Back to Journals » International Journal of Women's Health » Volume 14
Effects of HIV Infection on Pregnancy Outcomes Among Women Attending Antenatal Care in Referral Hospitals of the Amhara Regional State, Ethiopia: A Prospective Cohort Study
Authors Worku WZ, Azale T, Ayele TA, Mekonnen DK
Received 21 July 2022
Accepted for publication 15 September 2022
Published 23 September 2022 Volume 2022:14 Pages 1405—1423
DOI https://doi.org/10.2147/IJWH.S382685
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Everett Magann
Workie Zemene Worku,1 Telake Azale,2 Tadesse Awoke Ayele,3 Dawit Kassahun Mekonnen4
1Department of Community Health Nursing, School of Nursing, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Department of Health Education and Behavioural Sciences, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 3Department of Epidemiology and Biostatistics, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 4Department of Gynaecology and Obstetrics, School of Medicine, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Workie Zemene Worku, University of Gondar, P.O. Box 196, Gondar, Ethiopia, Tel +251 9187713 63, Email [email protected]
Background: Human immunodeficiency virus (HIV) has remained to be a significant public health problem worldwide mainly affecting women. Despite a 2 to 3 times higher risk of adverse pregnancy outcomes, around 2 million HIV positive women give birth each year globally. However, there is a dearth of evidences in Ethiopia about the effect of maternal HIV infection on pregnancy outcomes where adverse birth outcomes are still a significant health problem. This study is, therefore, aimed to examine the effect of HIV on the risk of adverse pregnancy outcomes in Amhara Regional State, Ethiopia.
Methods: A prospective cohort study was conducted among 704 pregnant women (352 women with HIV and 352 women without HIV infection). Systematic random sampling technique was employed to select the study participants. Data on socio-demographic, obstetric, clinical, as well as behavioral and psychosocial characteristics were collected using a validated tool. Data on the outcome variables were also collected following delivery. Modified Poisson regression was employed to estimate the relative risk (RR) of HIV on low birth weight (LBW), preterm birth, and still birth at 95% confidence level. Attributable fraction (AF) was used to report the impact of HIV infection on pregnancy outcomes.
Results: Of the total 704 pregnant women enrolled for the study, 96.3% (678) completed the study. The mean age of the study participants was 30.8 (SD ± 5.4) for HIV positive and 27 (SD ± 5.4) for HIV negative women. The cumulative incidence of low birth weight, preterm birth, and stillbirth were 21.4%, 9.4%, and 4.1%, respectively. The incidence of LBW was 24.7% among HIV positive and 17.8% among HIV negative women. The incidence of preterm birth was 10.7% among HIV positive and 7.9% among HIV negative women. And the incidence of stillbirth was 3.7% and 4.6% among HIV positive and those HIV negative women. New-borns from women with HIV infection had a higher risk of low birth weight and preterm birth than those HIV negative women (Adjusted Relative Risk (ARR) = 1.47; 95% CI: 1.06– 2.03) and (ARR = 1.74; 95% CI: 1.08– 2.79), respectively. The attributable risk of HIV on low birth weight was 32% (Attributable Fraction (AF) = 32%, 95% CI: 23– 46%), and 43% (AF = 43%, 95% CI: 23– 46%) for preterm birth.
Conclusion: Maternal HIV infection increased the risk of low birth weight and preterm birth. This implies due attention is required while providing maternal health services primarily antenatal care and delivery services. These services should be aimed at reducing adverse pregnancy outcomes with more attention given to women with HIV infection. Moreover, reinforcement of HIV prevention intervention strategies should be considered at all levels.
