Back to Journals » OncoTargets and Therapy » Volume 13
Effects of Huaier Extract on Ameliorating Colitis-Associated Colorectal Tumorigenesis in Mice
Authors Zou Y, Rong Y, Chen Z, Shen Z , Chen X, Tan Y, Weng J , Huang X, Lin X
Received 12 March 2020
Accepted for publication 5 August 2020
Published 26 August 2020 Volume 2020:13 Pages 8691—8704
DOI https://doi.org/10.2147/OTT.S253598
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Yi-feng Zou,1,2,* Yu-ming Rong,3,* Ze-xian Chen,1,2,* Zhi-hong Shen,4 Xi Chen,1,2 Ying-xin Tan,1,2 Jing-rong Weng,1,2 Xiao-ming Huang,2,5 Xu-tao Lin2,6
1Department of Colorectal Surgery, The Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, People’s Republic of China; 2Guangdong Institute of Gastroenterology; Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, People’s Republic of China; 3Department of Very Important Person Region, Cancer Center of Sun Yat-sen University, Guangzhou, Guangdong, People’s Republic of China; 4Department of General Surgery, Jieyang Hospital of Traditional Chinese Medicine, Jieyang, Guangdong, People’s Republic of China; 5Department of Hepatobiliary Surgery, The Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, People’s Republic of China; 6Department of Gastrointestinal Endoscopy, Department of Colorectal Surgery, The Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xu-tao Lin; Xiao-ming Huang Email [email protected]; [email protected]
Background: Huaier extract has been a part of traditional Chinese medicine (TCM) for roughly 1600 years and may serve as a potential anti-cancer drug as it is associated with good efficacy and low toxicity. Individuals with inflammatory bowel disease (IBD) are at a higher chance of being diagnosed with colorectal cancer (CRC) and as Huaier extract may potentially influence tumorigenesis, we set out to determine the effect of Huaier extract on colitis-associated CRC.
Methods: The CRC mouse model, established through azoxymethane (AOM) and dextran sulfate sodium (DSS), was administered Huaier extract. Weight loss, colon length, tumor number and tumor size were evaluated macroscopically. Pro-inflammatory cytokine expression and STAT3 phosphorylation were assessed in the colon using ELISA, Western blot and/or immunohistochemistry.
Results: Huaier extract improved the severity of colitis-associated tumorigenesis compared with control group, with attenuated weight loss and longer colons. Tumor number, size and load were drastically decreased in mice treated with Huaier. Histological assessment suggested that Huaier could decrease histological injury of the colon tissue. Additionally, Huaier extract treatment led to reduced pro-inflammatory cytokine levels (TNF-α, IL-6, IFN-γ and IL-1β) and a decrease of STAT3 phosphorylation in colon tissue. Additionally, present findings demonstrated that Huaier extract inhibited cell proliferation and induced apoptosis in CRC cells HCT116 and HCT8. The migration and invasion of CRC cells were markedly inhibited upon exposure to Huaier treatment. The apoptosis-associated protein levels (P53, Bax, Bcl-2, pro-caspase-3 and cleavage caspase-3) showed significant differences after the administration of Huaier extract in HCT116 and HCT8 cells. In vivo, the administration of Huaier extract to mice inhibited tumor growth and yielded a similar profile of apoptotic proteins expression p53, Bcl-2, pro-caspase-3 and cleaved caspase-3 while no significant differences in Bax were observed. Moreover, the ratio of TUNEL-positive/apoptotic cells was markedly increased in the Huaier-treated mice.
Conclusion: Huaier extract may reduce the IBD-associated tumor development by suppressing pro-inflammatory cytokine levels and STAT3 stimulation.
Keywords: colorectal cancer, Huaier extract, colitis, traditional Chinese medicine
Introduction
Cancer is a prominent cause of death globally. Despite the fact that colitis-associated CRC is rare, it is the most severe complication among patients with inflammatory bowel diseases (IBD). IBD is a disorder that develops due to immune system dysregulation and autoimmunity. Crohn’s disease and ulcerative colitis, two major types of IBD, are characterized by an overactive immune response to intestinal flora. Colitis-associated CRC does not show a typical adenoma-carcinoma sequence. The excessive repair response and enhanced inflammatory in mouse models made the mice significantly more susceptible to colitis-associated CRC.1–4 In different animal models, the occurrence and development of colitis-associated CRC have been affected by bacteria, tumor necrosis factor (TNF) and interleukin (IL).5–7 Despite the fact that IBD animal models have delivered vital data about the function of inflammatory molecules and events that lead up to CRC formation, current treatments still remain to be improved.4,8 The key to improving CRC therapy is to explore drug candidates with good efficacy and low treatment toxicity.
