Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 11 » Issue 1
Effects of long-term bronchodilators in bronchiectasis patients with airflow limitation based on bronchodilator response at baseline
Authors Jeong HJ, Lee H , Carriere KC, Kim JH, Han JH, Shin B , Jeong BH, Koh WJ, Kwon OJ, Park HY
Received 22 June 2016
Accepted for publication 14 September 2016
Published 7 November 2016 Volume 2016:11(1) Pages 2757—2764
DOI https://doi.org/10.2147/COPD.S115581
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Ho Jung Jeong,1,* Hyun Lee,1,* Keumhee C Carriere,2,3 Jung Hoon Kim,1 Jin-Hyung Han,1 Beomsu Shin,1 Byeong-Ho Jeong,1 Won-Jung Koh,1 O Jung Kwon,1 Hye Yun Park1
1Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University of Medicine, Seoul, South Korea; 2Department of Mathematical and Statistical Sciences, University of Alberta, Edmonton, AB, Canada; 3Biostatistics and Clinical Epidemiology Center, Samsung Medical Center, Seoul, South Korea
*These authors contributed equally to this work
Purpose: The association between positive bronchodilator response (BDR) at baseline and the effect of long-term bronchodilator therapy has not been well elucidated in patients with bronchiectasis. The aims of our study were to explore the association between positive BDR at baseline and lung-function improvement following long-term (3–12 months) bronchodilator therapy in bronchiectasis patients with airflow limitation.
Materials and methods: The medical records of 166 patients with clinically stable bronchiectasis who underwent baseline pre- and postbronchodilator spirometry and repeated spirometry after 3–12 months of bronchodilator therapy were retrospectively reviewed. For analysis, patients were divided into two groups, responders and poor responders, based on achievement of at least 12% and 200 mL in forced expiratory volume in 1 second (FEV1) following bronchodilator therapy from baseline FEV1.
Results: A total of 57 patients (34.3%) were responders. These patients were more likely to have positive BDR at baseline than poor responders (38.6% [22 of 57] vs 18.3% [20 of 109], P=0.004). This association persisted after adjustment for other confounding factors (adjusted odds ratio 2.298, P=0.034). However, we found FEV1 improved significantly following long-term bronchodilator therapy, even in patients without positive BDR at baseline (change in FEV1 130 mL, interquartile range -10 to 250 mL; P<0.001).
Conclusion: Positive BDR at baseline was independently associated with responsiveness to long-term bronchodilator therapy in bronchiectasis patients with airflow limitation. However, FEV1 improvement was also evident in bronchiectasis patients without positive BDR at baseline, suggesting that these patients can benefit from long-term bronchodilator therapy.
Keywords: bronchodilator effect, bronchodilator agents, bronchiectasis, airway obstruction
Introduction
Bronchiectasis is a suppurative airway disease that has been defined as an abnormal and permanent bronchial dilatation.1 Although the exact pathogenesis of bronchiectasis is still not clear, it is believed to be due to the development of a vicious cycle of chronic inflammation and altered response to infection by compromised mucociliary clearance, leading to progressive airway destruction and distortion.2 Furthermore, airway-wall thickening, chronic colonization of Pseudomonas aeruginosa in the bronchial epithelium, excessive airway collapse during expiration, and bronchial hyperreactivity may contribute to deterioration of lung function in patients with bronchiectasis.3–6
One mainstay of bronchiectasis management is pharmacologic treatment.7,8 While there is accumulating evidence supporting the use of anti-inflammatory therapy (eg, inhaled corticosteroids9,10 and macrolides11–13) and antibiotics, data supporting bronchodilator use in patients with bronchiectasis are scarce and recommendations based mainly on expert opinion. The British Thoracic Society guideline for non-cystic fibrosis bronchiectasis suggests that bronchodilator use may be appropriate for patients with bronchiectasis who have reversible airflow limitation.7 However, data supporting this recommendation are from small-scale studies showing a significant reversibility of airflow limitation in response to short-acting bronchodilator use in patients with bronchiectasis.5,14 Moreover, short-term bronchodilator response is a poor predictor of long-term treatment benefit for lung function in chronic obstructive pulmonary disease (COPD).15 On the other hand, the long-term effect of bronchodilator therapy on lung function has not been adequately investigated in patients with bronchiectasis.16–18 Therefore, the aims of our study were to explore the relationship between reversible airflow limitation, ie, positive bronchodilator response (BDR) at baseline, and lung-function improvement after long-term (3–12 months) bronchodilator therapy in bronchiectasis patients with airflow limitation.
