Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Effects of liraglutide on lipolysis and the AC3/PKA/HSL pathway
Authors Li Z , Yang P , Liang Y, Xia N, Li Y, Su H, Pan H
Received 20 May 2019
Accepted for publication 29 July 2019
Published 3 September 2019 Volume 2019:12 Pages 1697—1703
DOI https://doi.org/10.2147/DMSO.S216455
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Zhengming Li1,*, Pijian Yang2,*, Yuzhen Liang1,*, Ning Xia2,*, Yingrong Li1, Hongye Su1, Hailin Pan1
1Department of Endocrinology and Metabolism, Second Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China; 2Department of Endocrinology and Metabolism, The First Affiliated Hospital of Guangxi Medical University, Guangxi Medical University, Nanning, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuzhen Liang
Department of Endocrinology and Metabolism, Second Affiliated Hospital of Guangxi Medical University, Nanning 530007, People’s Republic of China
Email [email protected]
Ning Xia
Department of Endocrinology and Metabolism, The First Affiliated Hospital of Guangxi Medical University, Guangxi Medical University, Nanning 530021, People’s Republic of China
Email [email protected]
Background: Liraglutide reduces blood glucose, body weight and blood lipid levels. Hormone-sensitive lipase (HSL) is a key enzyme in lipolysis. Evidence from our and other studies have demonstrated that adenylate cyclase 3 (AC3) is associated with obesity and can be upregulated by liraglutide in obese mice. In the present study, we investigated whether hepatic HSL activity is regulated by liraglutide and characterized the effect of liraglutide in the AC3/protein kinase A (PKA)/HSL signalling pathway.
Methods: Obese mice or their lean littermates were treated with liraglutide or saline for 8 weeks. Serum was collected for the measurement of insulin and lipids. We investigated hepatic AC3, HSL and phosphorylated HSL Ser-660 (p-HSL(S660)) protein expression levels andAC3 and HSL mRNA expression levels and cyclic adenosine monophosphate (cAMP), PKA activity in liver tissue.
Results: Liraglutide treatment decreased triglycerides (TGs) and free fatty acids (FFAs), increased glycerol, and upregulated hepatic AC3 and p-HSL(s660) levels and cAMP and PKA activities.
Conclusion: The results suggest that liraglutide can upregulates AC3/PKA/HSL pathway and may promotes lipolysis.
Keywords: liraglutide, obesity, lipolysis, adenylate cyclase 3, hormone-sensitive lipase
Introduction
In recent years, as the proportion of obese people in the world’s population has grown rapidly, it has attracted more and more attention. A key feature of obesity is excessive lipid accumulation in adipose tissue and ectopic localizations (especially liver and muscle).1 Liraglutide treatment can augment weight loss and improve blood lipid levels.2–5 Lipolysis is considered to be the process of hydrolysis of triglycerides (TGs) to free fatty acids (FFAs) and glycerin. Hormone-sensitive lipase (HSL) in adipose tissue has been suggested to be a key regulatory enzym in controlling lipolysis.6 Endogenous glucagon-like peptide 1 (GLP-1) binds to and activates the glucagon-like peptide 1 receptor (GLP-1R). Upon its activation, GLP-1R stimulates adenylate cyclase (AC) to promote cAMP production,7 leading to activation of protein kinase (PKA).8 Activated PKA phosphorylates hormone-sensitive lipase (p-HSL).9–11 AC3 is a member of the ACs family, genome-wide association studies studies suggest that obesity-related genes are located on or near the AC3 gene.12–15 In both Swedish and Han Chinese populations, additional genetic evidence supports that single nucleotide variation of AC3 gene was found to be closely related to obesity.16,17 It is also emphasized in animal models that AC3 signalling plays an important role in maintaining energy homeostasis, AC3 mutation can protect mice from diet-induced obesity. Adult weight of AC3 knockout mice is significantly higher than that of wild-type mice. It is also found that TG of AC3 knockout mice is increased, and the fat mass of epididymal adipocytes of AC3 knockout mice is more than that of control mice.18 Our previous studies have shown that liraglutide can upregulate hepatic GLP-1R and AC3 levels in obese mice, and the levels of AC3 were negatively correlated with body weight.19,20 Thus, we hypothesize that liraglutide treatment can enhance lipolysis and affect the AC3-cAMP-PKA-HSL signalling pathway. To test our hypothesis, we employed an obese mouse model in which C57BL/6J mice were fed a high-fat diet (HFD). TGs, glycerol, FFA in serum and PKA activity were analysed in the liver, and the expression levels of proteins associated with lipolysis were determined in liver. The results of this study will increase our knowledge of the effect of liraglutide on lipolysis, leading to a more comprehensive understanding of the mechanisms underlying the physiological actions of liraglutide on obesity.
