Back to Journals » Journal of Pain Research » Volume 16
Effects of Hypnotic Analgesia and Transcranial Direct Current Stimulation on Pain Tolerance and Corticospinal Excitability in Individuals with Fibromyalgia: A Cross-Over Randomized Clinical Trial
Authors Schein B, Beltran G, França BR, Sanches PR, Silva DP Jr, Torres IL , Fegni F, Caumo W
Received 30 August 2022
Accepted for publication 28 October 2022
Published 24 January 2023 Volume 2023:16 Pages 187—203
DOI https://doi.org/10.2147/JPR.S384373
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Andrea Tinnirello
Bruno Schein,1,2 Gerardo Beltran,1– 3 Bárbara Regina França,1,2 Paulo RS Sanches,4 Danton P Silva Jr,4 Iraci Lucena Torres,5,6 Felipe Fegni,7 Wolnei Caumo1,2,5,8
1Post-Graduate Program in Medical Sciences, School of Medicine, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Rio Grande do Sul (RS), Brazil; 2Laboratory of Pain and Neuromodulation, Hospital de Clínicas de Porto Alegre (HCPA), Porto Alegre, RS, Brazil; 3Institute of Neurosciences, Universidad Catolica de Cuenca, Cuenca, Ecuador; 4Laboratory of Biomedical Engineer, HCPA, Porto Alegre, RS, Brazil; 5Pain and Palliative Care Service, HCPA, Porto Alegre, RS, Brazil; 6Laboratory of Pharmacology of Pain and Neuromodulation, Experimental Research Center, HCPA, Porto Alegre, RS, Brazil; 7Laboratory of Neuromodulation and Center for Clinical Research Learning, Physics and Rehabilitation Department, Spaulding Rehabilitation Hospital, Boston, MA, USA; 8Department of Surgery, School of Medicine, UFRGS, Porto Alegre, RS, Brazil
Correspondence: Wolnei Caumo, Laboratory of Pain and Neuromodulation, Hospital de Clínicas de Porto Alegre at UFRGS, Ramiro Barcelos, 2350 - CEP 90035-003 Bairro Rio Branco, Porto Alegre, RS, Brazil, Tel/Fax +55 51- 33598083, Email [email protected]
Objective: We compare the effect of HAS, a-tDCS on the left dorsolateral prefrontal cortex (l-DLPFC), and rest-testing on pain measures [(cold pressor test (CPT) (primary outcome) and heat pain threshold]. We also compare their effects on the motor evoked potential (MEP) (primary outcome), short intracortical inhibition (SICI), intracortical facilitation (ICF), and cortical silent period (CSP).
Methods: This randomized, blind, crossover trial included 18 women with fibromyalgia, aged from 18 to 65 years old. They received at random and in a crossover order a-tDCS over the l-DLPFC (2mA), HAS, or a rest-testing.
Results: HAS compared to a-tDCS increased the pain tolerance with a moderate effect size (ES) [Cohen’s f=− 0.78; (CI 95%; − 1.48 to − 0.12)]. While compared to rest-testing, HAS increased the CPT with a large ES [Cohen’s f=− 0.87; (CI 95%; − 1.84 to − 0.09)]. The a-tDCS compared to HAS increased the MEP amplitude with large ES [Cohen’s f=− 1.73 (CI 95%; − 2.17 to − 0.17)]. Likewise, its ES compared to rest-testing in the MEP size was large [Cohen’s f=− 1.03; (CI 95%; − 2.06 to − 0.08)].
Conclusion: These findings revealed that HAS affects contra-regulating mechanisms involved in perception and pain tolerance, while the a-tDCS increased the excitability of the corticospinal pathways. They give a subsidy to investigate their effect as approaches to counter regulate the maladaptive neuroplasticity involved in fibromyalgia.
Clinical Trial Registration: www.ClinicalTrials.gov, identifier – NCT05066568.
Keywords: tDCS, hypnosis, hypnotic analgesia, chronic pain, fibromyalgia, pain threshold, cold pressor test, CPM-test
Plain Language Summary
There is a lack of data to comprehend the difference between the effects of hypnotic analgesia suggestion and a-tDCS in fibromyalgia. This research was done to better understand the effects of anodal transcranial direct current stimulation (a-tDCS) and hypnotic suggestion analgesia (HAS) on corticospinal pathways and cortical excitability. Compared to a-tDCS and rest-testing, HAS increased tolerance to nociceptive stimulus. On the other hand, the a-TDCS raised excitability in cortical and cortico-spinal pathways. These results support the distinction between the effects of HAS and a-tDCS in corticospinal excitability. Also, they may be clinically relevant to creating a roadmap for customizing treatment in FM based on an individual’s characteristics. An exploratory analysis also revealed that patients with higher hypnotic susceptibility are likely to exhibit an increased effect of a-tDCS on MEP and SICI.
Introduction
Fibromyalgia encompasses widespread musculoskeletal pain, fatigue, sleep disorders and cognitive dysfunctions involving memory and attention.1 Although the precise mechanisms underlying fibromyalgia remain incompletely understood, it is a nociplastic pain condition associated with a central sensitization syndrome (CSS).1 CSS encompasses the impaired functioning of neurons and circuits in nociceptive pathways with neuronal excitability, increased synaptic efficacy, and reduced inhibition in the descending pain modulatory pathways.2 Its multiple components include sensory, emotional, cognitive, and behavioral elements. It involves activity in various networks in the central nervous system (CNS).2
The dysfunction of pain processing pathways in fibromyalgia has been linked with a deteriorated function of cortical inhibition.3,4 In a previous study, using transcranial magnetic stimulation (TMS) measures, we found an increase in short intracortical inhibition (SICI) compared to healthy subjects.3 However, other studies found lower intracortical facilitation (ICF) and SICI than controls.4 Despite these mixed results, these data indicate that motor cortex excitability might be an index to comprehend cortical dysfunction related to pain. Motor evoked potentials (MEPs) might be a valuable tool to investigate the dysfunction of corticospinal excitability.5 In the therapeutic expect, according to a meta-analysis, anodal stimulation on the M1 effectively reduces the intensity of various pain conditions, including fibromyalgia.6 This effect has been related to influences in the sensory-discriminative networks involved in pain sensitivity processing. On the other hand, the prefrontal cortex (PFC) has been associated with regulating top-down modulation and maintaining pain inhibition.2 PFC is responsible for driving behavioral responses and is involved in cognitive processes, including attention, value encoding, working memory, creativity, decision-making, and emotional regulation.2
An earlier study found that patterns in fibromyalgia's left prefrontal cortex (PFC) activation indexed by oxy-hemoglobin concentration using near-infrared spectroscopy might differentiate fibromyalgia patients from controls and discriminate subjects with more severe CSS symptoms.7 Hence, the differential cortical activation in chronic pain has been linked to an imbalance between excitability and disinhibition by GABA activity reduction.8 In contrast, with an increase in NMDA receptor activity by glutamate.9 Although advances in dysfunctional processes in fibromyalgia are ongoing, conventional medical treatment in fibromyalgia has a limited impact on improving cognitive and emotional aspects related to chronic pain.10 Based on the multimodal concept of pain management, there is a growing interest in interventions that can complement conventional medicine, such as hypnotic suggestions.