Plant-derived natural medicines are thought to have lower toxicity and fewer adverse effects compared to synthetically produced medicines.9 Thus, interest in chemotherapeutics originating from natural sources has been rising. Trametes robiniophila murr (Huaier) is a sandy beige mushroom found on the trunks of trees and has been widely used in traditional Chinese medicine (TCM) for more than 1600 years. Huaier granules, the aqueous product of Huaier extract, are mainly composed of proteoglycans and have been approved for use in TCM by the Chinese State Food and Drug Administration (SFDA), for clinical treatment for various cancer types, including gastric and colon cancer.10–12 Study showed that the active constituents of Huaier granules is a neutral water-soluble polysaccharide with a molecular weight of 2.5×104 Da that was composed of galactose (Gal), arabinose (Ara) and glucose (Glc), with a relative molar ratio of 4.2:2.5:0.7 by Gas chromatography (GC).13 Previously published studies have validated the antitumor activity of Huaier extract in several cancers, including cancers of the breast, liver, gastrointestinal system, cervix, lungs and ovary.11,14-17 Therefore, Huaier extract could possibly serve as an alternative or complementary therapeutic drug with good efficacy and reduced toxicity against CRC. However, few studies have evaluated the antitumorigenic effect of Huaier in CRC and the mechanisms that underlie the inhibitory influence of Huaier continue to be elusive. In this study, we examined the influence of Huaier on CRC and explored potential antitumor mechanisms.
Materials and Methods
Huaier Aqueous Extract Preparation
The trametes robiniophila murr (Huaier) were extracted with 95% anhydrous ethanol at 100°C for 1.5 h to remove grease, and the residue was processed twice with boiling water for 3 h each time. The extract was centrifuged. And we took the supernatant and concentrate it 10 times. After that, the supernatant was precipitated with 95% anhydrous ethanol at 4°C overnight. Then, we centrifuged the sediment and suspended it in distilled water, and the protein was removed by the Sevage method. The obtained polysaccharide was thoroughly dialyzed with water for 2 days, and then the dialysate was precipitated with 95% absolute ethanol, washed with absolute ethanol, acetone and ether to get the crude polysaccharide. Then, 8 g of crude polysaccharides was dissolved in 100 mL of distilled water and transfer to a 2×40 cm DEAE-52 cellulose chromatography column. After eluting with distilled water once, the column was eluted again with five gradients of sodium chloride solution (0.2, 0.5, 0.8, 1.0 and 2.0 M). Then, Sepharose CL-6B and Sephadex G-100 gel-permeation chromatography was used to collect, dialyze, freeze-dry, and purify the neutral water-soluble polysaccharide, guided by phenol-sulfuric acid method. The water-soluble polysaccharide was prepared into 100mg/mL solution with distilled water, sterilized using a 0.22um filter, and then stored at −20°C for further use.
Animal Experiments and AOM/DSS Inflammation-Induced CRC Mode
All female mice (C57BL/6, 6–8 weeks, 18–20 g) were acquired through Laboratory Animal Center at Sun Yat-sen University in China. Animals remained in sterile housings with filter-topped cages. Mice were kept in an area that was 22±1°C, had humidity levels of 50–70%, and a 12-h light/dark cycle. The study was performed after the mice were allowed to acclimatize for 1 week. All mice received humane care, as directed by study protocols approved by the Animal Care Committee at the University and in compliance with the guidelines on animal welfare set by the National Committee for Animal Experiments. Protocol was granted approval by the ethics committee at the university. The mice were euthanized through cervical dislocation.
The CRC mouse model was established using AOM (Sigma-Aldrich, St. Louis, USA) and DSS (MP Biomedicals, Solon, USA) as previously described.18 In brief, mice were injected into the peritoneum with one 12 mg/kg dosage of AOM. Next, they were given 2% DSS dissolved in drinking water for 1 week, after which normal drinking water was given for 2 weeks over a course of 3 cycles. After injection with AOM, the mice were assigned, on random, into two groups (six mice per group): the control group and Huaier group. Huaier extract was given only to the Huaier group by gavage at a daily dose of 4g/kg, while normal saline was given to the control group at the same dose and time. Evaluation of AOM/DSS-treated animal was conducted every day to record information regarding overall appearance, food intake, weight, stool consistency and anal bleeding.
Enzyme-Linked Immunosorbent Assay (ELISA)
TNF-α, IL-6, IFN-γ and IL-1β expression in mouse sera were analyzed according to the manufacturer’s instructions by ELISA (eBiosciences, San Diego, CA, USA). Analysis of all samples was conducted three times. Absorbance was determined at 450 nm through the use of a microplate reader (iMark™, Bio-Rad, USA).
Cell Culture
Two kinds of human CRC cell lines (HCT116 and HCT8) were obtained from the Chinese Academy of Science Cell Bank (Shanghai, China) and cultured in DMEM medium containing 10% FBS, 100U/mL penicillin and 100 ug/mL streptomycin as well as 1% Non-Essential Amino Acids and 1% L-Gu at 37°C in a moist incubator (Thermo Fisher Scientific, Inc, Waltman, MA, USA) under 5% CO2. The medium was replaced regularly every 2–3 days. The cells were digested with 0.25% trypsin for 2–3 min at before the digestion process was ended by DMEM medium with 2% fetal bovine serum (FBS). The cells were placed in plastic petri dishes (Nest, Shanghai, China) and cultured in complete medium at a density of 1x106 cells/mL.
