Back to Journals » Drug Design, Development and Therapy » Volume 16
Effects of Lactobacillus plantarum HFY15 on Lupus Nephritis in Mice by Regulation of the TGF-β1 Signaling Pathway
Authors Cheng L , Yao P, Wang H , Yuan Q, Wang X, Feng W, Sun F, Wang Q
Received 25 February 2022
Accepted for publication 20 August 2022
Published 26 August 2022 Volume 2022:16 Pages 2851—2860
DOI https://doi.org/10.2147/DDDT.S363974
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Lin Cheng, Pu Yao, Hongping Wang, Qian Yuan, Xiaowen Wang, Wei Feng, Fengjun Sun, Qian Wang
Department of Pharmacy, The First Affiliated Hospital of Third Military Medical University (Army Medical University), Chongqing, 400038, People’s Republic of China
Correspondence: Qian Wang; Fengjun Sun, Email [email protected]; [email protected]
Objective: In this study, the Lactobacillus plantarum HFY15 (LP-HFY15) strain isolated from naturally fermented yak yogurt was investigated. An animal model of lupus nephritis was established by pristane to verify the interventional effect of LP-HFY15 on mouse lupus nephritis by regulating the transforming growth factor-β 1 (TGF-β 1) signaling pathway.
Materials and Methods: Indexes in mouse serum and tissues were detected by kits, pathological changes in mouse kidney were observed by hematoxylin-eosin (H&E) staining, and quantitative polymerase chain reaction (qPCR) was used to detect TGF-β 1-related expression in mouse kidney tissue, which further elucidated the mechanism of LP-HFY15.
Results: LP-HFY15 decreased the elevation of urinary protein and the levels of interleukin-6 (IL-6), IL-12, tumor necrosis factor alpha (TNF-α), and interferon γ (IFN-γ) in serum and kidney tissue. LP-HFY15 also reduced serum creatinine (SCr), blood urea nitrogen (BUN), total cholesterol (TC), triglyceride (TG), and raised total protein (TP), and albumin (ALB) levels in mice with nephritis. In addition, LP-HFY15 inhibited the positive rate of double-stranded deoxyribonucleic acid (dsDNA) antibodies in mice with nephritis. The observation of H&E sections showed that LP-HFY15 alleviated the glomerulus morphological incompleteness and inflammatory infiltration caused by nephritis. Further results showed that LP-HFY15 downregulated the mRNA expression of TGF-β 1, vascular endothelial growth factor (VEGF), and nuclear factor kappa-B (NF-κB) in the kidneys of lupus nephritis mice, and the expression of inhibitor of NF-κB (IκB-α), copper/zinc superoxide dismutase (Cu/Zn-SOD), and manganese superoxide dismutase (Mn-SOD) was also upregulated.
Conclusion: These results indicated that LP-HFY15 plays a significant role in experimental intervention for lupus nephritis. The effect of LP-HFY15 was positively correlated with its concentration, and the effect was similar to that of prednisone at 109 CFU/kg.
Keywords: lactic acid bacteria, lupus nephritis, TGF-β 1, dsDNA, pristane
Introduction
The Qinghai-Tibet Plateau is the highest plateau in the world. The temperature is low, but the sunshine is strong, and a large area of natural grassland formed that is conducive to the development of animal husbandry. Thus, it has been traditional for Tibetan herdsmen on the Qinghai-Tibet Plateau to raise yaks and eat yak beef and milk.1 The most common effect of probiotics is to regulate the intestinal flora, so as to maintain intestinal health and inhibit constipation.2 Probiotics have shown a variety of bioactive effects and have many benefits to the human body. After the effect of probiotics on aging mice, it was found that probiotics can slow down the aging state of mice, thereby improving the effect of exercise ability of aging mice.3 The regulatory effect of probiotics on the body is also reflected in the protection of other metabolic organs, including the ability to reduce the degree of liver damage and protect liver tissue.4 In addition, probiotic products also have good activity. Bacteriocin produced by probiotics can play an antibacterial role and may be used as a substitute for some antibiotics.5 Probiotics can produce more beneficial substances and play a better role after fermenting food, and can be used as a starter of health food.6 The naturally fermented yak milk from the Qinghai-Tibet Plateau contains diverse microorganisms that have the potential for development and utilization as a source of probiotics.7
Lupus nephritis is a type of systemic lupus erythematosus. It is a complex immunological disorder of nephritis.8 Lupus nephritis can cause weakened immunity, lymph node hyperplasia, and glomerulonephritis. Glomerulosclerosis or diffuse hyperplasia of kidney tissue can seriously impair metabolism and detoxification, and eventually lead to chronic renal failure, which can be life-threatening in severe cases.9 In most patients with lupus nephritis disease, there is excessive activation of B cells and T cells. In T cell differentiation of helper T cells, the presence of probiotic bacteria can stimulate additional T cells to develop, which can decrease inflammation and can be used as a direct intervention for nephritis.10