The hypnotic analgesia suggestion trains patients to manipulate focus and self-regulation to change pain experience, cognition, thought, and behavior.11 Its benefits on pain perception have been demonstrated in several clinical conditions, such as skin burns, leukemia, labor pain, and cancer, including fibromyalgia.12,13 In addition, the hypnotic analgesia effect improved the emotional and cognitive symptoms of fibromyalgia.13 In an earlier study of healthy women with high susceptibility to hypnosis, we found that it increased heat pain threshold (HPTh), heat pain tolerance (HPTo), and cold pressor test (CPT) tolerance. However, in healthy women, hypnotic suggestion paradoxically reduced the inhibition of the descending pain modulatory system (DPMS) by conditioned pain modulation (CPM)-task. In contrast, the same study showed that anodal transcranial direct-current stimulation (a-tDCS) improved the efficiency of DPMS.14 Additional studies proved that anodal transcranial direct current stimulation (a-DCS) improves fibromyalgia symptoms related to pain and disability due to pain.15,16
The main target for tDCS to treat pain is the primary motor cortex (M1), influencing the sensory-discriminative networks evolved in pain sensitivity processing.6 Anodal stimulation over DLPFC, on the other hand, has revealed beneficial effects on mood regulation, cognitive functions (eg, decision-making), and mechanisms underlying adaptive and maladaptive emotional functioning.15 a-tDCS over M1 enhances the strength of the descending pain modulating system in chronic pain.17 In fibromyalgia, a-tDCS over DLPFC reduced pain sensations and improved either fatigue or cognitive performance. In addition, anodal stimulation on DLPFC led to increased orienting and executive attention networks.16,18 However, there is a lack of data to comprehend the difference between the effects of hypnotic analgesia suggestion and (a)-tDCS on DLPFC in fibromyalgia. Hence, as a concept proof test of the merit of a-tDCS and hypnotic suggestion in clinical practice, it is reasonable to determine if their effects are superior to the participants’ behavior alteration due to being observed (Hawthorne effect).19 A strict, controlled experimental paradigm is needed to control this possible bias and comprehend its therapeutic effect on pain processing. In the context of the scientific progress in complementary therapy, control group design is among the crucial methodological issues in hypnotic suggestion research, likewise in the development of yoga, tai chi, and mind-body therapy research.20
Thus, we plan a randomized clinical trial to examine the effect of a-tDCS, a hypnotic analgesia suggestion (HAS), and a rest testing condition on psychophysical pain measures and cortical excitability measures. From a pragmatic viewpoint, we compared the impact of HAS on a-tDCS and rest-testing on psychophysical measures related to pain [(cold pressor test (CPT) (primary outcome) and heat pain threshold (HPTh)]. We also compared their effects on cortical excitability measures by transcranial magnetic stimulation [motor evoked potential (MEP) (primary outcome), short intracortical inhibition (SICI), intracortical facilitation (ICF), and cortical silent period (CSP) (secondary outcomes)]. We hypothesize that HAS would be more effective in reducing pain perception than a-tDCS and rest-testing. In contrast, we hypothesize that a-tDCS would be more effective than HAS and rest-testing to improve the excitability of the motor cortex.
Materials and Methods
Design Overview, Setting, and Participants
The protocol of this randomized, single-center study, crossover trial was approved by the Research Ethics Committee at the Hospital de Clínicas de Porto Alegre (HCPA), Brazil – Institutional Review Board IRB (CAAE 29940720.7.00005327) in accordance with the Declaration of Helsinki. All participants provided oral and written informed consent before participating, and they did not receive payment in exchange for their participation. Recruitment was undertaken in time from July 2020 to December 2021. De-identified data relating to intervention and primary outcomes will be made available on request to Caumo W ([email protected]) with no time restriction.
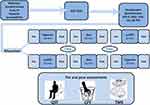
Patients were randomly assigned 1:1 to treatment order in a 3-phase crossover trial: in the first phase, a-tDCS or HAS; in the second phase, all subjects received the rest testing; and in the third phase, those who received HAS in the first phase crossed to a-tDCS or vice-versa. This way, every intervention was administered once. To prevent carry-over effects, we established a washout time of a minimum of 7 days between each phase, based on a previous proof of concept study with a similar design.21 The study is presented according to CONSORT 2010 guidelines (Appendix I). The timeline of this study is presented in Figure 1.
Protocol Change
According to the initial protocol plan, all assessments had been planned to occur face-to-face. However, due to the Covid-19 pandemic, we changed some procedures to maintain social distance, such as obtaining online: (i) formal online consent and (ii) a questionnaire about sociodemographic and previous medical history data. (iii) We excluded patients who contracted a covid-19 infection that reported some sequel or if they were in the first 3 months of grief. These changes occurred after the 12 months from the recruitment start, and this modification is shown in the timeline.
Recruitment, Inclusion, and Exclusion Criteria
All patients were recruited by directly contacting them from the institutional chronic pain clinic and referrals from other Basic Health Units of the Public Health System at Porto Alegre, Brazil. We also call volunteers registered in the database of fibromyalgia at the Laboratory of Pain and Neuromodulation at Hospital de Clínicas de Porto Alegre. They could also call the phone number of the research project announced through advertisements in groups and associations with patients with Fibromyalgia in Porto Alegre, Brazil. Firstly, we contact volunteers by phone to answer a screening questionnaire. Inclusion criteria were fibromyalgia women between 18 and 65 years old, with fibromyalgia diagnosis, according to the American College of Rheumatology revised criteria (ACR) 2016.22 They needed to be literate, with the capacity to read and write in Brazilian Portuguese, and have pain scores equal to or greater than six on Numerical Pain Scale (NPS) 0–10 on most days for the last 3 months. If they met the inclusion criteria, they were invited to answer the Waterloo-Stanford Group C Scale of Hypnotic Susceptibility (WSGC), validated in Portuguese.23 Volunteers should also present a high hypnotic susceptibility, defined as a score ≥8 on the susceptibility scale. After this initial assessment by phone call, we invited them face-to-face to confirm the fibromyalgia diagnosis by a physician with pain specialization with Brazilian Board-Certified. In this assessment, we collect medical history and detailed descriptions of their symptoms. We excluded volunteers if they presented some of the following conditions: Pregnancy; history of alcohol or drug abuse in the previous 6 months; history of uncontrolled systemic diseases (ie, ischemic heart disease, renal disease, hepatic disease, hypothyroidism, etc.); history of cancer treatment in the last year, history of chronic inflammatory disease (eg, lupus, rheumatoid arthritis, and Reiter’s syndrome); had COVID-19 infection or presence of similar symptoms in the last 14 days; suicide ideation with present risk and other uncontrolled psychiatric diseases that can interfere with the study. Contraindications to tDCS include a history of brain surgery, tumor, stroke, or intracranial metallic implants.24
Settings and Locations Where the Data Were Collected
The experimental protocol was conducted under standardized conditions, including controlling environmental temperature and noise, at the Laboratory of Pain and Neuromodulation at Hospital de Clínicas de Porto Alegre, Brazil. Interventions (HAS, a-tDCS or rest testing) are described below:
a. Hypnotic analgesia session: The protocol consisted of 20-min-long induction of hypnotic analgesia according to the standardized hypnotic induction guide developed by Professor Jensen.25 It starts with an induction, with suggestions for subjects to focus their attention on a single stimulus. Thus, they were encouraged to control their breathing, guiding subjects to progressive relaxation. After these initial instructions, the professional gave suggestions for comfort, in which the patient had to imagine being in a quiet and peaceful place. In the 10 final minutes of the induction, the hypnotic suggestions for analgesia target decreased the subject’s pain and control over her sensations. According to the hypnotic analgesia suggestion, as told to the patient, who no longer would feel pain. So, his mind would be able to control the sensations of his own body, preventing pain. We used the protocol of HAS previously published by Patterson, D. R., and Jensen, which follows standardized steps (see the hypnotic analgesia suggestion protocol in Appendix II).25 The duration of experimental manipulation (induction + suggestions) was 20 min.