MTS Assays
One hundred and fifty μL of cell suspension was added into each well in a 96-well plate. Each well contained 5000 cells. Following 24 h of incubation, the cells were treated with 0, 1, 2, 4, 8 or 10 mg/mL Huaier extract for 24, 48 and 72 h in a quintuplicate. Following treatment, 20μL of MTS solution was added followed by incubation for an additional 3 h. A microplate reader (Bio-Rad, Hercules, CA, USA) was used to detect the absorbance of the solution at 490 nm.
Colony Forming Assays
Colony-forming assays were performed to explore the ability of cell proliferation. Briefly, 500 cells were planted in each well in six-well plates. Cell culture mediums containing either 0, 4 and 8 μg/mL Huaier extract were replaced every 3 days. After 10 days, the cellular colonies were stained with crystal violet and analyzed with a scanner (Canon, China).
Invasion Assays and Migration Assays
Transwell chambers coated with 50uL Matrigel (BD Bioscience, Oxford, UK) were used to determine the in vitro invasion abilities. Briefly, 50uL Matrigel was used to coat the chambers and left to incubate for 4 h. Two hundred uL of cell medium containing 4×104cells per chamber was incubated for 36 h at 37°C,5 % CO2 and 95% humidity.
Huaier extract (4, 8mg/mL) was added to the upper chambers of the Huaier-treated group. Paraformaldehyde and crystal violet were used to fix and stain the cells, respectively. For analysis, the white light microscope (Olympus, BX53) was used and the total number of cells that invaded to the bottom chamber was quantified manually by a random selection of four random fields per chamber under × 400 magnifications. The process of migration assays was similar to the above invasion assays, except that no Matrigel was added to the Transwell chambers.
Scratch Assays
2.5×105 cells were seeded in 6-well plates and grown in cell medium until a confluent monolayer was achieved. A p20 pipette tip was used to generate a linear wound and the cell was then incubated with DMEM medium containing 0, 4 and 8 mg/mL Huaier extract and 2%FBS. The fluorescence inversion microscope system (Leica, USA) was used to take photographs at 0, 24 and 48 h, respectively. The percentage of wound closure (%) was calculated as migrated cell surface area/total surface area ×100%.
Flow Cytometry for Apoptosis Identification
PI-annexin-V double-staining assays were performed to detect cell apoptosis. Briefly, we resuspended 1x105 cells in 100 ul binding buffer and then added 5ul Annexin V-FITC and 10 ul propidium iodide (PI) in the solution. Then, the above solution was incubated in a dark for 15 min at room temperature. Following the incubation, another 400ul of binding buffer was added before FACScan flow cytometry was performed.
Mouse Xenograft Tumor Models and TUNEL Assays
Our animal experiments were approved by the Ethics Committee of the Institute of Sun Yat-sen University. Ten male nude BALB/c mice (4 weeks old) were obtained from the Experimental Animal Center of Sun Yat-sen University, where we conducted animal experiments. 1x106 HCT116 cells were subcutaneously injected to generate tumors. They were randomly assigned to two groups based on the tumor volume which was assessed after the tumors were palpable. Huaier extract was administrated daily to one group by gavage at a concentration of 4g/kg. The tumor size and the weight of the mice were measured every 3 days and the tumor volume was calculated using πls2/6, where l and s represented the long side and short side, respectively. The apoptotic index was measured by TUNEL assay according to the operating manual. The mice were euthanized through cervical dislocation without any anesthetics.
Western Blotting
Protein was isolated by utilizing RIPA buffer that contained a concoction of protease and phosphatase inhibitors. Subsequent to identifying the concentration of protein through a BCA experiment read a microplate spectrophotometer (Thermo, Massachusetts, USA), protein was ran on a SDS-PAGE, and moved onto nitrocellulose membranes (Millipore Corp, MA, USA) through a Trans-Blot System (Bio-Rad, CA, USA). Then, membrane was placed in blocking buffer and incubated for at least 12 h with primary antibody directed against STAT3 (1:1000, CST, Boston, USA), p-STAT3 (Tyr 705, 1:1000, CST, Boston, USA), p53 (1:1000, Abcam, Cambridge, UK), Bcl-2 (1:1000, Abcam, Cambridge, UK), Bax (1:1000, Abcam, Cambridge, UK) and GAPDH (1:5000, Sigma Aldrich). The membranes were placed overnight with secondary antibodies (1:5000, IRDye Goat IgG, LI-COR Bioscience NE, USA) at room temperature for 1 h. Bands were observed by utilizing the Odyssey Imaging System (LI-COR Biosciences, NE USA).