Materials and methods
Animal husbandry and analytic procedures
4-week-old C57BL/6J mice (male) were purchased from the Medical Laboratory Animal Centre of Guangzhou Province (Guangzhou, China). The experimental mice were maintained at the Animal Experiment Centre of Guangxi Medical University. The experimental mice were maintained in a specific pathogen-free (SPF) room with a 12-h light/dark cycle. In the first week, all mice were fed with a normal rodent chow diet (5% fat wt/wt). After then, the mice were randomly divided into two groups. ie N group: the mice fed with a normal chow diet (5% fat wt/wt) and O group: the mice fed with HFD (34.9% fat wt/wt). Body weight (BW) and blood glucose of all experimental mice were observed and recorded at the same time every week. Mice with fasting blood glucose (FBG) levels >16.7 mmol/L were considered to be diabetic.21 Mice with BW that exceeded normal weight by at least 20% were considered obese. After 12 weeks of chow diet or HFD feeding, the obese mouse model was successfully established, all mice were divided into the following four groups: N + saline (N + S), N + liraglutide (N + L), O + saline (O + S) and O + liraglutide (O + L). In N + L and O + L groups, liraglutide was injected subcutaneously at a dose of 0.1 mg/kg/12 h. N + S and O + S groups were subcutaneously injected with the same volume of saline as controls. After 8 weeks of treatment, the experimental mice were fasted overnight and anaesthetized with sodium pentobarbital (50 mg/kg, i.p.). The blood was obtained through the angular vein and centrifuged by a 4 °C centrifuge to separate the serum, which was stored at −20 °C. At the same time, the liver was dissected immediately and frozen in liquid nitrogen, then transferred to −80 °C refrigerator for preservation until analysis. All animal experiments and care procedures were conducted under the Guidelines of the Animal Ethics Committee of First Affiliated Hospital of Guangxi Medical University (the National Standard GB/T35892-2018 of the People’s Republic of China). The Animal Ethics Committee of First Affiliated Hospital of Guangxi Medical University also approved this study. Liraglutide was kindly provided by Novo Nordisk (Bagsvaerd, Denmark).