Keywords: HIV infection, low birth weight, preterm birth, stillbirth, Ethiopia
Background
HIV continues to be a major public health issue around the world affecting women and children disproportionately. In 2020, an estimated 37.7 million individuals worldwide were reportedly living with HIV. Of these, 53% were women of reproductive age, and Sub-Saharan African countries accounted for around 40% of those reported.1–4 Ethiopia is known to be hit hard by the HIV pandemic ranking among the top 25 countries in the world in terms of new HIV infections.5 In 2020, Ethiopia had a total of 620,000 HIV-positive people of whom around 60% were women of reproductive age.6,7
HIV infection has two effects on the foetus: vertical transmission and an increased risk of adverse pregnancy outcomes, both of which have a significant impact on a new-born’s life.8 Although HIV-positive pregnant women are taking antiretroviral therapy (ART), adverse pregnancy outcomes are 2 to 3 times more common in HIV-positive pregnant women compared to HIV-negative pregnant women.9,10 Specifically, low birth weight (LBW), preterm birth, and stillbirths are higher among HIV exposed than unexposed foetus.10–12 These adverse pregnancy outcomes pose the greatest risk of death and long-term complications for the new born worldwide.13,14 The impact extends to their parents, causing them to suffer from long-term psychological and financial problems, which can also affect the developmental, social, and cognitive growth of the new born.13,15
Preterm birth and LBW are the most important biological determinants of an infant’s survival and are used to understand the quality of an infant’s gestation.16–19 An estimated 20 million newborns are identified annually with low birth weight.18,20 Developing countries account for more than 95% of the world’s LBW infants.18,21 It is the leading cause of neonatal morbidity and mortality20 due to getting born immature and unprepared for life outside the womb. In this connection, maternal HIV infection was identified as an independent risk factor for LBW with relative risks ranging from 1.73 to 4.2.10,12,22–24
Preterm birth is the leading cause of death in the first month of life for an estimated 2.9 million babies worldwide and is also the primary cause of disability and chronic illness in later life.25 In Ethiopia, 320,000 children are born prematurely each year with 24,400 dying before the age of five due to complications attributable to preterm delivery.15,26 In this connection, evidences revealed that maternal HIV infection has been recognized as an independent risk factor for the occurrence of preterm birth with relative risks ranging from 1.41 to 2.77.10,12,23,27
Stillbirth is also a serious public health issue in all low, middle and high-income countries.28 However, over 2.6 million deliveries worldwide are stillbirths with 98% occurring in low and middle-income countries.13,15,29 The incidences of stillbirth among HIV positive mothers were reported to be high.12,30
Risk Factors Associated with Adverse Birth Outcomes
In addition to maternal HIV infection, a range of factors including maternal under nutrition,14,31 gestational hypertension,14,32 unplanned pregnancy,33,34 premature rupture of membranes,31,35 intimate-partner violence (IPV),36–38 and household food insecurity39,40 were identified as predictors for adverse birth outcomes.
Although adverse pregnancy outcomes are significant public health problems in Ethiopia, there is a dearth of evidence on the effects of HIV infection on adverse birth outcomes. Previous researches report inconsistent findings regarding the effects of HIV infection on LBW, preterm birth and still birth worldwide.30,35,41 Therefore, this study investigated the effect of maternal HIV infection on pregnancy outcomes in women with and without HIV in referral hospitals of the Amhara Regional State, Ethiopia where a significant number of people living with HIV (PLWHIV) resides.6
Methods
Study Design and Setting
A prospective cohort study was conducted from December 2020 to December 2021 at Referral Hospitals of the Amhara Regional State, Ethiopia, to measure the effects of maternal HIV infection on adverse pregnancy outcomes. There are six referral hospitals serving 3.5 to 5 million people in the region in which focused antinatal care is provided.42 The study was conducted in randomly selected three referral hospitals (the University of Gondar Comprehensive Specialized Referral Hospital, Felege Hiwot Comprehensive Specialized Hospital and Debre Tabor Referral Hospital) from which we got representative participants.
Participants
The study participants were pregnant women attending Antenatal Care (ANC) follow up in the selected hospitals. The participants were assigned into exposed and unexposed groups based on their HIV status. The exposed groups were those pregnant women infected with HIV while the unexposed group constituted of HIV negative pregnant women. While the unexposed group were recruited from the ANC clinics of the respective hospitals, those of the exposed group were recruited from the Prevention of Mother to Child Transmission (PMTCT) clinics of the hospitals. Both the exposed and the unexposed women were followed prospectively from the last week of second trimester of pregnancy until immediately after the end of delivery. The study had two follow up phases. The first phase was undertaken at the beginning of the follow-up period during the last week of second trimester of pregnancy and the second phase was immediately after delivery. We excluded those pregnant women who came for ANC and PMTCT follow up with multiple pregnancies, those who had uncertain HIV sero-status, and those who had uncertain gestational age after checking their last normal menstrual period (LMP) as well as ultrasound results.