Materials and methods
Patients
The medical records of 206 patients aged 18 years or older at a university-based tertiary hospital were evaluated. All patients had bronchiectasis on chest computed tomography (CT) scan, and had performed pre- and postbronchodilator spirometry when they were clinically stable and repeated spirometry after 3–12 months of bronchodilator therapy between January 1995 and February 2015. Among these patients, we excluded those who had more than a 6-month interval between baseline spirometry and initiation of bronchodilator therapy (n=34) and those without airflow limitation at baseline (n=6). A final total of 166 patients with concomitant bronchiectasis and airflow limitation were included (Figure 1). The institutional review board of Samsung Medical Center approved this study and waived the requirement for informed consent due to the retrospective nature of the study.
  | Figure 1 Study flowchart. |
Pulmonary function test
Spirometry was performed as recommended by the American Thoracic Society/European Respiratory Society using a Vmax 22 (SensorMedics, Yorba Linda, CA, USA).19 The highest measured forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) among three or more tests with acceptable curves were used. Absolute values of FVC and FEV1 were obtained, and the percentage of predicted values for FEV1 and FVC were calculated from equations obtained in a representative South Korean sample.20 Airflow limitation was defined as prebronchodilator FEV1/FVC <70%. Positive BDR at baseline was defined as postbronchodilator increase in FEV1 and/or FVC of at least 12% and 200 mL from baseline values at 15 minutes after inhalation of 400 μg of salbutamol.21
Definitions
Bronchiectasis was diagnosed when the following two key imaging findings were present on CT scan: 1) the internal diameter of the bronchus was larger than the accompanying vessels and 2) no bronchial tapering was present in the periphery of the lungs.22,23 All CT scans were reviewed by two pulmonologists (HJJ and JHK) to confirm the diagnosis of bronchiectasis. In this study, long-term bronchodilator use was defined as administration of bronchodilator therapy for longer than 3 months. Responders were defined as those whose FEV1 values improved at least 12% and 200 mL from baseline FEV1 following 3–12 months of bronchodilator therapy. Patients who received long-term bronchodilator but did not meet these benchmarks were defined as poor responders.
Bronchodilators included inhaled long-acting β2-agonists (LABAs) and inhaled long-acting muscarinic antagonist (LAMAs). Inhaled corticosteroids (ICS) exert an anti-inflammatory response rather than bronchodilation, but have beneficial effects on lung-function improvement;24 therefore, this category was counted as one of the inhalers.
Statistical analysis
Data are presented as medians and interquartile ranges (IQRs; first to third) for continuous variables and as numbers and percentages for categorical variables. Data were compared using the Mann–Whitney U-test for continuous variables and Pearson’s χ2 test or Fisher’s exact test for categorical variables. To determine whether positive BDR at baseline was an independent factor for being a responder and to appreciate the influence of demographic, clinical, and treatment variables, a series of multiple logistic regression analyses were performed on variables with P<0.2 based on univariate results or clinically important: model 1 contained the demographic variables age (continuous), sex, and body mass index (BMI) (continuous); model 2 additionally included pulmonary-related variables, which are generally considered important in pulmonary diseases (smoking history [non- vs ex- or current smokers] and prebronchodilator FEV1 <50% predicted); and finally model 3 additionally included the treatment variable and number of inhalers, as well as all of the aforementioned variables. The incremental values of each addition of variables in the three models were evaluated via χ2 test. Next, we considered the stepwise selection of variables in a multiple logistic model to obtain a parsimonious prediction model containing only the relevant variables based on clinical and statistical significance. We used the Hosmer–Lemeshow test to verify the goodness of fit for each model. All statistical tests were performed using SPSS version 22.0 (IBM, Armonk, NY, USA).
Results
Clinical characteristics of participants
The baseline characteristics of the 166 patients with bronchiectasis are shown in Table 1. Participants comprised 113 men (68.1%) and 53 women (31.9%) with a median age of 64 years (IQR 56–70 years). The median BMI was 22.8 kg/m2 (IQR 20.7–25.4 kg/m2), and 93 patients (57.4%) were current or ex-smokers. A total of 74 patients (44.6%) had a history of pulmonary tuberculosis, and common coexisting pulmonary diseases included nontuberculous mycobacterial lung disease (n=13, 7.8%) and chronic pulmonary aspergillosis (n=5, 3%). The most common extrapulmonary comorbidity was hypertension (n=51, 30.7%), followed by malignant disease (n=23, 13.9%), diabetes mellitus (n=21, 12.7%), chronic kidney disease (n=7, 4.2%), and cerebrovascular disease (n=7, 4.2%). The median FEV1/FVC, FVC, and FEV1 were 53.7%, 2.8 L (70.5% predicted), and 1.4 L (50% predicted), respectively. Positive BDR at baseline was observed in 42 patients (25.3%).