Biochemical analysis of serum and liver
The mouse TGs, glycerol, FFA and insulin ELISA kit (Shanghai JiNing Industrial Co., Ltd., China) was used to measure serum TGs, glycerol, FFA and insulin levels . Liver samples were prepared according to the instructions provided. A PKA Kinase Assay Kit (Enzo Life Sciences Inc. USA) was used to measure PKA activity in liver samples. A glucose meter (Johnson & Johnson, New Brunswick, NJ, USA) was used to measure blood glucose levels. The HOMA-IR was used to assess IR. The HOMA-IR score was calculated as [fasting insulin (mU l−1) × fasting glucose (mmol l−1)]/22.5.22
Real-time reverse transcription-PCR
Total RNAs in liver were extracted, and a Thermo Reverse Transcriptase Kit (Thermo Scientific, Waltham, MA, USA) was used to synthesize cDNA. Quantitative real-time PCR was performed using FastStart Universal SYBR Green Master (ROX) (Roche Diagnostics, Indianapolis, IN, USA) on an Applied Biosystems StepOnePlus Real-Time PCR System (Thermo Fisher Scientific, Foster City, CA, USA). The primers included the following: AC3 forward primer (5′-GGACACGCTCACAAACATC-3′) and reverse primer (5′-GCCACATTGACCGTATTGC-3′). HSL forward primer (5′- TCCTGGAACTAAGTGGACGCAAG-3′) and reverse primer (5′-CAGACACACTCCTGCGCATAGAC-3′), The glyceraldehyde 3-phosphatedehydrogenase (gapdh) was used as the internal control. Gapdh forward primer (5′-TGTGTCCGTCGTGGATCTGA-3′) and reverse primer (5′-TTGCTGTTGAAGTCGCAGGAG-3′). The relative copy number was calculated using the threshold crossing point (Ct) as calculated by the LightCycler software combined with the ∆∆Ct calculations.
Western blotting
Western blot analyses were performed as previously described.23 The primary antibodies included antibodies against the AC3 (1:1000; Sigma-Aldrich Co., LLC., China), cAMP (1:500; Abnova Co., Inc., USA), HSL (1:500; Bioworld Technology Co., Ltd., China) and phosphorylated-HSL Ser660 (p-HSL(S660)) (1:500; Cell Signal Technology Inc., USA). Horseradish peroxidase-conjugated goat anti-rabbit lgG was used as the secondary antibody. The membranes were scanned with the Odyssey infrared imaging system (LICOR Biosciences).
Statistical analysis
Quantitative values are shown as the means ± SD. Comparisons were performed using Student’s t-test, the one-way analysis of variance (ANOVA) or a factorial analysis, where appropriate. In all cases involving the use of Student’s t-test, one-way ANOVA analysis or factorial analysis, a homogeneity of variance test was applied. Values of p<0.05 were considered statistically significant. All analyses were performed using SPSS 17.0 (Chicago, IL, USA).
Results
Effects of liraglutide on the general condition and metabolism of the mice
BW, FBG, HOMA-IR, and the serum levels of TGs, glycerol, FFA were to evaluate the metabolic response of liraglutide in mice. Mice in the N group exhibited significantly reduced BW compared with mice in the O group (Table 1). FBG levels were elevated in the O group relative to those in the N group (Table 1). Factorial analysis showed that BW and HOMA-IR scores were significantly different between normal control mice and obese mice, and there were significant differences between liraglutide-treated mice and saline-treated mice (Table 2). There was no significant difference in FBG between normal control group and obesity group, nor between liraglutide-treated mice and saline-treated mice (Table 2). The serum levels of TGs, glycerol and FFA were significantly higher in obese mice than in normal control mice (Table 2). The liraglutide treatment significantly decreased the serum levels of TGs, FFA and significantly increased serum glycerol levels (Table 2).
 |
Table 1 Body weights and fasting blood glucose levels of mice before liraglutide treatment |
 |
Table 2 Serum metabolic parameters of mice after treatment with liraglutide or saline |
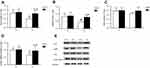
Effects of liraglutide on hepatic AC3, HSL, p-HSL(S660), and cAMP levels in mice
We examined AC3, HSL, p-HSL(S660) protein levels and AC3, HSL mRNA levels and cAMP levels in liver tissue; the results are summarized in Figures 1 and 2. The hepatic levels of AC3 and p-HSL(S660) protein andAC3 mRNA and cAMP were significantly different between normal control mice and obese mice. The hepatic levels of AC3 and p-HSL(S660) protein and AC3 mRNA and cAMP were lower in obese mice than in normal control mice. No significant differences in HSL protein and HSL mRNA levels were observed between normal control mice and obese mice. Liraglutide treatment significantly increased hepatic AC3, p-HSL(S660) and cAMP levels but not HSL protein and mRNA levels in normal contol and obese mice. The increase of hepatic AC3, p-HSL(S660) and cAMP levels in obese mice were more obviously.