b. The device and the cap used for the tDCS were developed by the Biomedical Engineering Department at the Hospital de Clínicas de Porto Alegre, Brazil, in partnership with the Laboratory of Pain & Neuromodulation. It is approved by ANVISA (Brazilian National Health Surveillance Agency) up to registration number N°80079190028. In this link it is possible to see details about the device and its validation, as well as the step-by-step self-administration process of the tDCS used at home: Home-Based Transcranial Direct Current Stimulation Device Development: An Updated Protocol Used at Home in Healthy Subjects and Fibromyalgia Patients (jove.com). The scalp electrodes were positioned according to the 10–20 system for EEG (F3 anode, l-DLPFC) and the cathode at r-DLPFC (F4). a-tDCS current applied was 2mA for 20 min.26 The 35cm2 electrodes were coated in a sponge and placed inside a neoprene cap adjusted for the subject’s head size. Before starting the intervention, we moistened the electrodes with a saline solution with two silicone cannulas. tDCS devices were programmed before the session by a medical engineer.
The protocol to choose the size of the cap to keep adequate contact of electrodes with the scalp followed these procedures: (i). The researcher measured the head circumference to define the size of the cap: Small-cap if the head circumference was 38 cm × 55 cm, medium if the head circumference was 39 cm × 57.5 cm, and large if the head circumference was 40 cm × 59 cm. The cap has a Velcro strap attached to adjust the contact with the head. Volunteers put the cap on and checked if the size cap was adequate to keep maintaining electrodes in touch with the scalp. (ii) The participants put the cap on their heads, and the researcher measured to localize the positions of the electrodes according to the 10–20 system for EEG. (iii) The electrodes from 35cm2 were inserted on the sponges to deliver the current to the scalp. The same researcher applied the a-tDCS session at the Laboratory of Pain and Neuromodulation of Hospital de Clinicas de Porto Alegre, Brazil.
c. Rest-testing: Volunteers sat in a comfortable chair in the same room used to apply the hypnotic suggestion or tDCS. The room’s environment was the same as that used for other interventions. They needed to keep their eyes open, for 20 min, without performing any other task. However, the same research team member who applied the hypnotic analgesia suggestion or tDCS was in the room as a mute observer.
Primary and Secondary Outcomes: Definitions and Measures
The primary outcomes were the CPT and the MEP amplitude. The secondary outcomes were HPTh, SICI, ICF, and CSP.
Psychophysical Pain Measures
- In the Cold pressor test, the volunteer was asked to immerse her dominant hand in cold water (0° to 1°C) for up to 2 min. Volunteers could withdraw her hand at any time when the pain was intolerable.14 Cold pressor value was the total time in seconds during which the subject maintained her hand submerged in the cold water.
- HPTh was assessed by quantitative sensory testing (QST) using a standardized protocol. The measure applies the method limits with a computer Peltier-based device thermode (30X30mm) attached to the skin on the mid-forearm ventral aspect. The temperature thermode was set at 32 degree Celsius (°C), and it increased at a rate of 1oC/s to a maximum of 52oC. The HPTh of each patient was defined as the means of three assessments performed with an inter-stimulus interval of 40 s. The mean of three HPTh assessments was defined as the outcome.27
Neuropsychological Measures by Transcranial Magnetic Stimulation Parameters
The cortical excitability and integrity measures of the corticospinal tract were taken by magnetic stimulation parameters using the machine Neuro-MS/D (2800V, peak 133 magnetic field – up to 4T, Neurosoft, Ivanovo – Russia). Participants sat in a comfortable reclining chair and were informed about the TMS procedure and possible sensations they might experience. To identify the motor “hot spot”, the coil was placed over the left M1 at 45°angle to the sagittal line tangential to the scalp to stimulate the area of the cerebral cortex that represents the hand.28 We found an optimal spot by moving the coil in 0.5–1 cm increments on the scalp, starting from approximately 4 cm lateral and 1 cm anterior from the head’s vertex. By 10–20 system, this point corresponds approximately with the area that characterizes the primary motor cortex.29 Thus, this location was used as an origin, and pulses were applied 0.5 cm away in four cardinal directions using frameless stereotaxy (Brainsight: Rogue Research Inc., Montreal, Canada). We used, on average, three pulses at each test location. If no larger EMG resulted at these test sites, the origin was considered the optimal site. To ensure the proper placement of the coil during cortical excitability assessments, researchers marked the site with a soft-tipped pen. To reduce variability, the same researcher performed all TMS assessments. Measurements of TMS, such as amplitudes of single and paired-pulse, latency, and duration of cortical silent period (CSP) measurements, were recorded on a spreadsheet and later uploaded to an online database.
The surface electromyography was recorded by the EMG Neuro-MEP (4-channel amplifier NCS, EMG, and Multi-modality EP System). A pair of Ag-AgCl surface electrodes were placed on the right first dorsal interosseous (FDI) belly muscle and its corresponding tendon on the distal phalanx of the index finger. A neutral and circular electrode (ground electrode with cable GE-2, adult, 400 mm) was placed in the forearm, ipsilateral to the other electrodes, and linked to an EMG. The stimuli were applied using single or paired pulses during the resting state or contraction of the target muscle. Excitability measures were conducted immediately before and after interventions. They comprised single-pulse (RMT, MEP and CSP) and paired-pulse (ICF and SICI). The inter-stimulus interval (ISIs) of 2ms was inhibitory, and 12ms was facilitatory.30 However, the testing intensity of single pulse MEP was the same before and after each treatment session.