The structural components of probiotics can also directly stimulate and activate the immune system via antigens, and contribute to discharging toxic substances in the body and reducing inflammation.11 In experimental studies, phytane has been successfully used to construct a lupus nephritis animal model. In the scientific research of universal recognition, phytane can cause inflammation and strengthen the immune response by activation of T cells and significantly increasing the reactivity of B cells. This triggers the animal to produce a variety of autoantibodies, resulting in lupus nephritis.12,13 Lactobacillus plantarum HFY15 was isolated and identified by our research team from naturally fermented yogurt made by Tibetan herdsmen in Hongyuan, Sichuan province in China (Figure 1S and 2S). Its basic in vitro resistance was satisfactory, its survival rate was greater than 80% in pH 3.0 artificial gastric juice, and its growth efficiency was greater than 50% in 0.3% bile salt (Table 1S).14
Prednisone can be used to treat kidney diseases. Most kidney diseases have immunological mechanisms, including the formation of immune cells and antigen antibody complexes. In this case, patients may suffer from glomerular filtration membrane damage, hematuria, proteinuria, etc., so the application of hormones at this time is beneficial to the treatment of patients. Therefore, prednisone was used as a positive control in this study. This study observed the interventional effect of Lactobacillus plantarum HFY15 on lupus nephritis by establishing an animal model. The effect of Lactobacillus plantarum HFY15 on lupus nephritis was confirmed by measuring the relevant indexes and inflammatory cytokines in the serum of mice, and the mechanism of action of Lactobacillus plantarum HFY15 was further elucidated by pathological observation and tissue mRNA gene expression detection.
Materials and Methods
Preparation of the Experimental Strain
Lactobacillus plantarum HFY15 was isolated and identified from naturally fermented yak yogurt originating from Hongyuan County, Aba Tibetan and Qiang Autonomous Prefecture, Sichuan Province, China. The strain was stored in the China General Microbiology Culture Collection Center (Beijing, China), and the patent number is CGMCC No. 16633.
Establishment of a Mouse Model of Lupus Nephritis
SPF six-week-old C57BL/J6 mice, half male and half female, weighing 23±2 g, were purchased from the Experimental Animal Center of Chongqing Medical University (Chongqing, China) with the production license number SCXK(Yu)2018–0003. The animal experiments in this study were approved by the Animal Experiment Ethics Committee of Chongqing Collaborative Innovation Center for Functional Food (Chongqing, China), with an approval number of 2021050008B. C57BL/J6 mice were housed at a temperature of 20±1°C and humidity of 30%-40%, with food and water offered ad libitum. After 7 days of adaptive feeding, the experiment began, with the random division of fifty mice into 5 groups, and 10 mice in each group: (i) normal group, (ii) model group, (iii) drug positive control group, (iv) low concentration treatment with LP-HFY15 (LP-HFY15-L) group, and (v) high concentration treatment with LP-HFY15 (LP-HFY15-H) group. On the first day after the experiment, the mice in the normal group were intraperitoneally injected with normal saline solution, and the mice in the other groups were intraperitoneally injected with 0.5 mL pristane (Shanghai Maclin Biochemical Technology Co., Ltd., Shanghai, China).15 Prednisone solution at 10 mg/kg daily was intragastrically administered to the mice in the drug positive control group, LP-HFY15 at 108 CFU/kg daily was intragastrically administered to the mice in the LP-HFY15-L group, and LP-HFY15 at 109 CFU/kg daily was administered to the LP-HFY15-H group. After 12 weeks of treatment, the mice were killed by amputation at the neck, and their heart blood was reserved and viscera were dissected for further testing.
Urine Protein Test
Starting at the beginning of the experiment, mice were reared in metabolic cages for a 24-hour period every two weeks. Urine excretion of mice was collected during 1 day, and the protein content in the urine of mice within 24 h was determined by a total protein kit (Coomassie brilliant blue method, Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China).
