is associated with cardiovascular disease. Int J Clin Pract. 2011;65:939–944. | |
Libby P, Okamoto Y, Rocha VZ, et al. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74:213–220. | |
Mehta NN, Heffron SP, Patel PN, et al. A human model of inflammatory cardio-metabolic dysfunction; a double blind placebo-controlled crossover trial. J Transl Med. 2012;10:124. | |
Stanford KI, Middelbeek RJ, Townsend KL, et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes. 2015;64:2002–2014. | |
Shah R, Hinkle CC, Ferguson JF, et al. Fractalkine is a novel human adipochemokine associated with type 2 diabetes. Diabetes. 2011;60:1512–1518. | |
Gerhardt CC, Romero IA, Cancello R, et al. Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Mol Cell Endocrinol. 2001;175:81–92. | |
Fain JN, Madan AK. Regulation of monocyte chemoattractant protein 1 (MCP-1) release by explants of human visceral adipose tissue. Int J Obes (Lond). 2005;29:1299–1307. | |
Dahlman I, Kaaman M, Olsson T, et al. A unique role of monocyte chemoattractant protein 1 among chemokines in adipose tissue of obese subjects. J Clin Endocrinol Metab. 2005;90:5834–5840. | |
Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes (Lond). 2005;29:146–150. | |
Damås JK, Boullier A, Waehre T, et al. Expression of fractalkine (CX3CL1) and its receptor, CX3CR1, is elevated in coronary artery disease and is reduced during statin therapy. Arterioscler Thromb Vasc Biol. 2005;25:2567–2572. | |
Njerve IU, Pettersen AA, Opstad TB, et al. Fractalkine and its receptor (CX3CR1) in patients with stable coronary artery disease and diabetes mellitus. Metab Syndr Relat Disord. 2012;10:400–406. | |
Palmefors H, DuttaRoy S, Rundqvist B, et al. The effect of physical activity or exercise on key biomarkers in atherosclerosis–a systematic review. Atherosclerosis. 2014;235:150–161. | |
Leggate M, Carter WG, Evans MJ, et al. Determination of inflammatory and prominent proteomic changes in plasma and adipose tissue after high-intensity intermittent training in overweight and obese males. J Appl Physiol. 1985;2012(112):1353–1360. | |
Sjogren P, Sierra-Johnson J, Kallings LV, et al. Functional changes in adipose tissue in a randomised controlled trial of physical activity. Lipids Health Dis. 2012;11:80. | |
Bruun JM, Helge JW, Richelsen B, et al. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006;290:E961–E967. | |
Christiansen T, Paulsen SK, Bruun JM, et al. Exercise training versus diet-induced weight-loss on metabolic risk factors and inflammatory markers in obese subjects: a 12-week randomized intervention study. Am J Physiol Endocrinol Metab. 2010;298:E824–E831. | |
Byrkjeland R, Njerve IU, Anderssen S, et al. Effects of exercise training on HbA1c and VO2peak in patients with type 2 diabetes and coronary artery disease: a randomised clinical trial. Diab Vasc Dis Res. 2015;12:325–333. | |
Diabetes Trial Unit, The Oxford Centre for Diabetes, Endocrinology and Metabolism. HOMA2 Score Calculator. 2013. Available from: http://www.dtu.ox.ac.uk/homacalculator/index.php. Accessed May 1, 2013. | |
Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. | |
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) Method. Methods. 2001;25:402–408. | |
Byrkjeland R, Edvardsen E, Njerve IU, et al. Insulin levels and HOMA index are associated with exercise capacity in patients with type 2 diabetes and coronary artery disease. Diabetol Metab Syndr. 2014;6:36. | |
Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. | |
Müller KA, Chatterjee M, Rath D, et al. Platelets, inflammation and anti-inflammatory effects of antiplatelet drugs in ACS and CAD. Thromb Haemost. 2015;114(3):498–518. | |
Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–1719. | |
Cimato TR, Palka BA. Fractalkine (CX3CL1), GM-CSF and VEGF-a levels are reduced by statins in adult patients. Clin Transl Med. 2014;3:14. | |
Fain JN. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review. Mediators Inflamm. 2010;2010:513948. | |
Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. | |
Analia Panek C, Victoria Ramos M, Pilar Mejias M, et al. Differential expression of the fractalkine chemokine receptor (CXCR1) in human monocytes during differentiation. Cell Mol Immunol. 2015;12(6):669–680. | |
Huber J, Kiefer FW, Zeyda M, et al. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab. 2008;93:3215–3221. | |
Catoire M, Mensink M, Kalkhoven E, et al. Identification of human exercise-induced myokines using secretome analysis. Physiol Genomics. 2014;46:256–267. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.