Other Instruments and Assessments
Clinical and Psychological Measurements: CSS Symptoms, Anxiety, Depressive Symptoms, Pain Score, and Analgesic Use
Two independent trained evaluators blinded to interventions whose subjects had been assigned conducted assessments of pain, psychological measures, and psychophysical tests. The State-Trait Anxiety Inventory (STAI) was used to assess anxiety.33 Beck Depression Inventory – Second Edition (BDI-II) was used to measure the severity of depressive symptoms, and sleep quality was assessed by Pittsburgh Sleep Quality Index (PSQI).34,35 The severity of symptoms related to central sensitization syndrome (CSS) was evaluated by the Central Sensitization Inventory (CSI).36 The Brazilian Portuguese Pain Catastrophizing Scale (BP-PCS) was used to assess catastrophizing related to pain.37 All scales used in this study have been validated for the Brazilian population.
We evaluated demographic data and medical comorbidities using a standardized questionnaire. We requested subjects to provide information about their age, sex, years of education, and lifestyle habits. Patients also provided information about their health status, including clinical and psychiatric diagnoses. A specific questionnaire evaluated all medications used and their daily doses (eg, antidepressants, anticonvulsants, hypnotics, non-opioid analgesics, opioids, etc.).
Sample Size
We used the G*Power software to estimate the sample size. We considered the average percent change from before to after intervention, either in CPT or in MEP.14 The data relating to MEP was obtained from a pilot study, with fibromyalgia using a-tDCS with a similar montage used in the present study (data not published). For the outcome CPT, the effect size (d) was 0.4 for a standard deviation equal to 4.44. For the MEP, the effect size (d) was 0.5 for a standard deviation equal to 0.6. To obtain the effect sizes “f”, the “d” values were transformed using the Platform https://www.psychometrica.de/effect_size.html. Thus, we estimate the sample size equal to 16 by a two-tailed hypothesis test considering three response variables, three groups in a crossover three-phases study for a type I error of 5% and a type II error of 20%. Since the initial estimation of sample size was based on data of studies with distinct characteristics from the current study, for example, healthy females, long-term tDCS treatment, and the possibility of dropouts; we decided to increase the sample by 12.5%. Thus, the final sample size was 18 individuals.
Randomization
The randomization to allocate each participant has been generated by a computer program (Randomlogue) in a ratio of 1:1 to receive the following interventions in an incomplete crossover manner: a-tDCS or HAS. Before the recruitment phase, two investigators not involved in the patient’s assessments made the randomization. They prepared the envelopes that contained the randomization number. These envelopes were sealed and numbered sequentially, and they were opened after the participant consented to participate in the trial, according to the numerical order registered outside. The engineer who opened the envelopes and programmed the devices was not involved with the clinical measures, subjects, or evaluators.
Blinding
We established the following strategies to control the possible bias of assessments and allocation: (i) Two trained independent evaluators performed the assessments, including transcranial magnetic stimulation parameters and psychophysical and pain measures. These evaluators were unaware of the intervention received in each study phase during the entire protocol. (ii) Two biomedical engineers (PRS and DPS) who were not involved in patients assessment prepared the a-tDCS device to provide active stimulation according to the randomization code. They saw the randomization code when they opened the envelopes according to a numerical order. (iii) The next step was for the medical engineers to communicate the subject’s name and the number of each envelope opened to the principal researcher to control the intervention sequence according to the randomization process.
Statistical Analyses
The values are presented as the mean (standard deviation) or frequency for descriptive statistics. To test continuous variables for normality, Shapiro–Wilks test was used. In the pre-specified analysis plan, endpoints related to cortical excitability (MEP, SICI, ICF and CSP) and psychophysical measures (CPT and HPTh) were analyzed using mixed-effects model repeated measures (MMRM), including sequence, period, treatment as fixed effect factors, and subject within the sequence and within-subject error as random factors. The difference between interventions (HAS, a-tDCS and rest testing) was tested using within-subject variability as the error term. We performed all analyses using two-tailed tests. In case of significant findings, post-hoc contrasts by the Bonferroni test were used to adjust for multiple comparisons. The standardized mean difference (SMD) was used to compare the changes in the outcome measures within groups from pre-intervention to post-intervention and the magnitude of differences in outcome measures between groups. The effect size (ES) based on the SMD was interpreted as follows: small, 0.20–0.4; moderate, 0.50–0.70; and large, 0.80 or higher. Spearman correlation (Rho) was used to evaluate the correlation between the hypnotic susceptibility score on WSGC with CPT and the MEP size. We accepted a type I error of 5%. To perform the analyses, we used the software SPSS version 22.0 (SPSS, Chicago, IL, United States).
Results
Demographics and Clinical Characteristics
A total of 49 subjects were recruited. Eighteen did not get the cutoff point (8/12) in the WSGC scores, which were used to define high susceptibility to hypnosis. Thirteen patients did not find inclusion criteria for the following reasons: contraindications to TMS, neurologic disorders, left hand, uncontrolled psychiatric disorders, covid-19 infection, or pandemic restrictions. In the end, we included 18 patients in the study. Socio-demographic and clinical characteristics are presented in Table 1.
 |
Table 1 Epidemiological and Clinical Characteristics of the Sample at Baseline, Values are Given as the Mean (SD) or Frequency (%) (n=18) |
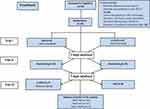
The allocation was in a crossover manner. The flow of patients through each stage of the study is presented in Figure 2. This means that the allocation in a crossover manner in the first trial was HAS (n=9) and a-tDCS (n=9), respectively. In the second trial, 16 received rest-testing. In the third trial (n=8) HAS and (n=7) a-tDCS, respectively. The experimental design and interventions in each phase are presented in Figure 2.
Generalized Mixed Model to Analyze Sequence, the Effect of Group, Time, and Interaction Time vs Group in the Primary Outcomes
GLMMs did not reveal a significant carry-over effect on the primary outcomes across the study periods. Thus, averages from three periods of each intervention group were combined and presented in Table 2.
HAS produced a considerable effect in the tolerance during the CPT from pre- to post-intervention, with a large ES Cohen’s f=0.96; (mean difference) 16.17/16.84 (pooled standard deviation); CI 95% 0.90 to 1.89. HAS produced a significant increase in the CPT with a statistically significant effect of group (F=3.84, P=0.026) and an interaction between group and time (F=4.982=3, P=0.010). HAS increased the pain tolerance compared to a-tDCS with a moderate ES [Cohen’s f=−0.70; (CI 95%; −1.65 to −0.25)]. At the same time, HAS produced a significant increase in the CPT compared to rest-testing, with a moderate ES [Cohen’s f=−0.87; (CI 95%; −1.84 to −0.09)].