Among the 166 patients, there were 57 responders (34.3%). Compared to poor responders, responders to treatment were more likely to be male (78.9% [45 of 57] vs 62.4% [68 of 109], P=0.03), current or ex-smokers (69.6% [39 of 56] vs 50.9% [54 of 106], P=0.022) and have lower median FEV1/FVC (50.5% [IQR 43.5%–58%] vs 55% [IQR 47%–62.4%], P=0.011) and positive BDR at baseline (38.6% [22 of 57] vs 18.3% [20 of 109], P=0.004). There were no significant differences with respect to age, BMI, previous history of pulmonary tuberculosis, coexisting pulmonary or extrapulmonary comorbidities, or baseline pulmonary function tests, including FVC (percentage predicted, liters) and FEV1 (percentage predicted, liters) between the responders and poor responders.
Comparison of CT findings and effect of inhaler therapy between responders and poor responders
As shown in Table 2, the median number of involved lobes was three (IQR 2–5). Between responders and poor responders, there were no significant differences in the number of involved lobes or the locations of the involved lobes. A median of two inhalers (IQR 1–2) were used, and there were no significant differences in the number of inhalers used between the responders and poor responders. There were no significant differences in ICS use, LABA ± ICS, or LAMA ± ICS between responders and poor responders. However, LABA/LAMA ± ICS was more frequently used by responders than poor responders (31.6% vs 16.5%, P=0.025).
Association between positive BDR at baseline and being a responder following bronchodilator therapy
The increase in FEV1 was more significant in patients with positive BDR at baseline compared to those without positive BDR at baseline (Figure 2; median 210 mL [IQR 130–430 mL] vs 130 mL [IQR −10 to 250 mL], P=0.001). In addition, patients with positive BDR at baseline were more likely to be responders to long-term bronchodilator therapy compared to those without positive BDR at baseline (Figure 3; 52.4% vs 28.2%, P=0.004). However, the increase in FEV1 following bronchodilator therapy was statistically significant in patients without positive BDR at baseline (130 mL [IQR −10 to 250 mL], P<0.001), as well as in those with positive BDR at baseline (210 mL [IQR 130–430 mL], P<0.001) (Figure 4).
  | Figure 2 Comparison of change in FEV1 between patients with and without positive BDR at baseline. |
  | Figure 3 Relationship between presence of BDR at baseline and response to long-term bronchodilator therapy in bronchiectasis patients. |
  | Figure 4 Comparison of FEV1 at baseline and following long-term (3–12 months) bronchodilator therapy. |
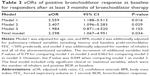
The three sequential models were compared for their incremental importance to predicting responsiveness to bronchodilator therapy. Compared to using only demographic variables (age, sex, and BMI) in model 1, model 2, which included pulmonary-related variables, including FEV1 <50% predicted and smoking history, was marginally better (χ2=0.47, P=0.79), while model 3, including inhalers in addition to all previous variables, was significantly better in predicting the probability of responders (χ2=6.03, P=0.049). As shown in Table 3, positive BDR at baseline remained consistently a significant predictive factor for being a responder to long-term bronchodilator therapy, with the exception of model 3, due to multiple insignificant variables making the model unstable.
We then considered risk-factor modeling to predict responsiveness by removing irrelevant variables, and found that positive BDR at baseline was 2.3 times more (confidence interval 1.067–4.951, P=0.034) significantly and independently associated with being a responder to long-term bronchodilator therapy than being a poor responder, in addition to the number of inhalers (P=0.047). The goodness of fit of the model was confirmed by the Hosmer–Lemeshow test (P>0.2).
Discussion
In the present study of bronchiectasis patients with airflow limitation, we found that approximately one-third of patients were responders to long-term bronchodilator therapy, and positive BDR at baseline was significantly associated with an increase in FEV1 following long-term bronchodilator therapy. However, the increase in FEV1 was evident not only in patients with positive BDR at baseline but also in those without positive BDR at baseline. Therefore, our findings suggest that patients with bronchiectasis who demonstrate poor BDR at baseline can benefit from long-term bronchodilator therapy.