Determination of Inflammatory Cytokines in Serum and Tissues
The whole blood of mice was collected from the heart and centrifuged at 1500 rpm and 4°C for 10 min. The upper serum was removed for experimentation. In addition, 0.1 g kidney tissue was weighed, and 0.9 mL normal saline was added to the kidney tissue, which was then homogenized at 4°C. After centrifugation (4000 rpm, 10 min), the supernatant was removed for experimentation. Finally, the levels of inflammatory cytokines IL-6, IL-12, TNF-α, and IFN-γ in serum and tissues were determined by kits (Shanghai Enzyme Linked Biotechnology Co., Ltd., Shanghai, China).
Determination of SCr, BUN, TC, TG, and ALB Levels in Serum
The serum levels of SCr, BUN, TC, TG, and ALB were determined by kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) after the serum was obtained by the above method (Varioskan LUX multifunctional microplate reader, Thermo Fisher Scientific, Waltham, MA, USA).
Determination of Anti-dsDNA Antibody
Starting at the beginning of the experiment, mouse blood was obtained by retro-orbital sinus collection every two weeks. Then, the mouse serum was prepared according to the above method, and the anti-dsDNA antibodies were measured by microtiter plate and indirect immunofluorescence test.16
Tissue Section Observation
After dissecting the mice, the kidney tissues were removed and fixed with 10% formalin. After 48 h of dehydration, tissue samples were embedded in paraffin and sectionalized. Finally, the tissues were stained with hematoxylin and eosin (H&E, Shanghai Shangbao Biotechnology Co., Ltd, Shanghai, China), and histopathological changes were observed by light microscope (BX43, Olympus Corporation, Tokyo, Japan).17
qPCR Experiment
First, 0.9 mL of normal saline was added to 0.1 g of mouse kidney tissue, and the tissue mixture was homogenized. Then, RNAzol (1.0 mL, Invitrogen, New York, USA) was used to extract RNA from mouse kidney tissue. The absorbance value of the extracted RNA was measured at 260 nm and 280 nm, the purity and concentration of RNA were calculated, and then, the concentration of RNA was adjusted to 1 μg/μL. After cDNA was generated by reverse transcription, the reaction system solution was prepared by mixing 1 μL cDNA, 10 μL SYBR Green PCR Master Mix, 7 μL sterile distilled water, and 1 μL forward and reverse primer solution (Thermo Fisher Scientific). The PCR reaction conditions consisted of initial denaturation at 95°C for 60s, and then, 40 cycles at 95°C for 15s; 55°C for 30s; 72°C for 35s; 95°C for 30s; the reaction was carried out at 55°C for 35s, and the related genes were quantitatively analyzed by the 2−ΔΔCt method. GAPDH was used as an internal reference in the experiment (Table 1).18
 |
Table 1 Primer Sequences Used in This Experiment |
Statistical Analysis
After the end of the animal experiment, all the indicators were measured three times in parallel, and the results obtained are expressed as the mean value and the standard deviation (mean±SD). The index values of each group obtained by one-way ANOVA were significantly different at P<0.05.
Results
Mouse Urine Protein
During the experiment, urine protein levels in the normal group did not significantly change, but urine protein levels in the other groups increased over time. After 2 weeks, proteinuria was observed in 7, 5, and 4 mice treated with LP-HFY15-L, LP-HFY15-H, and prednisone, and proteinuria was observed in all mice in the model group. After 12 weeks, the amount of urine protein in the lupus nephritis mice (model group) was higher than that in the LP-HFY15- and prednisone-treated mice and normal mice (Table 2). The urine protein output of mice treated with high concentrations of LP-HFY15 and prednisone was closest to that of normal mice, and the urine protein output of mice in the LP-HFY15-H group was only higher than that in the prednisone group.
 |
Table 2 Urinary Protein Content of Mice in Each Group During the Experiment |
Levels of IL-6, IL-12, TNF-α, and IFN-γ Cytokines in Serum and Kidney Tissues of Mice
As shown in Table 3, the levels of IL-6, IL-12, TNF-α, and IFN-γ cytokines in the serum and kidney tissues of normal group mice were significantly lower than those in other groups (P<0.05). Compared with the model group, the levels of IL-6, IL-12, TNF-α, and IFN-γ in the lupus nephritis mice (model group) were significantly downregulated after LP-HFY15-H and prednisone treatment (P<0.05).
