Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Effects of Intravitreal Bevacizumab Therapy in Patients with Proliferative Diabetic Retinopathy
Authors Ribeiro de Carvalho G , Loduca Lima V, da Veiga GL , Adami F, da Costa Aguiar Alves B, Cristiano Pereira E , Feder D , Fonseca FLA
Received 27 December 2019
Accepted for publication 25 March 2020
Published 10 September 2020 Volume 2020:13 Pages 3149—3155
DOI https://doi.org/10.2147/DMSO.S243873
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Giuliana Ribeiro de Carvalho,1,2 Vagner Loduca Lima,2 Glaucia Luciano da Veiga,1 Fernando Adami,3 Beatriz da Costa Aguiar Alves,1 Edimar Cristiano Pereira,4 David Feder,1 Fernando Luiz Affonso Fonseca1,4
1Clinical Analysis Laboratory, Centro Universitário Saúde ABC/Faculdade De Medicina Do ABC, Santo André, Brazil; 2Ophthalmology Discipline, Centro Universitário Saúde ABC-Faculdade de Medicina do ABC, Santo André, Brazil; 3Epidemiology Department, Centro Universitário Saúde ABC/Faculdade De Medicina Do ABC, Santo André, Brazil; 4Pharmaceutical Sciences Department, Universidade Federal De São Paulo, Diadema, Brazil
Correspondence: Fernando Luiz Affonso Fonseca 2000, Lauro Gomes Avenue, Santo Andre CEP: 09060-650, Brazil
Tel +5511 4993-5488
Email [email protected]
Introduction: Diabetes mellitus (DM) stands out as one of the chronic diseases with the highest morbidity and mortality rates worldwide. Among the many complications of DM, diabetic retinopathy (DR) is one of the causes of blindness in patients aged between 20 and 64 years. At least 90% of the new cases showed to have the retinal structure and function restored when proper treatment was provided.
Aim: To evaluate the efficacy of the antiangiogenic bevacizumab in the treatment of DR according not only to the clinical laboratory parameters for glycated hemoglobin (HbA1C) and capillary glycemia but also to the ophthalmological parameters for optical coherence tomography (OCT) and best-corrected visual acuity (BCVA).
Patients and Methods: A total of 11 individuals were included and followed up for 12 months after 3 administrations of bevacizumab.
Results: Upon associating the ophthalmological and laboratory variables throughout the treatment, no significant alterations could be seen regarding the analyzed variables. However, it was observed that HbA1c values and the total leukocyte count negatively interfered with the treatment response.
Conclusion: The current study showed that HbA1c values and the amount of leukocytes negatively interfere with the therapeutic response. Therefore, laboratory analyses of these parameters are recommended for diabetic patients undergoing the above-mentioned treatment.
Keywords: diabetic retinopathy, bevacizumab, macular edema
Introduction
Diabetes mellitus (DM) stands out as a cause for morbidity and mortality. Global estimates indicate that 382 million people live with DM (8.3%), and this number can reach 592 million by 2035.1 In 2015, the International Diabetes Federation (IDF) estimated that 8.8% of the world population aged between 20 and 79 years (415 million people) have DM, and this figure is projected to increase to 642 million by the year 2040.2 Around 75% of the cases occur in developing countries, where an increase in number of DM patients is expected in the next decades.2 Until the year 2045, the prevalence of DM can be estimated at 9.9%.3
One of the complications resulted from DM is diabetic retinopathy (DR). Actually, it is one of the major causes of blindness in patients aged between 20 and 64 years, which represent around 12% of new cases.4 Besides, 80% of DM patients who have lived with the disease for over 20 years are frequently diagnosed with DR. At least 90% of the new cases, however, showed to have the retinal structure and function restored when proper treatment was provided.5
DR is characterized by the progressive damage of the retinal vessels, and it can be classified as non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR).6 The former, associated with diabetic macular edema (DME), brings about alterations in the retinal vessels, and it is further divided into mild, moderate and severe stages.7 The latter is related to the angiogenesis under hypoxia condition.8 Therefore, the new formed vessels are fragile, and without proper treatment, visual function may be lost. In most cases, DR is also characterized by an increase in vascular permeability, which leads to fluid accumulation and retinal hemorrhages in the macula, all of which referred to DME.6–8
Currently, the three main effective treatments for the reduction of visual impairment in DR are laser photocoagulation, pharmacological and surgical therapies. Intraocular pharmacotherapy, especially the administration of anti-inflammatory and antiangiogenic agents, vascular endothelial growth factor (VEGF) inhibitors, have become the treatment of choice for DR. The advantage of this kind of therapy is the fact that it may reduce the treatment load in some cases, but in others, with chronic DME, long-term intensive therapy may be required.9