The a-tDCS produced a considerable effect in the MEP, from pre- to post-intervention, with a large ES Cohen’s f=−1.02; (mean difference) 0.40/0.39 (pooled standard deviation); CI 95% −1.51 to −0.66. The a-tDCS increased the cortical excitability indexed by MEP size with a statistically significant effect on the group (F=3.26, P=0.004) and an interaction between time and group [Time vs group (F=5.74, P=0.005)]. The ES when subjects received a-tDCS compared to HAS in the increase in MEP amplitude was large [Cohen’s f=−1.73 (CI 95%; −2.17 to −0.17)]. Likewise, the ES produced by a-tDCS compared to rest-testing in the MEP size was large [Cohen’s f=−1.03; (CI 95%; −2.06 to −0.08)].
Figure 3 presents the CPT presented mean pre-intervention and post-intervention (primary outcomes) according to intervention groups. The means were compared using GLMM, and post hoc adjusted for multiple comparisons using Bonferroni correction (the model is presented in Table 2). HAS increased the CPT with difference statistically significant compared to a-tDCS and rest-testing.
Figures 4 presents MEP amplitude presented as mean pre-intervention and post-intervention according to intervention groups. The means were compared using GLMM and post hoc adjusted for multiple comparisons using Bonferroni correction (the model is presented in Table 2). a-tDCS increased the MEP with difference statistically significant compared to HAS and rest-testing.
GLMM to Analyze Sequence, the Effect of Group, Time, and Interaction Time vs Group in the Secondary Outcomes
GLMMs did not reveal a significant carryover effect on the secondary outcomes across the study periods. Thus, each intervention group’s average from three periods was combined and presented in Table 3. The a-tDCS reduced the SICI considerably from pre- to post-intervention, with a moderate ES [Cohen’s f=−0.65; (CI 95%; −0.93 to −0.43)].
The a-tDCS reduced the SICI with a statistically significant effect of intervention group (=5.39, P=0.007), and an interaction between group and time (F=3.38, P=0.024). The ES when subjects received a-tDCS compared to HAS was moderate [Cohen’s f=−0.79; (CI 95%; −1.49 to 0.12)]. Likewise, a-tDCS compared to rest-testing reduced the SICI with a large ES [Cohen’s f=−1.04; (CI 95%; −1.76 to −0.36)].
Secondary Analysis: The Relationship Between Hypnotic Susceptibility and the Corticospinal Excitability Pathways and Pain Tolerance
The Spearman correlation (Rho) between the hypnotic suggestion susceptibility and MEP or CPT and according to interventions is presented in Table 4. The hypnotic susceptibility score on WSGC showed a low positive correlation with the change on CPT independently of intervention. While the scores on WSGC showed a moderate positive non-parametric correlation with change in CPT and MEP size (change from pre-intervention to post-intervention). Such non-parametric correlation means that patients that presented higher hypnotic susceptibility showed a higher response in the measure of corticospinal excitability indexed on MEP size by a-tDCS. However, we did not find a statistically significant relationship between the hypnotic susceptibility and either effect of rest testing or HAS.
Discussion
HAS increased CPT, indicating higher tolerance to nociceptive stimulus than a-tDCS and rest-testing. In contrast, the a-TDCS increased the MEP size and decreased the SICI. So, novelty of these results is to extend the literature to distinguish the effect of HAS from a-tDCS in neuroplasticity markers indexed by cortical excitability measures. These results are relevant to comprehending how these therapeutic approaches might change the maladaptive neuroplasticity of neural networks involved in chronic pain. Also, they may be clinically relevant to creating a roadmap for customizing treatment in FM based on an individual’s characteristics, such as, for example, in the corticospinal excitability measure. In addition, an exploratory analysis showed that patients with higher hypnotic susceptibility are prone to present a higher impact of a-tDCS on MEP and SICI.
HAS increased pain tolerance. However, this effect on pain perception did not associate with cortical excitability measure changes. To date, we do not have a clear explanation for the underpinning mechanisms of HAS on pain tolerance, but this finding is congruent with previous studies on experimental pain in healthy subjects.14,38 It also agrees with the results of our group’s earlier factorial clinical trial, which compared the effect between HAS and tDCS in healthy women, where HAS alone increased CPT compared to a-tDCS applied over left-DLPFC. It is plausible to hypothesize that the hypnotic suggestion shifted attention and altered the brain processing of pain. This hypothesis agrees with an earlier study, which observed that the hypnotic suggestion turned into attentional focus.39 According to a neuroimaging study, this effect of hypnotic suggestion involves the anterior cingulate and insular cortex, which are areas that modulate pain perception at the supraspinal level.40
Although the underlying mechanism is unclear, the effect on CPT is congruent with findings from a meta-analysis of studies on experimental pain, which revealed that HAS could increase pain tolerance and overall pain intensity.38 In fibromyalgia, another meta-analysis showed the effect of hypnosis/guided imagery in reducing pain levels and other symptoms such as fatigue and sleep disturbances.41 Thus, the impact of hypnosis on pain tolerance might be explained by its effect on the affective-motivational components of pain. Likewise, its benefits on other symptoms such as catastrophizing, sleep quality and depressive symptoms.42 According to a meta-analysis of 85 experimental pain studies, hypnosis produced a moderate to a large ES on all pain outcomes.38 Besides, neuroimaging studies found that hypnosis changed activity in supraspinal areas responsible for the processing of pain. Such sites included the thalamus, somatosensory cortex, insula, anterior cingulate cortex, and prefrontal area.40
The present study shows that a-tDCS has a critical role in the corticospinal pathway, as demonstrated by the increase in MEP amplitude while decreasing the SICI. The MEP amplitude slope indicates the excitability of the primary M1 to be unregulated and the strength of the corticospinal connections.5 In this way, larger amplitudes in the MEP indicate higher excitability of the motor cortex, as well as higher transmission efficiency of corticospinal neurons, with up-regulation of the descending pain modulatory system.43 Although the mechanisms underlying these findings are unclear, this is consistent with previous studies in humans with the therapeutic use of TMS.44 Hence, these findings suggest that the a-tDCS effect was not limited to the targeted cortical area but also to distant interconnected sites, including a contra-regulatory effect in the dysfunctional processing of the corticospinal pathways. Although the relationship of MEP measure in the physiopathology of fibromyalgia is still not understood, the increase in MEP size immediately after a-tDCS over M1 was already observed in various studies and reported in a recent systematic review.45 In summary, the increase in the MEP amplitude is a surrogate marker that reflects the latency of depolarization of the spinal motor neuron pool and the integrity and function of conduction along the efferent pathway. The change in its amplitude after a-tDCS suggests the potential impact of this intervention on the DPMS.