To the best of our knowledge, this is the first study to investigate the relationship between positive BDR at baseline and the long-term effects of bronchodilator therapy in patients with bronchiectasis. In the treatment of patients with bronchiectasis, bronchodilator use is recommended for patients with positive BDR at baseline.7 This recommendation is based on results from two previous studies that demonstrated significant BDR (greater than 15% improvement in FEV1) in a subset of patients with bronchiectasis after use of a short-acting bronchodilator.5,14 However, these studies evaluated BDR in fewer than 30 patients with bronchiectasis (one study included 24 patients and the second included 16 patients), and did not confirm the long-term effect of bronchodilator therapy based on immediate BDR. In a relatively larger number of patients, our study confirmed and extended the previous findings, showing that about 25% of bronchiectasis patients with airflow limitation had positive BDR at baseline, and the presence of positive BDR at baseline was independently associated with improvement in lung function following long-term bronchodilator therapy in patients with bronchiectasis and airflow limitation. These findings are consistent with results from studies on COPD patients. The predictive ability of airflow reversibility in a short-acting bronchodilator test on long-term improvements in lung function following bronchodilator therapy in patients with COPD has been widely studied.15,25,26 A randomized controlled trial showed that COPD patients with immediate BDR after the first dose of tiotropium showed a larger improvement in FEV1 following 1 year of tiotropium use compared to COPD patients without immediate BDR.15 Other studies that evaluated the effects of salmeterol on COPD patients have also shown that patients with positive BDR at baseline had larger improvements in lung function at 12 weeks and 1 year following the use of salmeterol compared with those without positive BDR at baseline.25,26
However, special attention should be given to patients without positive BDR at baseline. We observed that patients without positive BDR at baseline also exhibited significant improvement in FEV1 following long-term bronchodilator therapy, although those with positive BDR at baseline had greater improvement in FEV1 than those without positive BDR at baseline. This pattern has also been recognized in COPD patients. Despite the positive correlation between short-term response to bronchodilator at baseline and improvement in lung function following long-term bronchodilator therapy, several studies of COPD patients have found that long-term bronchodilator therapy significantly improved lung function, dyspnea, and health status, irrespective of the presence or absence of positive BDR at baseline.15,25–29 These findings led to the current guidelines that do not recommend use of reversibility testing with short-acting bronchodilator use to predict long-term response to treatment. Therefore, our results suggest that long-term bronchodilator use needs to be extended to patients without positive BDR at baseline in patients with bronchiectasis and airflow limitation. However, a randomized clinical trial with a placebo group is needed to establish the efficacy of long-term bronchodilator use in patients with bronchiectasis.
The coexistence of bronchial asthma in bronchiectasis patients has been reported,30,31 and a recent study has shown that the existence of bronchial asthma is an independent risk factor for bronchiectasis exacerbations.30 Regarding lung function improvement following long-term bronchodilator therapy with ICS, our study showed that coexisting bronchial asthma was not associated with being a responder in patients with bronchiectasis. Our study included only bronchiectasis patients who had airflow limitation, which could have mitigated the effect of bronchodilator therapy with ICS on coexisting bronchial asthma patients with bronchiectasis. Further studies are necessary to investigate the effect of long-term bronchodilator therapy with ICS on the rate of exacerbations in patients with both bronchial asthma and bronchiectasis.
Limitations
This study has several limitations. First, it was retrospectively performed in a single tertiary hospital. Second, patients who had not undergone spirometry after 3–12 months following bronchodilator therapy were excluded, which may have led to selection bias. Third, the rate of exacerbations, changes in symptoms, and health-related quality of life following long-term use of bronchodilators were not evaluated, which could represent additional long-term benefits to maintenance therapy with bronchodilators. Fourth, the duration of bronchodilator use was different in each patient. However, the duration of 3–12 months was long enough to observe improvements in FEV1, and there was no significant difference in the median duration of bronchodilator use between responders and poor responders. Finally, although we showed that bronchodilators are useful in improving lung function, the optimal inhalers for bronchiectasis patients could not be evaluated from our study. Therefore, further studies are necessary to compare the effectiveness of each type of bronchodilator in patients with bronchiectasis.
Conclusion
In summary, we conclude that positive BDR at baseline was an independent predictive factor for improvement in lung function after long-term bronchodilator therapy in patients with bronchiectasis and airflow limitation. We further suggest that clinicians should consider long-term bronchodilator therapy for patients with poor BDR at baseline, as it can exhibit beneficial response.
Disclosure
The authors report no conflicts of interest in this work.