The antiangiogenic bevacizumab is an inhibitor of the VEGF. It is a humanized monoclonal antibody produced by recombinant Deoxyribonucleic acid (DNA) technology. This antibody, with molecular mass of approximately 149 kDa, is composed of 214 amino acids.10 Its mechanism of action consists of its binding to VEGF (one of the most important agents that stimulates the formation of new vessels), thus inhibiting VEGF binding to its receptors on the surface of endothelial cells. The neutralization of VEGF reduces vascularization.10
In order to treat all the injuries that lead to PDR and DME, intravitreal injection of bevacizumab is administered to reduce vascular permeability and retinal and choroidal neovascularization.11 Also, the treatment with intravitreal bevacizumab reduces the neovascularization of the iris. However, despite the positive response, results are short-termed since neovascularization occurrence could be observed two weeks after the administration of the drug. Intravitreal bevacizumab is frequently used as a clinical complement to laser photocoagulation in DR patients.12
Therefore, the analysis of therapies that improve the ophthalmological parameters and may contribute to biochemical evaluation of DM patients is of utmost importance.
The aim of this study was to evaluate the efficacy of the antiangiogenic bevacizumab in the treatment of DR according to clinical laboratory parameters for glycated hemoglobin (HbA1C), capillary glycemia and to the ophthalmological parameters for optical coherence tomography (OCT) and best-corrected visual acuity (BCVA).
Patients and Methods
This was a longitudinal observational study performed in an ophthalmological clinic in São Paulo, Brazil. A total of 11 individuals were included and followed-up for 12 months after 3 administrations of bevacizumab.
Ethical Aspects
The current study was approved by the Ethics and Research Committee of Faculdade de Medicina do ABC (FMABC) under the number 1.759.520. All participants filed out the Free Informed Consent Form (FICF) and were made aware of all the procedures they would have to undergo. Additionally, all the protocols adopted in the study were clearly explained. Blood samples were discarded after the analyses were performed. All the patients were given the information concerning the employed treatment as well as the possible unwanted or adverse effects resulting from the administration of the drug. The patients' consent to review their medical records was not required by the Institutional Review Board (IRB), we declare that patient confidentiality data are following the Declaration of Helsinki.
Participants and Eligibility
A total of 11 DM patients diagnosed with macular edema took part in this study. Inclusion criteria: (1) DM patients ≥18 years of age diagnosed with clinically significant diffuse macular edema (CSME) who had not been previously laser treated and not only with PDR; (2) no previous history of glaucoma; and (3) transparent ocular media. Exclusion criteria: (1) individuals <18 years; (2) laser-treated patients; (3) previous glaucoma diagnosis; (4) cataract; and (5) vitreous hemorrhage.
Treatment
All patients received doses of 4 mg/0.1 mL of bevacizumab. A total of 3 doses were injected at an interval of 30 days, followed by 3 sessions of laser photocoagulation in each eye of all participants.
Procedures and Ophthalmologic Evaluation
The patients underwent complete ophthalmologic exams before treatment was started, which included visual acuity measurement according to the ETDRS (Early Treatment Diabetic Retinopathy Study) table that is a logarithmic scale composed by 5 optotypes per line, and geometric progression in letter size and spacing,13 the letter size on each line is 1.2589, or 0.1 log units, times bigger than those on the next line and optotypes are the 10 Sloan letters (C, D, H, K, N, O, R, S, V, and Z). Additionally, a stereoscopic biomicroscopy was performed and the intraocular pressure (IOP) was measured using an applanation tonometer and an optical coherence tomography ((Stratus OCT-3), Carl Zeiss Meditec Inc./CA, USA). A macular thickness map was generated for macular analysis, and the central region of this map was evaluated. All patients were classified according to the ETDRS, and they all underwent 3 exams which were performed by the same professional. The best macular thickness was obtained according to the following criteria: (1) the best grade for the obtained scan image attributed by the software; (2) the best set of 6 scan images with the lowest error rate in the analysis of retinal thickness; and (3) the best map centered in the foveal pit, if present.