 |
Table 3 The IL-6, IL-12, TNF-α and IFN-γ in Serum and Renal Tissue of Mice |
Serum Levels of SCr, BUN, TC, TG, TP and ALB in Mice
The serum levels of SCr, BUN, TC, and TG in the model group were higher than those in other groups, while the serum levels of SCr, BUN, TC, and TG in the normal group were the lowest (P<0.05, Table 4). Compared with the model group, the levels of SCr, BUN, TC, and TG in LP-HFY15- and prednisone-treated mice also decreased, but they were higher than those in normal mice. After LP-HFY15 treatment, the levels of SCr, BUN, TC, and TG in nephritic mice were significantly improved (P<0.05), and the effect of high concentration (LP-HFY15-H) could make the levels of SCr, BUN, TC, and TG closer to the normal group than that of low concentration (LP-HFY15-L). In addition, the serum levels of TP and ALB showed an opposite trend, and the levels in each group were normal group, prednisone group, LP-HFY15-H group, LP-HFY15-L group and model group from high to low.
 |
Table 4 Serum SCr, BUN, TC, TG, TP and ALB Levels in Mice |
dsDNA Positive Rate
At 2, 4, 6, 7, 10, and 12 weeks after treatment, autoantibody dsDNA was detected by indirect immunofluorescence. The results showed that from the end of the 6th week, all mice in the model group were positive, and the induction of lupus nephritis was successful. Mice in the LP-HFY15-L group were positive at the end of the 8th week, and mice in the LP-HFY15-H and prednisone groups were positive at the end of the 10th week, suggesting that LP-HFY15 and prednisone slowed the rate of development of lupus nephritis in mice, and the effects of LP-HFY15 and prednisone were similar.
Renal Histopathological Observation
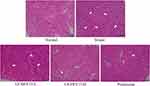
As shown in Figure 1, there were relatively severe lesions in the renal tissues of mice in the model group, with a large number of glomeruli showing irregular morphology, rupture of some glomeruli, and severe infiltration of inflammatory cells between tissues. The glomerular and cellular structures of the normal group were intact, and LP-HFY15 and prednisone alleviated the renal tissue lesions caused by lupus nephritis and the renal injury in the kidney group. A high concentration of LP-HFY15 (LP-HFY15-H) and prednisone exerted stronger effects, and both promoted morphology of renal tissue that was similar to that of the normal group.
mRNA Expression in Mouse Kidney Tissue
As shown in Figure 2, the mRNA expression of TGF-β1, VEGF, and NF-κB was the strongest in the renal tissues of the model group, while the mRNA expression of IκB-α, Cu/Zn-superoxide dismutase (SOD), and Mn-SOD was the weakest. The expression of TGF-β1, VEGF, and NF-κB was the weakest, and the expression of IκB-α, Cu/Zn-SOD, and Mn-SOD was increased in the normal group. Compared with the model group, LP-HFY15 and prednisone significantly upregulated the expression of IκB-α, Cu/Zn-SOD, and Mn-SOD and downregulated the expression of TGF-β1, VEGF, and NF-κB in the kidney tissues of lupus nephritis mice (P<0.05). The effect of high concentration LP-HFY15 (LP-HFY15-H) and prednisone was stronger than low concentration LP-HFY15 (LP-HFY15-L).
Discussion
Proteinuria plays an important role in the occurrence and development of renal disease. Proteinuria is one of the main symptoms in complex nephropathy, and the main clinical manifestation of lupus nephritis is also proteinuria.19 The lupus nephritis mice modeled in this study exhibited proteinuria, and LP-HFY15 and prednisone reduced the amount of protein in the urine of lupus nephritis mice, and played a role in alleviating lupus nephritis, with the effect of LP-HFY15 increasing with the increase in concentration. Serum creatinine and urea nitrogen are nitrogenous organic compounds, which are the final products of protein metabolism. When kidney function is normal, these small molecules filter through the glomeruli. When kidney damage is present, the glomerular filtration capacity decreases, and the serum creatinine and urea nitrogen levels increase. Therefore, the increased serum creatinine and urea nitrogen levels can be used as indicators for clinical diagnosis of kidney injury.20
Hyperlipidemia is caused by excess cholesterol and triglycerides, and features of hyperkalemia co-exist when kidney disease progresses to a certain level. Therefore, cholesterol and triglyceride can also be regarded as indicators of renal dysfunction and damage.21 In patients with nephrotic syndrome, the total protein in the serum is significantly reduced due to long-term proteinuria.22 Albumin is the most common protein in serum, and it is commonly used in the treatment of serious diseases, including the clinical treatment of edema caused by kidney disease, and the reduction of total protein and albumin in the case of renal dysfunction. Therefore, maintaining normal levels of serum total protein and albumin is an important method for maintaining normal renal function.23 In this study, LP-HFY15 and prednisone inhibited the increase in serum creatinine, urea nitrogen, cholesterol, triglyceride, and decrease in total protein and albumin caused by lupus nephritis, which can protect the kidneys by regulation of these nephropathy-related indicators.