Sample Size Determination
The sample size was determined using double population proportion formula43 using Epi info version 7.44 Incidences of composite pregnancy outcome, 48.3% from the HIV exposed and 30.3% from the HIV unexposed pregnant women were used23 with one-to-one ratio. A 5% significance level, 80% power, 15% non-response rate, effect size of 2, and design effect of 2 were considered. The final sample size calculated was 704 with 352 participants assigned to each group.
Sampling Techniques
Systematic random sampling technique was used to select 352 pregnant women with HIV and 352 from those without HIV in the selected study sites. The sample size for the three facilities was assigned based on proportional allocation to the number of pregnant women who attended ANC as well as PMTCT clinics. Following this, we recruited 128 from University of Gondar Comprehensive Specialized Hospital, 118 from Felege Hiwot Comprehensive Specialized Hospital, and 106 from Debre Tabor Referral Hospital for women without HIV. Similarly, 162, 140, and 50 women with HIV were included from University of Gondar Comprehensive Specialized Referral Hospital, Felege Hiwot Comprehensive Specialized referral Hospital and Debre Tabor Referral Hospital, respectively.
The sampling interval (k) was calculated using the sum total of women which is 2338 who attended ANC from the three referral hospitals. Therefore, k was calculated as: K = 2338/352 = 7. Based on this sampling interval, participants were recruited at seven intervals until the required sample was attained.
Data Collection Procedure and Quality Assurance
A standardized, structured and interviewer-administered questionnaire was used to collect the data. The questionnaire encompasses items on socio-demographic, obstetric, medical, behavioral and psychosocial characteristics of the participants. With the help of language and public health experts, the tool was first developed in English, then translated in to Amharic, the participants’ mother tongue to prevent communication difficulties, and finally translated back to English to ensure consistency. Face and content validity tests were performed with gynecologists, pediatricians, midwives, and infectious disease experts.
Baseline data were collected both retrospectively from the participants’ ANC charts and prospectively by interviewing the participants during enrolment in the second trimester. Data on the outcome variables including body weight measurement of the new born baby, recording gestational age at birth, recording whether the newborn was alive or still birth together with other related variables were collected from the mother and the newborn baby following delivery. Other data were collected from integrated antenatal, labour, delivery and postnatal care cards of the pregnant women following delivery using a checklist. The data was collected by twelve Bachelor of Science (BSc) holder midwifery nurses under the supervision of three clinical midwifery nurse and three public health professionals.
Data collectors and supervisors were trained. Daily supervision was made by the supervisors as well as the principal investigator throughout the data collection period. Appropriate health information was provided to the pregnant women on the importance of completing attendance of ANC services, and institutional delivery if possible at the health facility where the women had their ANC follow up.
Study Variables
Operational Definitions of Study Outcomes
The outcome variables of the study included low birth weight (LBW), preterm birth, and stillbirth. Following delivery a newborn baby was examined to determine whether she/he had low birth weight, a preterm birth, or a stillbirth based on the operational definition given below. According to the World Health Organization (WHO), LBW is defined as weight at birth less than 2500 g regardless of the gestational age of the infant.45,46 Birth weight was measured within an hour of delivery using a standard weight measuring instrument, for some cases taken from medical records measured with a similar standard weight measuring instrument. Preterm birth is defined as a live birth before the 37th week of gestation.13,47 Stillbirth is defined as birth of a foetus without sign of life at or after 28 weeks of gestational age.48
Exposure Variable
HIV status was considered as the exposure variable. According to WHO, HIV positive status is defined as a positive HIV antibody test, which is confirmed by a second HIV antibody test and/or positive virological test for HIV or its components confirmed by a second virological test obtained from a separate determination.49 HIV testing and diagnosis was conducted at the ANC clinics during the first ANC follow up based on the HIV counseling and testing guideline of Ethiopia.50
Independent Variables
The independent variables include socio-demographic, obstetric, medical, behavioral, and psychosocial factors of the study participants. Some of the independent variables were measured using standardized tools. Prenatal depression was assessed using 10 items Edinburgh Postnatal Depression Scale (EPDS) with responses categorized as depressed (a score of 12 and above) and not depressed (a score less than 12).51,52 Health related quality of life (HRQOL) was measured using a tool developed by WHO known as WHOQOL BREF which contains 26 items each (five point Likert scale) that fall on to four domains: physical, psychological, social relationships and environment domain.53 Social support was measured using the 6-item Maternity Social Support Scale (MSSS) with five Likert response scales developed by Joan Webster et al.54 Food insecurity was measured using three item Household Hunger Score with three point Likert response scales, classified as (little to no hunger, moderate hunger and severe hunger).55 Intimate partner violence (IPV) was measured by a 13-item WHO Violence against Women Questionnaire,56 and was claimed when the participants said “Yes” to any of the 13 questions, regardless of the legal status of the relationship.57
Outcome Ascertainment
Outcome ascertainment measures were carefully undertaken during the data collection period. The gestational age of the participants was ascertained using a combination of ultrasound done before 20 weeks of gestation (when available), and last menstrual period (LMP). In pregnant women who have both ultrasound done before 20 weeks of gestation and known LMP, we calculated the difference in days. While we used LMP when the difference was less than 7 days, we took the gestational age estimated by ultrasound when the difference was greater than 7 days. We used LMP for those who did not have ultrasound before 20 week. The birth weight of the newborn was measured using a standard beam balance weight scale within the first hour of birth.