The a-tDCS effect on HPTh did not get a statistically significant difference. The literature related to a-tDCS on HPTh is mixed. In an earlier study conducted in our laboratory in patients with fibromyalgia, we found that HPTh increased with a-tDCS on left DLPFC with cathodal on supraorbital contralateral area combined with an inhibitory task.18 In contrast, another study in fibromyalgia with the same montage used in the current study with a-tDCS applied for 3 months (sixty sessions) did not increase the HPTh.15 The controversy related to the a-tDCS application on DLPFC on the analgesic effects has also been indicated in recent meta-analyses.6,16 Indeed, this issue is in debate, and an argument to explain the discrepancy is that a-tDCS on DLPFC could be more prominent to reduce fatigue and improve emotional and cognitive symptoms, while pain relief of fibromyalgia and its effect on pain perception is more present with the application of tDCS in M1.6,16 From a theoretical perspective, DLPFC stimulation is mainly related to the modulation of the limbic system and the anterior cingulate cortex, which are responsible for the affective, motivational component of pain, including beneficial effects of up-regulating mechanisms underlying maladaptive emotions involved in the regulation of mood and cognitive functions (eg, decision-making).16 Although the mechanism to explain the effect of the DLPFC stimulation on pain is not clear, according to neuroimage with fMRI, a-tDCS on DLPFC effects activates downstream circuits, to the anterior insula, hypothalamus, periaqueductal gray substance, nucleus accumbens, and rostroventral medulla.46
As for the interpretation of HPTh results, we should realize that it is a measurement based on the subject’s responses. This way, we need to consider the intrinsic properties of these measures, which in the case of QST identify the functional deficit of small nerve fibers. Hence, the variability among studies can be explained by a reduced sensibility of these fibers to detect HPTh, which might vary among patients with fibromyalgia. This argument is supported by an earlier study, in which we found that peripheral sensory dysfunction is associated with the disengagement of DPMS in fibromyalgia.26 Therefore, it is possible that at least part of this discrepancy involving the HPTh assessed by the QST might be related to intrinsic properties of the test as mentioned. Additionally, we cannot discard an error type II that can explain this discrepancy between the pain threshold measures of the current study with the literature.
Some concerns about the design of our study must be addressed. First, although the effect of only one neuromodulatory therapy session was evaluated, it is essential to realize that this study aimed to understand the acute impact of one session of a-tDCS and HAS in cortical processing. So, to understand how the effect of these neuromodulatory techniques could improve the excitatory/inhibitory balance in the corticospinal way. Hence, these findings might support the planning of further studies to treat fibromyalgia, such as to comprehend what technique and if it is possible to have an additive effect when we combine strategies to induce top-down modulatory effects. Second, we did not find significant differences between groups in ICF and CSP measures. However, in the a-tDCS, the duration of the CSP pre- to post-intervention was shorter, a result linked to a reduction of intracortical inhibition mediated by GABAB receptors.47 In contrast, the ICF reflects the activity within glutamatergic circuits or a loss of GABAA modulation.48 Although the ICF did not get a variation with a statistically significant difference, the changes in their numerical values from pre- to post- a-tDCS are pointing to increased excitability. So, parsimony is needed in their interpretation because they represent an acute effect after one a-tDCS session in a small sample. Thereby, an error type-II cannot be excluded. Third, the crossover design in a small study population helped us to prevent overestimation of the benefits of the therapy being tested, making it likely that our results reflect a conservative assessment of the benefits to compare the effects of a-tDCS and the HAS to improve the excitability of the motor cortex and pain processing.49 Fourth, the strength of this design is that the interventions under investigation were evaluated within the same patients and thus eliminated between-subject variability.50 Given that patients act as their own controls, the analyses could be based on paired data (using paired tests). The number of patients who completed the three trials provided sufficient power (80%) to reach statistical significance (P<0.05), despite 16.66% (3/18) of patients dropping out after the first or second phases and thus not participating in the subsequent phase. Fifth, we included the resting test in the intermediate phase to increase the washout period. In this case, the resting test occurred at a specific point for all subjects during the protocol. In contrast, the HAS or tDCS were allocated randomly to mitigate possible bias in the therapeutic effects of these interventions. A potential argument to support the validity of these results is that we did not find a carryover effect or on the intervention’s sequence of application. We recognize that a limitation of the present study was to pre-assuming that the rest testing would not influence our outcomes. Another critical point of the experimental design is that we only assessed the a-tDCS effect on cortical excitability. One should consider this limitation in interpreting results because the literature about the tDCS effect immediately after in healthy subjects is mixed about significant differences in the cortical excitability measures between active and sham-tDCS. Although we cannot exclude the importance of this limitation in the study design, we balanced the potential impact of an increase in dropouts related to many assessments to the detriment of including an additional s-tDCS group. This argument finds support in earlier studies, which demonstrated that high level of psychological stress, the complexity of treatment regimens (eg, frequency of dosing), comorbid conditions, stressful events such as was the pandemic scenario, and fatigue would be related to less adherence.51 Specifically, we consider that a physical and emotional burden determined by many evaluations in a pandemic scenario could increase the dropout rate. These aspects are particularly relevant to a sample such as fibromyalgia subjects, who live with intense physical and emotional suffering determined by their disease. Although we realize that these are weaknesses in our study that need to be considered in interpreting these results and exploring them in future studies, we believe that our findings are reliable for comprehending differences in the neurophysiological process underlying the effects of a-tDCs compared to HAS. Sixth, we included only females, and we know that it is a limitation to extend them to other populations. We used this approach to reduce potential bias related to sex differences in the function of DPMS and motor cortex excitability according to sex. In this way, a higher amplitude in the MEP was positively correlated with the higher efficiency of DPMS.43 In addition, we observed that the level of hypnotizability might be a valuable index to predict individual response to a-tDCS. However, we know that these results are helpful for women with high hypnotic susceptibility. Finally, these findings extend the literature to comprehend the distinct neurobiological processes related to HAS and a-tDCS. However, they do not support therapeutic decision-making in a clinical setting. In conclusion, these findings revealed that HAS affects contra-regulating mechanisms involved in perception and pain tolerance, while the a-tDCS increased the excitability of the corticospinal pathways. This opens an avenue for customizing therapeutic approaches to counter regulating the maladaptive neuroplasticity involved in fibromyalgia.
Data Sharing Statement
After acceptance, the corresponding author accepts to submit the datasets underlying the results of this paper to the editorial office.
Acknowledgments
This manuscript was based on the author’s master dissertation intitulated “Effects of transcranial direct current stimulation and hypnotic suggestion in tolerance to pain and cortical excitability in fibromyalgia: a cross-over randomized clinical trial”. Reference: Corrêa BSC. Efeitos da estimulação transcraniana de corrente contínua e sugestão hipnótica na tolerância à dor e excitabilidade cortical na fibromialgia: um ensaio clínico randomizado cruzado. LUME UFRGS. 2022. http://hdl.handle.net/10183/238843. The authors would like to acknowledge Fundação de incentivo à Pesquisa (FIPE) at Hospital de Clínicas de Porto Alegre.