IL-12 plays an important role in the autoimmune response in lupus nephritis, and IL-12 levels are elevated during the onset of lupus nephritis. One of the characteristics of lupus nephritis is the presence of a large number of autoantibodies. IL-12 promotes the direct production of such autoantibodies by cells, and increased IL-12 further leads to the production of such autoantibodies, aggravating the disease.24 IFN-γ is an inflammatory mediator that is involved in the entire immune inflammatory process of nephritis, and it has been clinically shown that the level of IFN-γ in patients with glomerulonephritis is significantly increased.25 The occurrence of nephritis is accompanied by a significant increase in the levels of inflammatory cytokines in the blood, such as IL-6, IL-12, TNF-α, and IFN-γ.25 In this study, the levels of inflammatory cytokines IL-6, IL-12, TNF-α, and IFN-γ were significantly increased in lupus nephritis mice, and LP-HFY15 and prednisone significantly inhibited these changes.
The necessary process of autoreactive antibodies in lupus nephritis is through mutation and category conversion to IgG. The massive deposition of IgG anti-dsDNA antibodies and immune complexes in the plasma in the glomerulus leads to kidney damage, which then causes inflammation and leads to invasion of inflammatory cells. In addition, high concentrations of dsDNA antibodies were found almost exclusively in lupus nephritis, and because dsDNA antibodies show specificity for lupus nephritis, they can be used as an indicator for the diagnosis of systemic lupus nephritis.26 Clinically, the renal tissues of patients with lupus nephritis often show changes in glomerular cell proliferation and glomerular neutrophil infiltration.27 The experimental indicators in this study also confirmed these manifestations. LP-HFY15 and prednisone inhibit the emergence of dsDNA antibodies and protect against pathological changes in kidney tissues.
TGF-β1 is the most important pro-fibrosis factor in the body and indicates fibrosis of organs, including the kidney. Abnormal expression of TGF-β1 often occurs in renal diseases. TGF-β1 in the kidney is secreted by podocytes and glomerular mesangial cells (GMCs). Podocytes secrete TGF-β1 through a previous association with endothelial cells, while GMCs secrete TGF-β1 in response to immunoglobulin (IgA) stimulation.28 After renal damage, glomerular endothelial cells secrete TGF-β1 by stimulation of VEGF. Abnormal expression of TGF-β1 and VEGF is typical in lupus nephritis, and therefore, regulation of this expression can effectively control lupus nephritis and inhibit inflammatory cytokines such as IL-6, IL-12, TNF-α, and IFN-γ.29 NF-κB plays a decisive role in gene transcriptional regulation of many of the above immune inflammatory factors. When interstitial nephritis is accompanied by abundant proteinuria, NF-κB is activated in the renal cortex, and the expression of TNF-α, IL-6, and TGF-β is increased.30 Reduced NF-κB activity reduces renal interstitial injury and the expression of IL-6 and TGF-β. The effect of NF-κB on TGF-β plays an important role in the pathological accumulation of extracellular matrix and the formation of glomerulosclerosis in lupus nephritis.31 IκB-α regulates the activation and transcription of NF-κB, and indirectly intervenes in lupus nephritis by influencing TGF-β1.32
Glomerular inflammation caused by nephritis is closely related to the imbalance of free radicals in the body. Oxygen free radicals in the kidney produce inflammatory cells that enter the kidney through the blood, and when phagocytosis of foreign bodies or activation by immune complexes occurs, oxygen free radicals will appear in large quantities.33 SOD can participate in regulating TGF-β1 and VEGF expression in clinical inflammatory diseases.34,35 SOD in mammals plays a role in the form of Cu/Zn-SOD and Mn-SOD, and regulating these two types of SOD can control inflammation.36 LP-HFY15 and prednisone exhibited regulatory effects on the expression of TGF-β1, VEGF, NF-κB, IκB-α, Cu/Zn-SOD, and Mn-SOD, thus playing a role in the intervention of lupus nephritis, and the effect of LP-HFY15 was positively correlated with its concentration.