Biochemistry Analysis and Clinical Evaluation
Sample Collections
Blood samples were collected according to the standard operating protocol (SOP) adopted by the clinical analysis laboratory of Faculdade de Medicina do ABC (FMABC), where they were properly stored under refrigeration until the analyses were performed. These samples were obtained through venipuncture using the vacuum tube method after an eight-hour fasting period so that fasting glycemia and HbA1c levels could be analyzed. Besides, systemic blood pressure (SBP) and capillary glycemia were also measured.
Complete Blood Count
Hematologic analyses were performed at clinical analysis laboratory of FMABC using a Sysmex XE-2100™ automated hematology analyzer. Erythrocytes, leukocytes and platelets were counted by electrical impedance. Hematocrit count (Hct) was measured as well as the following hematimetric values: mean globular volume (MGV), mean globular hemoglobin concentration (MGHC), mean globular hemoglobin (MGH) and the red blood cell distribution width (RDW). Platelet-derived indices were also determined: mean platelet volume (MPV), platelet distribution width (PDW) and plateletcrit (PCT). Leukocyte differential counting was carried out by counting 100 cells per blood smear.
Analysis of Fasting Glycemia and HbA1c Levels
Values of fasting plasma glycemia were obtained through the evaluation of blood-glucose concentrations using the automated colorimetric enzymatic method after an overnight fasting period. The analysis of glycemic control was performed using the fasting glycemia values.
Analysis of Glycated Hemoglobin (HbA1c) Levels Through Capillary Electrophoresis in DM2 Patients
Glycated hemoglobin (HbA1c) was determined using the capillary electrophoresis technique performed on an ultra2 analyzer, through which the percentage of total hemoglobin is expressed and the mean blood glucose level is evaluated, over a period of 3 months. The samples were composed of 5 mL of blood and 1 mL of hemolysis reagent. HbA1c values over 7% were considered altered. The FASC position marker kit (Trinity Biotech 01–04-0042, Kansas, USA) was prepared from stabilized whole blood products containing hemoglobins F, A, S and C, which were combined and lyophilized to ensure stability.
Patients Follow-Up
Systemic blood pressure and capillary glycemia were measured after each drug administration (total of 3). Patients were requested to undergo glycated hemoglobin and fasting glycemia tests at diagnosis and 1, 3, 6, 9 and 12 months after the administrations.
Ophthalmologic exams were performed on the same day of the intravitreal injections (1 per month, 3 in total). Administrations were performed according to the guidelines previously published.14 They included the use of sterile gloves, blepharostat, povidone-iodine, and the injection of ophthalmic anesthetic drops with 30-gauge insulin needles in the inferior temporal site without anterior chamber paracentesis.
The patients were scheduled to return in 1, 3, 6, 9 and 12 months after the administrations. At each visit, IOP and visual acuity were measured and the macular map obtained (Stratus OCT). Visual and anatomic results were observed as well as the possible complications related to intravitreal injection procedures.
Statistical Analysis
In order to describe the qualitative variables, absolute and relative frequencies were used. For quantitative variables (Shapiro–Wilk <0.05), 95 confidence interval median was used. To study the association between biochemical markers and pre- and post-treatment moments, the Mann–Whitney test was applied. For the association between biochemical variables and the treatment moments, the Friedman test was used. The association of groups (pre- and post treatment, 0 or 1, respectively) was estimated according to a multiple regression model, adopting robust variance and stepwise forward strategy for variable selection. Stata 11.0 was used for statistical analysis. All values with p<0.05 were considered statistically significant.