Data Processing and Analysis
Data were cleaned, coded and entered to EpiData Manager V4.6.0.0 and exported to STATA version 14 for recoding and further analysis. Descriptive statistics such as frequencies, percentages, mean, and standard deviation were computed to summarize and present study participants’ characteristics. Categorical data of the participants were also computed and compared based on their exposure status using Pearson’s chi-square test.
Log-binomial regression model using a Generalized Linear Model (GLM) was used to estimate the effect size of the exposure variable on LBW, preterm birth, or stillbirth.58 Due to a convergence problem, we used modified Poisson regression which combines robust variance estimation with a log Poisson regression model.59–61 Factors with p ≤ 0.2 in the bivariable analysis were included in the multivariable model. Variables with p-value less than 0.05 in the multivariable model were considered to be statistically significant at 95% CI. Both crude and adjusted relative risk estimates were reported along with 95% confidence intervals. Model goodness-of-fit was assessed using Pearson goodness-of-fit for the three models. In both tests, the probability value of >0.05 demonstrated that the model fits the data adequately. The Attributable fraction (AF) is used to estimate how much the occurrence of LBW, preterm birth, or stillbirth among HIV positive women could be avoided by preventing HIV infection. AF was calculated as: AF = [(RR-1)/RR]*100.62
Results
Cohort Profiles
A total of 704 pregnant women were recruited at the ANC clinics of three referral hospitals of the Amhara Regional State. The participants were followed retrospectively since the first ANC visit and prospectively from the last week of second trimester until childbirth. We enrolled 352 pregnant women in the HIV-exposed group and 352 in the HIV-unexposed group. Of the total participants, 96.3% (678) of the women completed the study, with a 3.7% loss - to- follow up rate. The reason for these losses - to- follow up was related to the fact that the women did not give birth at the health facility. Therefore, 678 women were included in the final analysis.
Socio-Demographic Characteristics of the Study Participants
The mean age was 30.8 (SD ± 5.4), and 27 (SD ± 5.4) for HIV positive and negative women, respectively. A majority (96.4%) of HIV negative women and (88.8%) of HIV positive women were Orthodox Christian in religion. Most (98.8%) of the women without HIV and 93.1% of those with HIV were married. About half (45%) of the women with HIV and 129 (39.2%) women without HIV were house wives (Table 1).
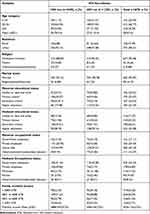 |
Table 1 Socio-Demographic Characteristics of Pregnant Women Attending ANC in Referral Hospitals of Amhara Regional State, Ethiopia, 2021 (n = 678) |
Obstetric Characteristics of the Study Participants
Two hundred seventy (77.4%) women with HIV and 189 (57.5%) women without HIV infection were multi-gravida. Regarding inter-pregnancy interval, 233 (86.3%) women with HIV and 142 (75.1%) those without HIV had spaced their pregnancy more than two years (Table 2).