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by increased expression of IFN and production of autoantibodies that trigger inflammation and damage to the kidneys. Animal experiments have shown that the interaction between gut microbes and the host is particularly important in the development of SLE, and changes in gut microbes are directly linked to kidney damage.37 TGF-β can regulate the proliferation of B lymphocytes, induce apoptosis of immature B lymphocytes, and block the activation and differentiation of B cells. TGF-β is a strong and favorable inhibitor of natural killer cells (NK cells), which can inhibit lupus nephritis by inhibiting the expression of IFN, thereby regulating the immune system.38 Probiotics can modulate host immune responses, and one probiotic (Lactobacillus gasseri SBT2055) has been shown to regulate TGF-β levels, thereby controlling B cell production of IgA.39 In this way, the probiotics can play a role in intervening in the pathological changes of the body. It can be seen that the regulatory effect of probiotics on lupus nephritis may be closely related to its regulation of TGF-β1 signaling pathway. Clinical studies have further verified the effect of probiotics on lupus nephritis. Bifidobacteria can interfere with lupus nephritis and inhibit lupus nephritis through immune regulation and anti-inflammatory mechanisms, and the effect is obvious.40
Conclusion
In this study, a new strain of Lactobacillus plantarum HFY15 was obtained from naturally fermented yak yogurt, and the interventional effect of LP-HFY15 on lupus nephritis was verified by animal model. The results showed that LP-HFY15 alleviated the inflammatory lesions in serum and tissues of mice caused by lupus nephritis, and in particular, LP-HFY15 regulated TGF-β1, the signature expression of lupus nephritis. There are unwanted side affects accompanying the drugs that are currently used for treating lupus nephritis. In the current study, prednisone was used as a positive drug control because there are less side effects associated with its use. Through comparison, it was also found that the effect of LP-HFY15 was similar to that of prednisone, which could be used as a safer intervention for lupus nephritis. In conclusion, LP-HFY15 can be used as a biological agent to intervene in lupus nephritis, which requires further study and promotion through human trials.
Data Sharing Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Ethical Statement
The animal experiments in this study were approved by the Animal Experiment Ethics Committee of Chongqing Collaborative Innovation Center for Functional Food (Chongqing, China), with an approval number of 2021050008B, and the experimental process was followed the 2010/63/EU directive.
Funding
There is no funding to report.
Disclosure
No conflicts of interest in this article.
References
1. Gan Y, Tong J, Zhou X, et al. Hepatoprotective effect of Lactobacillus plantarum HFY09 on ethanol-induced liver injury in mice. Front Nutr. 2021;8:684588. doi:10.3389/fnut.2021.684588
2. Tan Q, Hu J, Zhang Y, et al. Inhibitory effect of Lactococcus lactis subsp. lactis HFY14 on diphenoxylate-induced constipation in mice by regulating the VIP-cAMP-PKA-AQP3 signaling pathway. Drug Des Devel Ther. 2021;15:1971–1980. doi:10.2147/DDDT.S309675
3. Zhang J, Chen L, Zhang L, et al. Effect of Lactobacillus fermentum HFY03 on the antifatigue and antioxidation ability of running exhausted mice. Oxid Med Cell Longev. 2021;2021:8013681. doi:10.1155/2021/8013681
4. Li F, Lu D, Zhong Q, et al. Lactobacillus fermentum HFY06 reduced CCl4-induced hepatic damage in Kunming mice. RSC Adv. 2020;10(1):1–9. doi:10.1039/C9RA08789C
5. Xu C, Fu YY, Liu F, et al. Purification and antimicrobial mechanism of a novel bacteriocin produced by Lactobacillus rhamnosus 1.0320. LWT. 2021;137:110338. doi:10.1016/j.lwt.2020.110338