Results
A total of 11 individuals were included, and a total of 77 blood samples were obtained (28 from males and 49 from females). The obtained variables and laboratory evaluations are described in Table 1.
 | 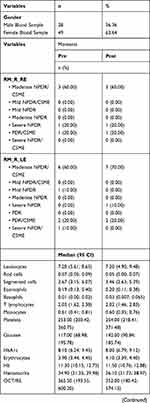 |  |
Table 1 Sample Characterization |
Upon evaluating the laboratory markers at pre- and post-treatment moments, no significant differences for laboratory variables (complete blood count parameters, HbA1c and glucose measurement) could be observed as seen in Table 2.
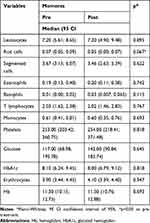 |
Table 2 Association Between Laboratory Markers and Treatment Moments |
When the multiple regression model was used to account for laboratory markers throughout the treatment, it could be observed that the total number of leukocytes and HbA1c determination interfere in the treatment. Table 3 shows the multiple regression model of the markers throughout the treatment.
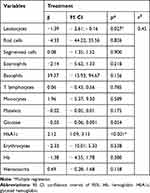 |
Table 3 Multiple Regression Model of the Biochemical Markers Throughout the Treatment |
A linear regression of the laboratory markers was performed during the treatment, and it was observed that HbA1c levels, leukocyte total count and eosinophil total count interfere with the treatment as seen in Table 4.
 |
Table 4 Multiple Linear Regression Model of the Biochemical Markers Throughout the Treatment |
Biochemical and clinical analyses at different moments of the treatment are described in Table 5.
 |
Table 5 Laboratory and Clinical Analyses at Different Treatment Moments |
Discussion
According to the results here found, DME patients on bevacizumab showed a subtle response regarding ophthalmologic, biochemical and hematological variables at different moments of the treatment. Jiao et al (2018)15 reveal that the treatment with bevacizumab has a preventive effect on DR. Some reports showed that the treatment with anti-VEGF agents reduces the severity of DR, and that this response is associated with lower rates of PDR development. Today, the management of NPDR consists of risk factor modification. Therefore, the use of anti-VEGF medications, in addition to risk factor modification, may prevent the development of PDR.16 It is known that the effects of anti-VEGF agents on the treatment of DR are still contradictory; thus, the investigation on the use of this medication and its repercussions in other DM parameters is of utmost importance.
In this study it was observed that bevacizumab lowered HbA1c levels and the global leukocyte count, resulting in an improvement in the overall condition of the patients. Those whose serum values of HbA1c were ≤7.0% and those with HbA1c >7.0% showed a statistically significant progress in BCVA. Central subfoveal thickness (CST) was significantly improved, but higher improvement rates could be observed in the group with better control over DM.17 Such data corroborate the findings of Mott et al (2019),18 who reported that the use of bevacizumab combined with glycemic control results in the achievement of better contrast sensitivity in DM2 patients with DME.6–8
The diabetic decompensation, due to the increase of HbA1c levels can be relevant for the staging of the macula and therapeutic responses. The mean levels of HbA1c remained relatively stable during the follow-up in both groups, but patients with improved glycemic control during the study duration had a significantly lower retinal thickness than that in patients who had stable or worsened HbA1c levels.17
The Brazilian National Health Agency (ANS, in Portuguese) has recently regulated the use of antiangiogenics for ocular treatments.19 However, there are no clinical recommendations for the investigation of HbA1c levels or the complete blood count parameters in DM patients on bevacizumab. In this study, it was possible to relate the biochemical data of glycemic control to the action of bevacizumab.
DM is an endocrine-metabolic disorder of heterogeneous etiology characterized by chronic hyperglycemia, which results from defects in insulin secretion or insulin action that degenerates the retina. Bevacizumab, in turn, is a medication that inhibits the VEGF through different actions. Therefore, despite the need for further laboratory and clinical analysis of patients on bevacizumab, the use of this antiangiogenic agent is recommended.