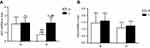
Effects of liraglutide on hepatic PKA activity levels
Liver PKA activity was significantly higher in normal control mice than in obese mice (Figure 3). Liraglutide treatment significantly increased liver PKA activity in normal control and obese mice (Figure 3).The increase in obese mice was more obviously.
Discussion
In this study, obesity in mice was induced by a high-fat diet. We found that treatment with liraglutide decreased the BW and HOMA-IR index in obese mice and normal control mice, though the decreases were stronger in obese mice. Our data showed that the serum levels of TGs, glycerol and FFA were significantly higher in obese mice than in normal control mice. Liraglutide treatment significantly decreased serum levels of TGs and FFA, and significantly increased serum glycerol levels.
It has been repeatedly demonstrated that liraglutide can reduce TG and FFA levels.2–5,24 Lipolysis is the process by which TGs are hydrolysed to FFAs and glycerol. Lipolysis was determined by assaying FFA and glycerol levels.25 Many studies have demonstrated that excessive FFAs play a pivotal role in insulin resistance.26–28 Our data show that liraglutide treatment decreased serum TGs and FFAs and increased serum glycerol. The decrease in TGs and the increase in glycerol indicated that liraglutide treatment promoted lipolysis. The decrease in FFAs is likely due to liraglutide increasing FFA uptake and increasing the oxidation rate of FFAs. Mells, Fu, Sharma, etc.29 found that liraglutide treatment significantly increased the mRNA and protein levels of genes related to fatty acid uptake and peroxisomal β-oxidation.
GLP-1 has been reported to reduce hepatic steatosis in animal models of non-alcoholic fatty liver disease (NAFLD).29–31 The mechanism underlying this effect of GLP-1 is not completely understood. Earlier studies have suggested that exenatide (exendine-4) had direct effects on the liver by enhancing lipid hydrolysis and oxidation.31,32 The mechanism by which liraglutide promotes lipolysis is not completely understood. Our previous study showed that liraglutide treatment upregulated GLP-1R and AC3 and that AC3 gene expression was negatively correlated with BW, HOMA-IR and the area ratio of hepatic fat deposition in the liver.19,20 We hypothesized that liraglutide treatment could enhance lipolysis and upregulate the AC3-cAMP-PKA-HSL pathway. Phosphorylation of HSL at Ser660 is critical for activation of the protein’s intrinsic enzymatic activity.33 Therefore, we compared the protein expression levels of hepaticAC3, HSL and p-HSL(S660), and the mRNA expression levels of AC3 and HSL, and cAMP levels and PKA activity in livers of obese and normal control mice with and without liraglutide treatment. We observed that the protein and mRNA expression of hepatic AC3 and the cAMP levels and PKA activity were significantly difference in obese mice than in normal control mice, and the protein and mRNA expression of hepatic AC3 and the cAMP levels and PKA activity were increased after liraglutide treatment in obese mice and in normal control mice. The increase in obese mice was more obviously. Liraglutide treatment did not affect HSL protein and mRNA expression, and hepatic HSL expression was not different between obese and normal control mice. Although total HSL expression was not affected, p-HSL(S660) was significantly increased by liraglutide treatment. Human genetic and animal models indicate that AC3 may play an important role in energy homeostasis. Our previous study data provide evidence that supports this concept.19,20 In this present study, AC3 was also upregulated by liraglutide treatment, and the increase in AC3 was paralleled by increases in cAMP, PKA activity and p-HSL(S660). Whether AC3 is the key regulator of this pathway remains to be investigated in future studies.