Funding
The present research was supported by the following Brazilian funding agencies: (i) Committee for the Development of Higher Education Personnel – CAPES PROEX (grant to BS with master scholarship, Grant #2020). (ii) National Council for Scientific and Technological Development – CNPq (grant to WC number: 420826/2018-1). (iii) Foundation for the Support of Research at Rio Grande do Sul (FAPERGS) Ministry of Science and Technology. National Council for Scientific and Technological Development – (CNPq)/ Health Secretary of state of Rio Grande do Sul, Brazil (SEARS) n. 03/2017 (PPSUS) (number: 17/2551-0001). (iv) Brazilian Innovation Agency (FINEP [Financiadora de Estudos e Projetos]) (process number 1245/13). (v) FINEP grant 0261/18 chamada pública MCTIC/FINEP/CT-INFRA 04/2018. (vi) Fundação de incentivo à Pesquisa (FIPE) at Hospital de Clínicas de Porto Alegre.
Disclosure
Dr Paulo RS Sanches reports a patent BR2020150164500 with royalties paid to Quark Medical. Dr Felipe Fegni reports grants from NIH and personal fees from Neurive, outside the submitted work. The authors declare no other conflicts of interest in this work.
References
1. Sarzi-Puttini P, Giorgi V, Marotto D, Atzeni F. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol. 2020;16(11):645–660. doi:10.1038/s41584-020-00506-w
2. Seminowicz DA, Moayedi M. The dorsolateral prefrontal cortex in acute and chronic pain. J Pain. 2017;18(9):1027–1035. doi:10.1016/j.jpain.2017.03.008
3. Cardinal TM, Antunes LC, Brietzke AP, et al. Differential neuroplastic changes in fibromyalgia and depression indexed by up-regulation of motor cortex inhibition and disinhibition of the descending pain system: an exploratory study. Front Hum Neurosci. 2019;13:138. doi:10.3389/fnhum.2019.00138
4. Passard A, Attal N, Benadhira R, et al. Effects of unilateral repetitive transcranial magnetic stimulation of the motor cortex on chronic widespread pain in fibromyalgia. Brain. 2007;130(Pt 10):2661–2670. doi:10.1093/brain/awm189
5. Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2(3):145–156. doi:10.1016/S1474-4422(03)00321-1
6. Zortea M, Ramalho L, Alves RL, et al. Transcranial direct current stimulation to improve the dysfunction of descending pain modulatory system related to opioids in chronic non-cancer pain: an integrative review of neurobiology and meta-analysis. Front Neurosci. 2019;13:1218. doi:10.3389/fnins.2019.01218
7. Donadel DG, Zortea M, Torres ILS, Fregni F, Caumo W. The mapping of cortical activation by near-infrared spectroscopy might be a biomarker related to the severity of fibromyalgia symptoms [published correction appears in Sci Rep. 2021 Oct 22;11(1):21257]. Sci Rep. 2021;11(1):15754. doi:10.1038/s41598-021-94456-2
8. Valdés M, Collado A, Bargalló N, et al. Increased glutamate/glutamine compounds in the brains of patients with fibromyalgia: a magnetic resonance spectroscopy study. Arthritis Rheum. 2010;62(6):1829–1836. doi:10.1002/art.27430
9. Wurtman RJ. Fibromyalgia and the complex regional pain syndrome: similarities in pathophysiology and treatment. Metabolism. 2010;59(Suppl 1):S37–S40. doi:10.1016/j.metabol.2010.07.008
10. Araya-Quintanilla F, Gutiérrez-Espinoza H, Muñoz-Yánez MJ, Cavero-Redondo I, Álvarez-Bueno C, Martinez-Vizcaíno V. Effectiveness of a multicomponent treatment versus conventional treatment in patients with fibromyalgia: study protocol. Medicine. 2020;99(4):e18833. doi:10.1097/MD.0000000000018833
11. Jensen MP, Patterson DR. Hypnotic approaches for chronic pain management: clinical implications of recent research findings. Am Psychol. 2014;69(2):167–177. doi:10.1037/a0035644
12. Kihlstrom JF. Hypnosis: applications. In: Reference Module in Neuroscience and Biobehavioral Psychology. Elsevier; 2018. doi:10.1016/B978-0-12-809324-5.21772-0
13. Castel A, Cascón R, Padrol A, Sala J, Rull M. Multicomponent cognitive-behavioral group therapy with hypnosis for the treatment of fibromyalgia: long-term outcome. J Pain. 2012;13(3):255–265. doi:10.1016/j.jpain.2011.11.005
14. Beltran Serrano G, Rodrigues LP, Schein B, et al. Comparison of hypnotic suggestion and transcranial direct-current stimulation effects on pain perception and the descending pain modulating system: a crossover randomized clinical trial. Front Neurosci. 2019;13:662. doi:10.3389/fnins.2019.00662
15. Brietzke AP, Zortea M, Carvalho F, et al. Large treatment effect with extended home-based transcranial direct current stimulation over dorsolateral prefrontal cortex in fibromyalgia: a proof of concept sham-randomized clinical study. J Pain. 2020;21(1–2):212–224. doi:10.1016/j.jpain.2019.06.013
16. Conde-Antón Á, Hernando-Garijo I, Jiménez-Del-Barrio S, Mingo-Gómez MT, Medrano-de-la-Fuente R, Ceballos-Laita L. Effects of transcranial direct current stimulation and transcranial magnetic stimulation in patients with fibromyalgia. A systematic review [published online ahead of print, 2020 Oct 15]. Efectos de la estimulación transcraneal por corriente directa y de la estimulación magnética transcraneal en pacientes con fibromialgia. Revisión sistemática [published online ahead of print, 2020 Oct 15]. Neurologia. 2020;S0213-4853(20)30278–4. doi:10.1016/j.nrl.2020.07.024
17. da Graca-Tarragó M, Lech M, Angoleri LDM, et al. Intramuscular electrical stimulus potentiates motor cortex modulation effects on pain and descending inhibitory systems in knee osteoarthritis: a randomized, factorial, sham-controlled study. J Pain Res. 2019;12:209–221. doi:10.2147/JPR.S181019
18. Silva AF, Zortea M, Carvalho S, et al. Anodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex modulates attention and pain in fibromyalgia: randomized clinical trial. Sci Rep. 2017;7(1):135. doi:10.1038/s41598-017-00185-w
19. Sedgwick P, Greenwood N. Understanding the Hawthorne effect. BMJ. 2015;351:h4672. doi:10.1136/bmj.h4672
20. Sherman KJ. Guidelines for developing yoga interventions for randomized trials. Evid Based Complement Alternat Med. 2012;2012:143271. doi:10.1155/2012/143271
21. Beltran Serrano G, Pooch Rodrigues L, Schein B, et al. The Hypnotic analgesia suggestion mitigated the effect of the transcranial direct current stimulation on the descending pain modulatory system: a proof of concept study. J Pain Res. 2020;13:2297–2311. doi:10.2147/JPR.S253747
22. Wolfe F, Clauw DJ, Fitzcharles MA, et al. 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46(3):319–329. doi:10.1016/j.semarthrit.2016.08.012
23. Carvalho C, Mazzoni G, Kirsch I, Leal I. Presentation of the Portuguese version of a hypnotic susceptibility assessment scale. Psychol Health Disord. 2006;7(1):3–11.