Conclusion
The current study showed that HbA1c values and the number of leukocytes negatively interfere with the therapeutic response. Hence, laboratory analyses of these parameters are recommended for diabetic patients undergoing the above-mentioned treatment.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE Global estimates of diabetes prevalence for 2013 and projections for 2035. Diab Res Clin Pract. 2014;103(2):137–149. doi:10.1016/j.diabres.2013.11.002
2. International Diabetes Federation. IDF Diabetes Atlas.
3. Cho NH, Shaw JE, Karuranga S, et al.. Global estimates of diabetes prevalence for 2017 and projections for 2045. Diab Res Clin Pract. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023.
4. Dodda D, Ciddi V. Plants used in the management of diabetic complications. Indian J Pharm Sci. 2014;76(2):97–106.
5. Frank RN. Diabetic retinopathy and systemic factors. Middle East Afr J Ophthalmol. 2015;22(2):151–156. doi:10.4103/0974-9233.154388
6. Mitchell P, Wong TY. Diabetic macular edema treatment guideline working group. Management paradigms for diabetic macular edema. Am J Ophthalmol. 2014;157(3):e1–8. doi:10.1016/j.ajo.2013.11.012
7. Stewart MW, Flynn HW, Schwartz SG, Scott IU. Extended duration strategies for the pharmacologic treatment of diabetic retinopathy: current status and future prospects. Expert Opin Drug Deliv. 2016;13(9):1277–1287. doi:10.1080/17425247.2016.1198771
8. Kumar B, Gupta SK, Saxena R, Srivastava S. Current trends in the pharmacotherapy of diabetic retinopathy. J Postgrad Med. 2012;58(2):132–139. doi:10.4103/0022-3859.97176
9. Lu L, Jiang Y, Jaganathan R, Hao Y Current advances in pharmacotherapy and technology for diabetic retinopathy: a systematic review. J Ophthalmol. 2018 17;2018:1694187.
10. Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2017;237(4):185–222. doi:10.1159/000458539
11. Blinder KJ, Dugel PU, Chen S, et al. Anti-VEGF treatment of diabetic macular edema in clinical practice: effectiveness and patterns of use (ECHO study report 1). Clin Ophthalmol. 2017;11:393–401. doi:10.2147/OPTH.S128509
12. Bahrami B, Hong T, Zhu M, Schlub TE, Chang A. Switching therapy from bevacizumab to aflibercept for the management of persistent diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2017;255(6):1133–1140. doi:10.1007/s00417-017-3624-y
13. Klein R, Klein BE, Moss SE, Cruickshanks KJ The Wisconsin epidemiologic study of diabetic retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology. 1998;105(10):1801–1815. doi:10.1016/S0161-6420(98)91020-X
14. Aiello LP, Brucker AJ, Chang S, et al. Evolving guidelines for intravitreous injections. Retina. 2004;24(5 Suppl):S3–19. doi:10.1097/00006982-200410001-00002
15. Lv J, Chen MM, Mu ZH, et al. Intravitreal bevacizumab injection attenuates diabetic retinopathy in adult rats with experimentally induced diabetes in the early stage. J Diab Res. 2018;9(2018):9216791.
16. Zhao Y, Singh RP The role of anti-vascular endothelial growth factor (anti-VEGF) in the management of proliferative diabetic retinopathy. Drugs Context 2018; 13(7):212532.
17. Matsuda S, Tam T, Singh RP, et al. The impact of metabolic parameters on clinical response to VEGF inhibitors for diabetic macular edema. J Diab Complications. 2014;28(2):166–170. doi:10.1016/j.jdiacomp.2013.11.009
18. Motta AAL, Bonanomi MTBC, Ferraz DA, et al. Short-term effects of intravitreal bevacizumab in contrast sensitivity of patients with diabetic macular edema and optimizing glycemic control. Diab Res Clin Pract. 2019;149:170–178. doi:10.1016/j.diabres.2019.02.002
19. PARECER TÉCNICO Nº 51/GEAS/GGRAS/DIPRO/2018. Available from: http://www.ans.gov.br/images/stories/parecer_tecnico/uploads/parecer_tecnico/_PARECER%20TCNICO%20N%2051-2018_TRATAMENTO%20OCULAR%20QUIMIOTERAPICO%20COM%20ANTIANGIOGENICO_VERSO%20FINAL_28122017%20verso%202%201%2031012018.pdf.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