Histological and Immunohistochemical Analysis
The mouse tumors were extracted, embedded in paraffin and divided into sections (4 μm). Hematoxylin and eosin (H&E) were conducted to determine the tissues’ histopathologic state and validate the presence of dysplasia. Expression of p-STAT3 (Tyr705, 1:200, CST, Boston, USA) was visualized using immunohistochemical staining. Paraffin was removed from the slides using dimethylbenzene and rehydrated using graded alcohols. The antigen was recovered by incubating the slide in sodium citrate buffer. Endogenous peroxidase was inhibited through hydrogen peroxide and treated overnight using antibody against p-STAT3 (Tyr 705, 1:200, CST, Boston, USA) at 4°C. Finally, slides marked using diaminobenzidine in an Envision System (Dako, Denmark) were further stained with hematoxylin.
Histopathological Evaluation
Four micron-thick formalin-fixed paraffin-embedded tissue sections were stained with hematoxylin and eosin (HE) to assess the severity of inflammation. Colitis was scored using a blind method, that is, a comprehensive score of tissue damage (0–3 points) and inflammatory cell infiltration (0–3 points).19 In short, for tissue damage, normal colonic mucosa was scored 0; discrete lymphoepithelial lesions were scored 1; surface mucosal erosions or focal ulcers were scored 2; extensive mucosal damage extends deep was 3 points. For inflammatory cell infiltration, occasional inflammatory cells in the lamina propria were scored 0; increased lamina propria inflammatory cells were scored 1; inflammatory cells extending to the submucosa were scored 2; infiltration transmural expansion was scored 3. The histological score was the sum of the above two parameters (from 0 to 6).
Statistical Analyses
SPSS 22.0 was utilized for all the statistical analyses. Student’s t-test helped assess statistical significance. P value <0.05 represents statistical significance.
Results
Huaier Extract Inhibited Malignant Transformation of Colitis
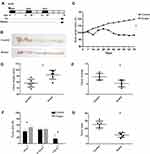
To investigate the function of Huaier extract in CRC, we used Huaier extract on the AOM/DSS experimental inflammation-stimulated CRC model (Figure 1A and B). After 70 days of post-AOM injection, the mice were euthanized, and their colon length and tumors formation were determined. All mice were alive during the experimental period, but went on to develop obvious clinical signs including weight loss and bloody diarrhea. The present study showed that weight reduction was drastically more significant in the controls (p < 0.001, Figure 1C). Colon swelling was seen across both cohorts that received AOM/DSS treatment. However, colons in the Huaier extract treatment group were substantially longer compared to the controls (92.17 ± 3.01 mm vs 78.33 ± 2.59 mm, p = 0.006, Figure 1D). The amount of tumors in mice administered Huaier extract was considerably decreased in comparison to the controls (5.33 ± 0.67 vs 8.67 ± 0.67, p = 0.005, Figure 1E). Furthermore, the diameter of tumor in mice treated with Huaier extract was reduced compared to controls (4.28 ± 0.32 mm vs 5.82 ± 0.51 mm, p = 0.029, Figure 1F). Finally, the tumor load (calculated as a total of tumor diameter per mouse) in Huaier extract treatment group was significantly lower (11.50 ± 1.26 mm vs 25.33 ± 1.84 mm, p < 0.001, Figure 1G).
Huaier Extract Decreased Histological Damage
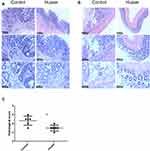
Colon tissue was H&E stained and tumors across the two cohorts were identified as adenomas associated with high-grade dysplasia (Figure 2A). To determine inflammation, tissue damage and penetration of inflammatory cells, the non-tumor tissues were examined using a microscope. Similar to the macroscopic findings conveyed earlier, Huaier extract effectively decreased severity of inflammation, as shown by the histological score between the two cohorts (3.00 ± 0.26 vs 4.67 ± 0.42, p = 0.007, Figure 2B and C).
Huaier Extract Reduces Pro-Inflammatory Cytokine Expression
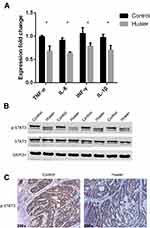
To determine the mechanism by which Huaier extract reduced colitis-associated colorectal tumorigenesis, changes in colonic contents of well-known pro-inflammatory cytokines were evaluated by ELISA. Compared to control mice, Huaier extract decreased pro-inflammatory cytokine expression in serum, such as TNF-α (P = 0.029), IL-6 (P = 0.007), IFN-γ (P = 0.010) and IL-1β (P = 0.021) (Figure 3A).
Huaier Extract Reduced STAT3 Phosphorylation
Next, we identified that Huaier extract decreased p-STAT3 expression in colon compared with the controls (Figure 3B). We also observed the down-regulation of p-STAT3 from immunohistochemistry results of tumor tissue (Figure 3C).