Conclusion
In this study, we found that liraglutide treatment decreased TGs and increased glycerol. Liraglutide treatment upregulated AC3 and p-HSL(S660) levels and cAMP and PKA activities in liver. Thus, we conclude that liraglutide can upregulate the AC3/PKA/HSL pathway and may promote lipolysis.
Acknowledgment
We are grateful for assistance from the Medical Laboratory Animal Centre of Guangxi Medical University. This work was supported by research grants from National Natural science of China (Grant No.81760346). The study was approved by the medical Ethics Committee of First Affiliated Hospital of Guangxi Medical University (the approval number: (No.2017-KY-130).
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lee JH, Moon MH, Jeong JK, et al. Sulforaphane induced adipolysis via hormone sensitive lipase activation, regulated by AMPK signaling pathway. Biochem Bioph Res Co. 2012;426:492–497. doi:10.1016/j.bbrc.2012.08.107
2. Astrup A, Rossner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi:10.1016/S0140-6736(09)61375-1
3. Cummings BP, Stanhope KL, Graham JL, et al. Chronic administration of the glucagon-like peptide-1 analog, liraglutide, delays the onset of diabetes and lowers triglycerides in UCD-T2DM rats. Diabetes. 2010;59:2653–2661. doi:10.2337/Db09-1564
4. Hermansen K, Baekdal TA, During M, et al. Liraglutide suppresses postprandial triglyceride and apolipoprotein B48 elevations after a fat-rich meal in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, cross-over trial. Diabetes Obes Metab. 2013;15:1040–1048. doi:10.1111/dom.12133
5. Kim SH, Abbasi F, Lamendola C, et al. Benefits of liraglutide treatment in overweight and obese older individuals with prediabetes. Diabetes Care. 2013;36:3276–3282. doi:10.2337/dc13-0354
6. Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res. 2002;43:1585–1594. doi:10.1194/jlr.r200009-jlr200
7. Buteau J. GLP-1 receptor signaling: effects on pancreatic beta-cell proliferation and survival. Diabetes Metab. 2008;34 Suppl 2:S73–S77. doi:10.1016/S1262-3636(08)73398-6
8. Quoyer J, Longuet C, Broca C, et al. GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a beta-arrestin 1-mediated ERK1/2 activation in pancreatic beta-cells. J Biol Chem. 2010;285:1989–2002. doi:10.1074/jbc.M109.067207
9. Stralfors P, Bjorgell P, Belfrage P. Hormonal regulation of hormone-sensitive lipase in intact adipocytes: identification of phosphorylated sites and effects on the phosphorylation by lipolytic hormones and insulin. Proc Natl Acad Sci U S A. 1984;81:3317–3321. doi:10.1073/pnas.81.11.3317
10. Garton AJ, Campbell DG, Cohen P, Yeaman SJ. Primary structure of the site on bovine hormone-sensitive lipase phosphorylated by cyclic AMP-dependent protein kinase. FEBS Lett. 1988;229:68–72. doi:10.1016/0014-5793(88)80799-3
11. Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 1998;273:215–221. doi:10.1074/jbc.273.1.215
12. Speliotes EK, Willer CJ, Berndt SI, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948. doi:10.1038/ng.686
13. Wen W, Cho YS, Zheng W, et al. Meta-analysis identifies common variants associated with body mass index in east Asians. Nat Genet. 2012;44:307–311. doi:10.1038/ng.1087
14. Monda KL, Chen GK, Taylor KC, et al. A meta-analysis identifies new loci associated with body mass index in individuals of African ancestry. Nat Genet. 2013;45:690–696. doi:10.1038/ng.2608
15. Cousminer DL, Berry DJ, Timpson NJ, et al. Genome-wide association and longitudinal analyses reveal genetic loci linking pubertal height growth, pubertal timing and childhood adiposity. Hum Mol Genet. 2013;22:2735–2747. doi:10.1093/hmg/ddt104