6. Li C, Liu H, Yang J, et al. Effect of soybean milk fermented with Lactobacillus plantarum HFY01 isolated from yak yogurt on weight. RSC Adv. 2020;10(56):34276–34289. doi:10.1039/D0RA06977A
7. Yi R, Tan F, Liao W, et al. Isolation and identification of Lactobacillus plantarum HFY05 from natural fermented yak yogurt and its effect on alcoholic liver injury in mice. Microorganisms. 2019;7(11):530. doi:10.3390/microorganisms7110530
8. Gao Y, Zeng Y, Xue W, et al. Anti−IL-12/23 p40 antibody attenuates chronic graft-versus-host disease with lupus nephritis via inhibiting Tfh cell in mice. Biomed Pharmacother. 2020;129:110396. doi:10.1016/j.biopha.2020.110396
9. Zhang L, Xiao B, Zhong M, et al. LncRNA NEAT1 accelerates renal mesangial cell injury via modulating the miR-146b/TRAF6/NF-κB axis in lupus nephritis. Cell Tissue Res. 2020;382(3):627–638. doi:10.1007/s00441-020-03248-z
10. Sfikakis PP, Boletis JN, Lionaki S, et al. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheum. 2005;52(2):501–513. doi:10.1002/art.20858
11. Lomax AR, Calder PC. Probiotics, immune function, infection and inflammation: a review of the evidence from studies conducted in humans. Curr Pharm Des. 2009;15(13):1428–1518. doi:10.2174/138161209788168155
12. Bonomini F, Dos Santos M, Veronese FV, et al. NLRP3 inflammasome modulation by melatonin supplementation in chronic pristane-induced lupus nephritis. Int J Mol Sci. 2014;20(14):3466. doi:10.3390/ijms20143466
13. Fu D, Senouthai S, Wang J, et al. Vasoactive intestinal peptide ameliorates renal injury in a pristane-induced lupus mouse model by modulating Th17/Treg balance. BMC Nephrol. 2019;20(1):350. doi:10.1186/s12882-019-1548-y
14. Long XY, Wang P, Zhou YJ, et al. Preventive effect of Lactobacillus plantarum HFY15 on carbon tetrachloride (CCl4)-induced acute liver injury in mice. J Food Sci. 2022;87(6):2626–2639. doi:10.1111/1750-3841.16171
15. Shen H, Zhu YQ, Kong Y, et al. Immune intervention effect of human-mouse chimeric antibody B7-1 against murine lupus nephritis model. Chin J Immunol. 2015;2015(9):1200–1205. doi:10.3969/j.issn.1000-484X.2015.09.011
16. Zheng ZW, Chen C, Wang HM, et al. Correlation of Serumβ2-microglobulin level with disease activity of systemic lupus erythematosus and degree of lupus nephritis. Chinese Gen Pract. 2019;22(17):2058–2063. doi:10.12114/j.issn.1007-9572.2018.00.310
17. Hu TT, Chen R, Qian Y, et al. Antioxidant effect of Lactobacillus fermentum HFY02-fermented soy milk on D-galactose-induced aging mouse model. Food Sci Human Wellness. 2022;11(5):1362–1372. doi:10.1016/j.fshw.2022.04.036
18. Long XY, Wu HB, Zhou YJ, et al. Preventive effect of Limosilactobacillus fermentum SCHY34 on lead acetate-induced neurological damage in SD rats. Front Nutr. 2022;9:852012. doi:10.3389/fnut.2022.852012
19. Hamilton P, Myers J, Gillham J, et al. Urinary protein selectivity in nephrotic syndrome and pregnancy: resurrection of a biomarker when renal biopsy is contraindicated. Clin Kidney J. 2014;7(6):595–598. doi:10.1093/ckj/sfu103
20. Li XY, Kong FY, Zhang HQ, et al. The duplication and identification of anti-glomerular basement membrane (GBM) nephritis model in mice. Acta Academiae Medicinae Xuzhou. 2011;31(8):527–530. doi:10.3969/j.issn.1000-2065.2011.08.007
21. Chou A, Zhou JY, Zhou Y, et al. Therapeutic effect of Zhenwu decoction on chronic glomerulonephritis rat model induced by cationization bovine serum albumin osmotic pump. Trad Chin Drug Res Clin Pharmacol. 2012;23(6):626–630. doi:10.3969/j.issn.1003-9783.2012.06.009
22. Zhou T, Lin S, Yang S, et al. Efficacy and safety of tacrolimus in induction therapy of patients with lupus nephritis. Drug Des Devel Ther. 2019;13:857–869. doi:10.2147/DDDT.S189156