Huaier Extract Inhibited the Proliferation of Human Colorectal Cancer Cells
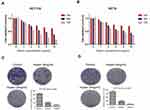
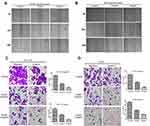
In order to evaluate the effect of Huaier extract on the proliferation of CRC cells, HCT116 and HCT8 were treated with Huaier extract (0, 1, 2, 4, 8 and 10 mg/mL) for 24, 48 and 72 h, respectively, and then MTS assays were used to measure cell viability. Huaier extract significantly inhibited both HCT116 and HCT8 cell growth in a concentration- and time-dependent manner (Figure 4A and B). When HCT116 and HCT8 cells were treated with 0, 1, 2, 4, 8 and 10 mg/mL Huaier extract for 48 h, the cell viabilities of HCT116 cells were 100.0%, 91.2%, 72.6%, 54.3%, 48.2% and 46.8%, respectively, and those of HCT8 cells were 100.0%, 94.8%, 83.5%, 59.2%, 51.0% and 47.6%, respectively. Based on these results, concentrations of 4 and 8mg/mL were selected for further studies. Furthermore, colony formation assays were carried out to further examine the effect of Huaier extract on the proliferation of CRC cells. It revealed that Huaier extract (4 and 8 mg/mL) reduced both abilities of HCT116 and HCT8 to form cell colonies (Figure 4C and D).
Huaier Extract Inhibited Cell Motility of HCT116 and HCT8 cells
To explore the effects of Huaier extract on cell migration, HCT116 and HCT8 were treated with 4 and 8 mg/mL Huaier extract, and cell migration ability was determined using scratch and migration assays. Consistent results were obtained from both of the assays. In the scratch assays, both 4 and 8 mg/mL Huaier extract significantly inhibited the process of wound closure compared with the untreated cells for both HCT116 and HCT8 cells (Figure 5A and B). In the migration assay, both 4 and 8 mg/mL Huaier extract significantly inhibited cell migration compared to the untreated cells (Figure 5C and D). To determine whether Huaier extract could affect cell invasion ability, we performed an in vitro invasive assay of HCT116 and HCT8 cells. The number of invading cells was significantly less than that of untreated cells when exposed to 4 and 8 mg/mL Huaier extract, indicating that Huaier extract reduced the invasive potential of CRC cells (Figure 5C and D).
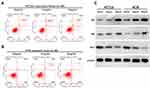
Huaier Extract Promoted Cell Apoptosis of HCT116 and HCT8
We performed the PI-annexin-V double-staining assays using flow cytometry to determine the rate of cell apoptosis after 48 h of exposure to Huaier extract (4 and 8mg/mL). Both early and late apoptosis rates were increased in a concentration-dependent manner for both HCT116 and HCT8 cells (Figure 6A and B). Given that p53 plays a vital role in the process of cell apoptosis, expression of p53 was measured with Western blotting after HCT116 and HCT8 cells were treated with 4 and 8 mg/mL Huaier extract for 48 h. The protein level of p53 significantly increased in both HCT116 and HCT8 cells. Previous studies reported that Bax and Bcl-2 were involved in p53-associated apoptosis processes. To further examine the impact of Huaier extract on cell apoptosis, the expressions of Bax and Bcl-2 were also measured with Western blotting. The trend of Bax protein levels was similar to levels of p53, while the administration of Huaier extract markedly down-regulated Bcl-2 expression (Figure 6C).
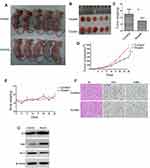
Huaier Extract Inhibited CRC Growth and Promoted Apoptosis in Xenograft Models
We further investigated the in vivo effects of Huaier extract on CRC cells. Based on the above results, the anti-proliferation effects of Huaier extract were more prominently seen in HCT116 cells than HCT8 cells. As such, HCT116 cells were selected for further studies in xenograft models. HCT116 (1 ×106) cells were injected subcutaneously in nu/nu mice to generate tumors and Huaier extract was administrated daily to the animals by gavage at a concentration of 4g/kg. These protocols were based on methods published in previous studies [11] (Figure 7A). Administration of Huaier extract inhibited tumor growth and resulted in lower tumor weights (Figure 7B–D). Additionally, no significant difference was demonstrated between the body weights of mice of the treated and control groups, indicating that the Huaier dose used in this study did not impart any significant toxicity (Figure 7E). Notably, no mice in the two groups tested positive for metastatic tumors in the lung, liver and lymph nodes. IHC analysis revealed that Huaier-treated tumors showed a markedly decreased ratio of Ki67-positive cells and increased ratio of TUNEL-positive/apoptotic cells compared with the control group (Figure 7F). Consistent with the in vivo experiments, the protein levels of apoptosis-related proteins p53, Bax and Bcl-2 were found to be the same in in vitro experiments (Figure 7G).