24. Russo C, Souza Carneiro MI, Bolognini N, Fregni F. Safety review of transcranial direct current stimulation in stroke. Neuromodulation. 2017;20(3):215–222. doi:10.1111/ner.12574
25. Patterson DR, Jensen MP. Hypnosis and clinical pain. Psychol Bull. 2003;129(4):495–521. doi:10.1037/0033-2909.129.4.495
26. Brietzke AP, Antunes LC, Carvalho F, et al. Potency of descending pain modulatory system is linked with peripheral sensory dysfunction in fibromyalgia: an exploratory study. Medicine. 2019;98(3):e13477. doi:10.1097/MD.0000000000013477
27. Schestatsky P, Stefani LC, Sanches PR, et al. Validation of a Brazilian quantitative sensory testing (QST) device for the diagnosis of small fiber neuropathies. Arq Neuropsiquiatr. 2011;69(6):943–948. doi:10.1590/s0004-282x2011000700019
28. van de Ruit M, Perenboom MJ, Grey MJ. TMS brain mapping in less than two minutes. Brain Stimul. 2015;8(2):231–239. doi:10.1016/j.brs.2014.10.020
29. Silva LM, Silva KMS, Lira-Bandeira WG, Costa-Ribeiro AC, Araújo-Neto SA. Localizing the primary motor cortex of the hand by the 10-5 and 10-20 systems for neurostimulation: an MRI study. Clin EEG Neurosci. 2021;52(6):427–435. doi:10.1177/1550059420934590
30. Di Lazzaro V, Restuccia D, Oliviero A, et al. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119(2):265–268. doi:10.1007/s002210050341
31. Chen M, Lixandrão MC, Prudente CN, Summers RLS, Kimberley TJ. Short interval intracortical inhibition responses to low-frequency repetitive transcranial magnetic stimulation under multiple interstimulus intervals and conditioning intensities. Neuromodulation. 2018;21(4):368–375. doi:10.1111/ner.12773
32. Valls-Solé J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85(6):355–364. doi:10.1016/0168-5597(92)90048-g
33. Fioravanti-Bastos ACM, Cheniaux E, Landeira-Fernandez J. Development and validation of a short-form version of the Brazilian State-Trait Anxiety Inventory. Psychology. 2011;24:485–494.
34. Gomes-Oliveira MH, Gorenstein C, Lotufo Neto F, Andrade LH, Wang YP. Validation of the Brazilian Portuguese version of the beck depression inventory-II in a community sample. Braz J Psychiatry. 2012;34(4):389–394. doi:10.1016/j.rbp.2012.03.005
35. Bertolazi AN, Fagondes SC, Hoff LS, et al. Validation of the Brazilian Portuguese version of the Pittsburgh Sleep Quality Index. Sleep Med. 2011;12(1):70–75. doi:10.1016/j.sleep.2010.04.020
36. Caumo W, Antunes LC, Elkfury JL, et al. The central sensitization inventory validated and adapted for a Brazilian population: psychometric properties and its relationship with brain-derived neurotrophic factor. J Pain Res. 2017;10:2109–2122. doi:10.2147/JPR.S131479
37. Sehn F, Chachamovich E, Vidor LP, et al. Cross-cultural adaptation and validation of the Brazilian Portuguese version of the pain catastrophizing scale. Pain Med. 2012;13(11):1425–1435. doi:10.1111/j.1526-4637.2012.01492.x
38. Thompson T, Terhune DB, Oram C, et al. The effectiveness of hypnosis for pain relief: a systematic review and meta-analysis of 85 controlled experimental trials. Neurosci Biobehav Rev. 2019;99:298–310. doi:10.1016/j.neubiorev.2019.02.013
39. Jamieson GA, Sheehan PW. A critical evaluation of the relationship between sustained attentional abilities and hypnotic susceptibility. Contemp Hypn. 2002;19(2):62–74. doi:10.1002/ch.243
40. Vanhaudenhuyse A, Laureys S, Faymonville ME. Neurophysiology of hypnosis. Neurophysiol Clin. 2014;44(4):343–353. doi:10.1016/j.neucli.2013.09.006
41. Zech N, Hansen E, Bernardy K, Häuser W. Efficacy, acceptability and safety of guided imagery/hypnosis in fibromyalgia - a systematic review and meta-analysis of randomized controlled trials. Eur J Pain. 2017;21(2):217–227. doi:10.1002/ejp.933
42. Caumo W, Alves RL, Vicuña P, et al. Impact of bifrontal home-based transcranial direct current stimulation in pain catastrophizing and disability due to pain in fibromyalgia: a randomized, double-blind sham-controlled study. J Pain. 2022;23(4):641–656. doi:10.1016/j.jpain.2021.11.002
43. Gasparin A, Zortea M, Dos Santos VS, et al. Brain-derived neurotrophic factor modulates the effect of sex on the descending pain modulatory system in healthy volunteers. Pain Med. 2020;21(10):2271–2279. doi:10.1093/pm/pnaa027
44. Maeda F, Gangitano M, Thall M, Pascual-Leone A. Inter- and intra-individual variability of paired-pulse curves with transcranial magnetic stimulation (TMS). Clin Neurophysiol. 2002;113(3):376–382. doi:10.1016/S1388-2457(02)00008-1
45. Biabani M, Aminitehrani M, Zoghi M, Farrell M, Egan G, Jaberzadeh S. The effects of transcranial direct current stimulation on short-interval intracortical inhibition and intracortical facilitation: a systematic review and meta-analysis. Rev Neurosci. 2018;29(1):99–114. doi:10.1515/revneuro-2017-0023
46. Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci. 2015;16(7):403–418. doi:10.1038/nrn3976
47. Poston B, Kukke SN, Paine RW, Francis S, Hallett M. Cortical silent period duration and its implications for surround inhibition of a hand muscle. Eur J Neurosci. 2012;36(7):2964–2971. doi:10.1111/j.1460-9568.2012.08212.x
48. Fedi M, Berkovic SF, Macdonell RA, Curatolo JM, Marini C, Reutens DC. Intracortical hyperexcitability in humans with a GABAA receptor mutation. Cereb Cortex. 2008;18(3):664–669. doi:10.1093/cercor/bhm100
49. Grizzle JE. The two-period change-over design and its use in clinical trials. Biometrics. 1965;21:467–480). PMID: 14338679.
50. Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133(2):144–153. doi:10.1093/oxfordjournals.aje.a115853
51. Cardenas-Rojas A, Castelo-Branco L, Pacheco-Barrios K, et al. Recruitment characteristics and non-adherence associated factors of fibromyalgia patients in a randomized clinical trial: a retrospective survival analysis. Contemp Clin Trials Commun. 2021;24:100860. doi:10.1016/j.conctc.2021.100860
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.