23. Li ZJ, Li YJ, Yang QQ, et al. Significance of levels of IL 12 and IgG in patients with lupus nephritis. Li. New Chin Med. 2002;33(1):19–20. doi:10.3969/j.issn.0253-9802.2002.01.011
24. Xiang L, Gao XX, Pan JR. Serum levels of interferon-gamma and interleukin-10 in patients with chronic glomerulonephritis and their clinical significance. Chin J Clin Med. 2006;13(2):269–270, 272. doi:10.3969/j.issn.1008-6358.2006.02.057
25. Córdova C, Lopes-E-Silva F Jr, Pires AS, et al. Long-term resistance training is associated with reduced circulating levels of IL-6, IFN-γ and TNF-α in elderly women. Neuroimmunomodulation. 2011;18(3):165–170. doi:10.1159/000323396
26. Du J, Wang QS, Jia RH. Glomerular cell proliferation and apoptosis in experimental glomerulosclerosis. J Pract Med. 2005;21(15):1623–1625. doi:10.3969/j.issn.1006-5725.2005.15.006
27. Yang SR, Hua KF, Chu LJ, et al. Xenon blunts NF-κB/NLRP3 inflammasome activation and improves acute onset of accelerated and severe lupus nephritis in mice. Kidney Int. 2020;98(2):378–390. doi:10.1016/j.kint.2020.02.033
28. Ravinal RC, Costa RS, Coimbra TM, et al. Mast cells, TGF-beta1 and myofibroblasts expression in lupus nephritis outcome. Lupus. 2005;14(10):814–821. doi:10.1191/0961203305lu2188oa
29. Edelbauer M, Kshirsagar S, Riedl M, et al. Soluble VEGF receptor 1 promotes endothelial injury in children and adolescents with lupus nephritis. Pediat Nephrol. 2012;27:793–800. doi:10.1007/s00467-011-2062-z
30. Zheng L, Sinniah R, Hsu SIH. Pathogenic role of NF-kappaB activation in tubulointerstitial inflammatory lesions in human lupus nephritis. J Histochem Cytochem. 2008;56(5):517–529. doi:10.1369/jhc.7A7368.2008
31. Pu Y, Zhao H, Wu X, et al. The long noncoding RNA Ptprd-IR is a novel molecular target for TGF-β1-mediated nephritis. Int J Biochem Cell Biol. 2020;122:105742. doi:10.1016/j.biocel.2020.105742
32. Wang Q, Sun P, Wang R, et al. Therapeutic effect of Dendrobium candidum on lupus nephritis in mice. Pharmacogn Mag. 2017;13(49):129–135. doi:10.4103/0973-1296.197653
33. Endreffy E, Túri S, Lászik Z, et al. The effects of vitamin E on tissue oxidation in nephrotoxic (anti-glomerular basement membrane) nephritis. Pediat Nephrol. 1991;5(3):312–317. doi:10.1007/bf00867490
34. Liu YN, Zha WJ, Ma Y, et al. Galangin attenuates airway remodelling by inhibiting TGF-β1-mediated ROS generation and MAPK/Akt phosphorylation in asthma. Sci Rep. 2015;5:11758. doi:10.1038/srep11758
35. Zhao J, Chen W, Wei J, et al. Protective effects of icariin on renal function in rats with diabetic nephropathy and the related mechanisms. Immunol J. 2020;36(1):74–79. doi:10.13431/j.cnki.immunol.j.20200014
36. Rui T, Cepinskas G, Feng Q, et al. Delayed preconditioning in cardiac myocytes with respect to development of a proinflammatory phenotype: role of SOD and NOS. Cardiovasc Res. 2003;59(4):901–911. doi:10.1016/s0008-6363(03)00502-9
37. Kim JW, Kwok SK, Choe JY, et al. Recent advances in our understanding of the link between the intestinal microbiota and systemic lupus erythematosus. Int J Mol Sci. 2019;20(19):4871. doi:10.3390/ijms20194871
38. Xia J, Jiang LH, Su X, et al. Effect of herbal formula for serum IFN-γ, IL-4, TGF-β1 in lupus nephritis. Jilin J Trad Chin Med. 2015;35(4):367–369. doi:10.13463/j.cnki.jlzyy.2015.04.016
39. Sakai F, Hosoya T, Ono-Ohmachi A, et al. Lactobacillus gasseri SBT2055 induces TGF-β expression in dendritic cells and activates TLR2 signal to produce IgA in the small intestine. PLoS One. 2014;9(8):e105370. doi:10.1371/journal.pone.0105370
40. Huang M, Huang CJ, Ou QJ, et al. Effect of probiotic intervention on class IV and V lupus nephritis. Chinese Gen Pract. 2022;25(20):2462–2467. doi:10.12114/j.issn.1007-9572.2022.02.001
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.