Discussion
Recently, TCM has acquired worldwide recognition and serves as a vital source for identifying potential anticancer medicines with good efficacy and low toxicity. Huaier, a form of officinal fungi, has been utilized in TCM for about 1600 years.20–22 The main functional substance of Huaier aqueous extract is a light-yellow powder that can be obtained through hot-water extraction, ethanol precipitation, deproteinization and lyophilization. The crude extract is made up of water-soluble polysaccharide, which is actually made up of 96.5% carbohydrate, including six sugars, L-fucose, L-arabinose, D-xylose, D-galactose, D-mannose and D-glucose.13 Given Huaier’s potential role as an anticancer agent, it has been extensively studied. Our current analysis aimed to establish the influence of Huaier extract on colitis-associated colorectal tumorigenesis and its antitumor effects on CRC. The key findings of the current study provide a novel understanding of the central function of Huaier extract as a regulator of inflammatory immune responses in the inhibition of CRC growth. In our study, we found that the number of tumors was substantially lower in mice treated with Huaier extract in comparison to the controls. Additionally, the tumor size in the Huaier extract treatment group was likely to be smaller. The results directly indicate that Huaier extract suppresses malignant transformation. Furthermore, this pilot study revealed that Huaier extract could inhibit cell proliferation and induce cell apoptosis in CRC cells, with both processes at least partially facilitated by activation of the apoptotic pathway.
Chronic inflammation may be a powerful force in tumor development by stimulating genetic mutations, encouraging growth and hindering apoptosis, which are pathways in which inflammatory cytokines have important functions.18,23 Indeed, TNF-α, IL-6, IL-1β and IFN-γ are vital pro-inflammatory cytokines that have a function in CRC.8 As the function of these cytokines contribute to a fundamental shift between inflammation and cancer, several lines of evidence point to a vital function of TNF-α, IL-6, IL-1β and IFN-γ in tumor development and progression.24,25 In this study, we demonstrated that pro-inflammatory cytokine expression in CRC mice treated with Huaier extract were reduced in comparison to the vehicle-control mice. These results suggest that Huaier extract can decrease pro-inflammatory response, leading to the inhibition of carcinogenesis. IL-6, a proinflammatory cytokine, can bind to gp130 and phosphorylate the downstream effector STAT3, which regulates expression and control of cell growth and apoptotic suppression in colitis-associated tumor development.26,27 Abnormal STAT3 stimulation increases certain survival factors, including Bcl-2 and Bcl-xL, which leads to encouragement of growth and reduction of apoptosis.28 Our analysis indicates that STAT3 phosphorylation was reduced post-treatment with Huaier extract, which, at least to some degree, may represent a powerful mechanism by Huaier extract acts as an antitumor agent.
Not only does Huaier extract inhibit inflammatory carcinogenesis, but it also has significant antitumor effects when tumors formed. Dysregulation of cellular proliferation and apoptosis are the hallmarks of cancer and aberrantly sustained active proliferative signaling and resistance against apoptosis are common events that often take place during the development of human tumors including CRC.29,30 Consequently, the modulation of proliferation and apoptosis in cancer cells has emerged as a promising approach in cancer therapy. In the present study, we demonstrated that Huaier aqueous extract markedly inhibited the growth and induced apoptosis of CRC cells both in vitro and in vivo. In particular, the antitumor effects of Huaier aqueous extract on HCT116 cells were more effective than that on HCT8 cells. The dismal prognosis and high mortality of CRC patients are the results of the highly malignant property of CRC cells. Consequently, inhibiting cancer metastasis may be the key in improving the prognosis of CRC patients. Herein, our results revealed that treatment with Huaier extract significantly represses migratory and invasive capacities of HCT116 and HCT8 cells. Based on our in vitro pilot results, it is rational and feasible that Huaier extract serves as a potential drug that targets CRC metastasis. However, further studies delineating the pathways underlying this phenomenon are necessary.
The mechanisms of antitumor effects of Huaier extract include inhibition of proliferation, induction of apoptosis, reversal of drug resistance in cancer cells, as well as regulation of immunity and anti-angiogenic functions.16,22,31 In the current investigation, we demonstrated that Huaier extract inhibited the proliferation of CRC cells in vitro and in vivo possibly via an apoptosis-associated pathway. According to a previous study, PI-annexin-V double staining can be used to detect cells in the early and late phases of apoptosis.32 Indeed, our results revealed that the rates for both late and early apoptosis were increased in a concentration-dependent manner. To further elucidate the mechanisms underlying Huaier-induced apoptosis, p53, Bax and Bcl-2 protein levels were measured by Western blotting and TUNEL assays were performed on the tumor tissues. The p53 protein generally serves as a kind of tumor suppressor protein, playing a vital role in the cellular response to DNA damage and other genomic aberrations. Herein, the up-regulation of p53 was observed after treatment with Huaier extract both in vitro and in vivo, partly explaining the mechanism underlying Huaier-induced apoptosis. Bcl-2 and Bax are generally considered as critical mediators of cell apoptosis which work through the mitochondrial pathway. In agreement with a previous report,20 our results revealed that the mitochondrial apoptosis pathway can be affected by treatment with Huaier extract. However, it remains unclear as to why no significant difference in Bax was observed in the Huaier-treated tumor tissues.