References
Amalakuhan B, Maselli DJ, Martinez-Garcia MA. Update in Bronchiectasis 2014. Am J Respir Crit Care Med. 2015;192(10):1155–1161. | ||
Cole PJ. Inflammation: a two-edged sword – the model of bronchiectasis. Eur J Respir Dis Suppl. 1986;147:6–15. | ||
Roberts HR, Wells AU, Milne DG, et al. Airflow obstruction in bronchiectasis: correlation between computed tomography features and pulmonary function tests. Thorax. 2000;55(3):198–204. | ||
Ho PL, Chan KN, Ip MS, et al. The effect of Pseudomonas aeruginosa infection on clinical parameters in steady-state bronchiectasis. Chest. 1998;114(6):1594–1598. | ||
Nogrady SG, Evans WV, Davies BH. Reversibility of airways obstruction in bronchiectasis. Thorax. 1978;33(5):635–637. | ||
Pang J, Chan HS, Sung JY. Prevalence of asthma, atopy, and bronchial hyperreactivity in bronchiectasis: a controlled study. Thorax. 1989;44(11):948–951. | ||
Pasteur MC, Bilton D, Hill AT. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65 Suppl 1:i1–i58. | ||
McShane PJ, Naureckas ET, Tino G, Strek ME. Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2013;188(6):647–656. | ||
Martínez-García MA, Perpiñá-Tordera M, Román-Sánchez P, Soler-Cataluña JJ. Inhaled steroids improve quality of life in patients with steady-state bronchiectasis. Respir Med. 2006;100(9):1623–1632. | ||
Tsang KW, Tan KC, Ho PL, et al. Inhaled fluticasone in bronchiectasis: a 12 month study. Thorax. 2005;60(3):239–243. | ||
Wong C, Jayaram L, Karalus N, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):660–667. | ||
Altenburg J, de Graaff CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA. 2013;309(12):1251–1259. | ||
Serisier DJ, Martin ML, McGuckin MA, et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA. 2013;309(12):1260–1267. | ||
Hassan JA, Saadiah S, Roslan H, Zainudin BM. Bronchodilator response to inhaled beta-2 agonist and anticholinergic drugs in patients with bronchiectasis. Respirology. 1999;4(4):423–426. | ||
Tashkin D, Kesten S. Long-term treatment benefits with tiotropium in COPD patients with and without short-term bronchodilator responses. Chest. 2003;123(5):1441–1449. | ||
Sheikh A, Nolan D, Greenstone M. Long-acting β2-agonists for bronchiectasis. Cochrane Database Syst Rev. 2001;(4):CD002155. | ||
Franco F, Sheikh A, Greenstone M. Short acting β2 agonists for bronchiectasis. Cochrane Database Syst Rev. 2003;(3):CD003572. | ||
Guan WJ, Gao YH, Xu G, et al. Bronchodilator response in adults with bronchiectasis: correlation with clinical parameters and prognostic implications. J Thorac Dis. 2016;8(1):14–23. | ||
Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. | ||
Choi JK, Paek DM, Lee JO. Normal predictive values of spirometry in Korean population. Tuberc Respir Dis. 2005;58(3):230–242. | ||
Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. | ||
Dodd JD, Lavelle LP, Fabre A, Brady D. Imaging in cystic fibrosis and non-cystic fibrosis bronchiectasis. Semin Respir Crit Care Med. 2015;36(2):194–206. | ||
Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. | ||
Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. | ||
Rennard SI, Anderson W, ZuWallack R, et al. Use of a long-acting inhaled β2-adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(5):1087–1092. | ||
ZuWallack RL, Mahler DA, Reilly D, et al. Salmeterol plus theophylline combination therapy in the treatment of COPD. Chest. 2001;119(6):1661–1670. | ||
Bleecker ER, Emmett A, Crater G, Knobil K, Kalberg C. Lung function and symptom improvement with fluticasone propionate/salmeterol and ipratropium bromide/albuterol in COPD: response by beta-agonist reversibility. Pulm Pharmacol Ther. 2008;21(4):682–688. | ||
Mahler DA, Donohue JF, Barbee RA, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest. 1999;115(4):957–965. | ||
Tashkin DP, Celli B, Decramer M, et al. Bronchodilator responsiveness in patients with COPD. Eur Respir J. 2008;31(4):742–750. | ||
Mao B, Yang JW, Lu HW, Xu JF. Asthma and bronchiectasis exacerbation. Eur Respir J. 2016;47(6):1680–1686. | ||
Saynajakangas O, Keistinen T, Tuuponen T, Kivela SL. Links between hospital diagnoses of bronchiectasis and asthma. Allergy. 1997;52(11):1120–1122. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.