16. Nordman S, Abulaiti A, Hilding A, et al. Genetic variation of the adenylyl cyclase 3 (AC3) locus and its influence on type 2 diabetes and obesity susceptibility in Swedish men. Int J Obes. 2008;32:407–412. doi:10.1038/sj.ijo.0803742
17. Wang H, Wu M, Zhu W, et al. Evaluation of the association between the AC3 genetic polymorphisms and obesity in a Chinese Han population. PLoS One. 2010;5:e13851. doi:10.1371/journal.pone.0013851
18. Wang Z, Li V, Chan GC, et al. Adult type 3 adenylyl cyclase-deficient mice are obese. PLoS One. 2009;4:e6979. doi:10.1371/journal.pone.0006979
19. Liang Y, Li Z, Liang S, et al. Hepatic adenylate cyclase 3 is upregulated by Liraglutide and subsequently plays a protective role in insulin resistance and obesity. Nutr Diabetes. 2016;6:e191. doi:10.1038/nutd.2015.37
20. Li Z, Liang Y, Xia N, et al. Liraglutide reduces body weight by upregulation of adenylate cyclase 3. Nutr Diabetes. 2017;7:e265. doi:10.1038/nutd.2017.17
21. Zhou XY, Zhang F, Hu XT, et al. Depression can be prevented by astaxanthin through inhibition of hippocampal inflammation in diabetic mice. Brain Res. 2017;1657:262–268. doi:10.1016/j
22. Chu X, Liu L, Na L, et al. Sterol regulatory element-binding protein-1c mediates increase of postprandial stearic acid, a potential target for improving insulin resistance, in hyperlipidemia. Diabetes. 2013;62:561–571. doi:10.2337/db12-0139
23. Wong ST, Trinh K, Hacker B, et al. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000;27:487–497.
24. Jendle J, Nauck MA, Matthews DR, et al. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab. 2009;11:1163–1172. doi:10.1111/j.1463-1326.2009.01158.x
25. Fine JB, DiGirolamo M. A simple method to predict cellular density in adipocyte metabolic incubations. Int J Obes Relat Metab Disord. 1997;21:764–768.
26. Hoeg LD, Sjoberg KA, Jeppesen J, et al. Lipid-induced insulin resistance affects women less than men and is not accompanied by inflammation or impaired proximal insulin signaling. Diabetes. 2011;60:64–73. doi:10.2337/db10-0698
27. Pereira S, Yu WQ, Moore J, Mori Y, Tsiani E, Giacca A. Effect of a p38 MAPK inhibitor on FFA-induced hepatic insulin resistance in vivo. Nutr Diabetes. 2016;6:e210. doi:10.1038/nutd.2016.11
28. Cree-Green M, Gupta A, Coe GV, et al. Insulin resistance in type 2 diabetes youth relates to serum free fatty acids and muscle mitochondrial dysfunction. J Diabetes Complications. 2016. doi:10.1016/j.jdiacomp.2016.10.014
29. Mells JE, Fu PP, Sharma S, et al. Glp-1 analog, liraglutide, ameliorates hepatic steatosis and cardiac hypertrophy in C57BL/6J mice fed a Western diet. Am J Physiol Gastrointest Liver Physiol. 2012;302:G225–G235. doi:10.1152/ajpgi.00274.2011
30. Svegliati-Baroni G, Saccomanno S, Rychlicki C, et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31:1285–1297. doi:10.1111/j.1478-3231.2011.02462.x
31. Lee J, Hong SW, Chae SW, et al. Exendin-4 improves steatohepatitis by increasing Sirt1 expression in high-fat diet-induced obese C57BL/6J mice. PLoS One. 2012;7:e31394. doi:10.1371/journal.pone.0031394
32. Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173–181. doi:10.1002/hep.21006
33. Rahmah N, Anuar AK, A’Shikin AN, et al. A Brugia malayi antigen specifically recognized by infected individuals. Biochem Biophys Res Commun. 1998;250:586–588. doi:10.1006/bbrc.1998.9360
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.