Previous studies have shown significant antitumor effects of Huaier extract on gastric cancer and inhibition of inflammation in DSS-induced experimental colitis.33,34 Our study further found that it also has a significant antitumor effect in CRC. Dysregulation of inflammatory immune responses has emerged as a hallmark of CRC and is common during the multistep development of CRC from IBD.35–37 To our knowledge, there are a few studies that have been carried out to determine the anticancer effects of Huaier extract on colitis-associated colorectal cancer. Given that Huaier extract possesses promising anticancer properties and may serve as an adjuvant therapy in CRC treatment, future studies should focus on the use of Huaier extract in CRC patients. In addition, Huaier extract may be helpful for IBD patients at high-risk factors for malignant transformation.
Conclusions
In conclusion, this pilot study revealed that Huaier extract could inhibit cell proliferation and induce cell apoptosis in CRC cells, with both processes at least partially facilitated by activation of the apoptotic pathway. And finding from our analyses indicates that Huaier extract can inhibit the development of colitis-associated CRC by regulating inflammatory immune responses of a mouse model. Present results help to additionally elucidate the antitumor influence of Huaier extract on CRC and provide a unique scientific rationale for clinical medication. Nevertheless, the development of IBD-associated cancer in humans is increasingly complicated compared to the mouse model and the mechanism of Huaier extract on this disease development is not clear. Additional preclinical and clinical analyses are needed to confirm these results.
Abbreviations
AOM, azoxymethane; CRC, colorectal cancer; DMEM, dulbecco’s modified eagle medium; DSS, dextran sulfate sodium; ELISA, enzyme-linked immunosorbent assay; H&E, hematoxylin and eosin; IBD, inflammatory bowel disease; IFN, interferon; IHC, immunohistochemistry; IL, interleukin; PI, propidium iodide; TCM, traditional Chinese medicine; STAT, signal transducer and activator of transcription; TNF, tumor necrosis factor; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling assay.
Data Sharing Statement
If necessary, please contact the corresponding authors to get additional data.
Ethics Approval
This study was approved by the ethics committees of the Sixth Affiliated Hospital of Sun Yat-sen University.
Consent
We have obtained consents to publish this paper from all the participants of this study.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Rogler G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014;345(2):235–241. doi:10.1016/j.canlet.2013.07.032
2. Wunderlich CM, Ackermann PJ, Ostermann AL, et al. Obesity exacerbates colitis-associated cancer via IL-6-regulated macrophage polarisation and CCL-20/CCR-6-mediated lymphocyte recruitment. Nat Commun. 2018;9(1):1646. doi:10.1038/s41467-018-03773-0
3. Yashiro M. Ulcerative colitis-associated colorectal cancer. World J Gastroenterol. 2014;20(44):16389–16397. doi:10.3748/wjg.v20.i44.16389
4. Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol. 2011;11(1):9–20. doi:10.1038/nri2891
5. Jobin C. Colorectal cancer: CRC–all about microbial products and barrier function? Nat Rev Gastroenterol Hepatol. 2012;9(12):694–696. doi:10.1038/nrgastro.2012.220
6. Arthur JC, Perez-Chanona E, Muhlbauer M, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science (New York, NY). 2012;338(6103):120–123. doi:10.1126/science.1224820
7. Grivennikov SI, Wang K, Mucida D, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491(7423):254–258. doi:10.1038/nature11465
8. Benson AB
9. Lee HN, Shin SA, Choo GS, et al. Anticancer effects of Ixeris dentata (Thunb. ex Thunb.) nakai extract on human melanoma cells A375P and A375SM. J Ethnopharmacol. 2016;194:1022–1031. doi:10.1016/j.jep.2016.11.010
10. Chen Q, Shu C, Laurence AD, et al. Effect of Huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial. Gut. 2018;67(11):2006–2016. doi:10.1136/gutjnl-2018-315983
11. Yan X, Lyu T, Jia N, Yu Y, Hua K, Feng W. Huaier aqueous extract inhibits ovarian cancer cell motility via the AKT/GSK3beta/beta-catenin pathway. PLoS One. 2013;8(5):e63731. doi:10.1371/journal.pone.0063731
12. Zou Y, Xiong H, Xiong H, et al. A polysaccharide from mushroom Huaier retards human hepatocellular carcinoma growth, angiogenesis, and metastasis in nude mice. Tumour Biol. 2015;36(4):2929–2936. doi:10.1007/s13277-014-2923-8
13. Sun Y, Sun T, Wang F, et al. A polysaccharide from the fungi of Huaier exhibits anti-tumor potential and immunomodulatory effects. Carbohydr Polym. 2013;92(1):577–582. doi:10.1016/j.carbpol.2012.09.006
14. Xie HX, Xu ZY, Tang JN, et al. Effect of Huaier on the proliferation and apoptosis of human gastric cancer cells through modulation of the PI3K/AKT signaling pathway. Exp Ther Med. 2015;10(3):1212–1218. doi:10.3892/etm.2015.2600
15. Li Y, Qi W, Song X, Lv S, Zhang H, Yang Q. Huaier extract suppresses breast cancer via regulating tumor-associated macrophages. Sci Rep. 2016;6:20049. doi:10.1038/srep20049
16. Hu Z, Yang A, Su G, et al. Huaier restrains proliferative and invasive potential of human hepatoma SKHEP-1 cells partially through decreased Lamin B1 and elevated NOV. Sci Rep. 2016;6:31298. doi:10.1038/srep31298
17. Wu T, Chen W, Liu S, et al. Huaier suppresses proliferation and induces apoptosis in human pulmonary cancer cells via upregulation of miR-26b-5p. FEBS Lett. 2014;588(12):2107–2114. doi:10.1016/j.febslet.2014.04.044
18. Chen Z, He X, He X, et al. Bone marrow mesenchymal stem cells ameliorate colitis-associated tumorigenesis in mice. Biochem Biophys Res Commun. 2014;450(4):1402–1408. doi:10.1016/j.bbrc.2014.07.002
19. Yang X, Zhang FY, Wang YJ. Oroxylin A inhibits colitis-associated carcinogenesis through modulating the IL-6/STAT3 signaling pathway. Inflamm Bowel Dis. 2013;19(9):1990–2000. doi:10.1097/MIB.0b013e318293c5e0
20. Zhang N, Kong X, Yan S, Yuan C, Yang Q. Huaier aqueous extract inhibits proliferation of breast cancer cells by inducing apoptosis. Cancer Sci. 2010;101(11):2375–2383. doi:10.1111/j.1349-7006.2010.01680.x
21. Yang A, Fan H, Zhao Y, et al. Huaier aqueous extract inhibits proliferation and metastasis of tuberous sclerosis complex cell models through downregulation of JAK2/STAT3 and MAPK signaling pathways. Oncol Rep. 2016;36(3):1491–1498. doi:10.3892/or.2016.4969
22. Wang X, Zhang N, Huo Q, Yang Q. Anti-angiogenic and antitumor activities of Huaier aqueous extract. Oncol Rep. 2012;28(4):1167–1175. doi:10.3892/or.2012.1961
23. Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659(1–2):15–30. doi:10.1016/j.mrrev.2008.03.002
24. Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41(16):2502–2512. doi:10.1016/j.ejca.2005.08.016
25. Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14(6):e218–e228. doi:10.1016/S1470-2045(12)70582-X
26. Bollrath J, Phesse TJ, von Burstin VA, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15(2):91–102. doi:10.1016/j.ccr.2009.01.002
27. Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. doi:10.1016/j.ccr.2009.01.001
28. Al Zaid Siddiquee K, Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008;18(2):254–267. doi:10.1038/cr.2008.18
29. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
30. Arora H, Qureshi R, Rizvi MA, et al. Study of apoptosis-related interactions in colorectal cancer. Tumour Biol. 2016;37(11):14415–14425. doi:10.1007/s13277-016-5363-9
31. Song XJ, Li YM, Zhang HW, et al. The anticancer effect of Huaier (Review). Oncol Rep. 2015;34(1):12–21. doi:10.3892/or.2015.3950
32. Engeland MV, Nieland LJ, Ramaekers FC, et al. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31(1):1–9. doi:10.1002/(SICI)1097-0320(19980101)31:1<1::AID-CYTO1>3.0.CO;2-R
33. Wang YP, Lv H, Xu ZY, et al. Huaier N-butanol extract suppresses proliferation and metastasis of gastric cancer via c-Myc-Bmi1 Axis. Sci Rep. 2019;9(1):447. doi:10.1038/s41598-018-36940-w
34. Wang LJ, Yu ZX, Wei C, et al. Huaier aqueous extract protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NLRP3 inflammasome activation. Oncotarget. 2017;8(20):32937–32945. doi:10.18632/oncotarget.16513
35. Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol. 2013;35(2):229–244. doi:10.1007/s00281-012-0352-6
36. Waldner MJ, Neurath MF. Mechanisms of immune signaling in colitis-associated cancer. Cell Mol Gastroenterol Hepatol. 2015;1(1):6–16. doi:10.1016/j.jcmgh.2014.11.006
37. Zhao W, Qi L, Qin Y, et al. Functional comparison between genes dysregulated in ulcerative colitis and colorectal carcinoma. PLoS One. 2013;8(8):e71989. doi:10.1371/journal.pone.0071989
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.