 |
Table 2 Obstetric Characteristics of Pregnant Women Attending ANC in Referral Hospitals of Amhara Regional State, Ethiopia, 2021 (n = 678) |
Behavioral and Psychosocial Characteristics
One hundred and seven (44.99%) women with HIV and 232 (70.5%) women without HIV consumed alcohol during pregnancy. Around one in seven (15.2%) of those with HIV and 26 (7.9%) of those without HIV experienced severe hunger in the household. One hundred and eighty nine (54.2%) women with HIV and 122 (37.1%) those without HIV received poor social support. A significant proportion (17.2%) of women with HIV and 29 (8.81%) without HIV experienced antenatal depression symptoms. Two hundred and twenty six (64.8%) exposed women and 137 (42%) non-exposed experienced at least one form of intimate partner violence (IPV). One hundred eighty-four (52.7%) women with HIV and 141 (42.9%) without HIV reported a poor overall quality of life (Table 3).
 |
Table 3 Behavioral and Psychosocial Characteristics of Pregnant Women Attending ANC in Referral Hospitals of Amhara Regional State, Ethiopia, 2021 (n = 678) |
HIV Related Characteristics of Pregnant Women Living with HIV
More than half (57.9%) of women were living with the virus for six and more years. Almost all (99.4%) of the participants were on ART, of these 324 (93.4%) started ART before the current pregnancy, and 331 (95.4%) were on 1st line regimens (see Supplementary Table S1).
Incidence of Adverse Pregnancy Outcomes Among Study Participants
From the total 678 study participants who completed the follow up, 203 (29.94%) (95% CI: 26.60–33.50,) had at least one type of adverse pregnancy outcome. Out of 203 adverse pregnancy outcomes, 123 (35.2%) were from women living with HIV infection and 80 (24.3%) were from those without the infection. The risk ratio (RR) for the composite adverse pregnancy outcome was 1.45 (95% CI: 1.14–1.84, p = 0.002). The incidence of low birth weight among women living with HIV infection was 83 (24.7%) and it was 56 (17.8%) among those without HIV infection. The RR for low birth weight was 1.39 (95% CI: 1.02–1.87, p = 0.033). The incidence of preterm birth was 36 (10.7%) and 25 (7.9%) among women with HIV infection and those without the infection, respectively. The RR for preterm birth was 1.35 (95% CI: 0.83–2.19, p = 0.23). The incidence of stillbirth was 13 (3.7%) among HIV positive and 15 (4.6%) among HIV negative women. The risk ratio (RR) for stillbirth was 0.817 (95% CI: 0.394–1.69, p = 0.59) (Figure 1; Supplementary Table S2)
 |
Figure 1 Adverse birth outcome (low birth weight, Preterm birth, and stillbirth) of pregnant women attending ANC in Referral Hospitals of Amhara Regional state, Ethiopia, 2021 (n=678). |
Low Birth Weight and Maternal HIV Infection
After adjusting possible confounder variables, maternal HIV infection was independently associated with LBW. The risk of having a low birth weight baby was 1.5 times higher among the HIV positive women than those with HIV negative (ARR = 1.47; 95% CI: 1.06–2.03). The attributable fraction of HIV infection was 32% (95% CI: 23–46%) (Table 4).
 |
Table 4 Bivariable and Multivariable Analysis of Low Birth Weight Among Pregnant Women Attending ANC in Referral Hospitals of Amhara Regional State, Ethiopia, 2021 (n = 678) |
Preterm Birth and Maternal HIV Infection
After adjusting possible confounder variables, maternal HIV infection was independently associated with preterm birth. The risk of preterm birth was 1.7 times higher among women infected with HIV than those without HIV infection (ARR = 1.74; 95% CI: 1.08–2.79). The attributable fraction of HIV infection was 43% (95% CI: 21–54%) (Table 5).
 |  |  |
Table 5 Bivariable and Multivariable Analysis of Preterm Birth Among Pregnant Women Attending ANC in Referral Hospitals of Amhara Regional State, Ethiopia, 2021 (n = 678) |
Stillbirth and Maternal HIV Infection
After adjusting possible confounder variables, maternal HIV infection was not independently associated with stillbirths (ARR = 0.96; 95% CI: 0.42–2.19) (Table 6).
 |
Table 6 Bivariable and Multivariable Analysis of Stillbirth Among Pregnant Women Attending ANC in Referral Hospitals of Amhara Regional State, Ethiopia, 2021 (n = 678) |
Discussion
In this prospective cohort study, a total of 678 participants completed the study follow up. Incidences of adverse pregnancy outcomes as well as the effects of HIV on adverse pregnancy outcomes were determined. The incidence of having at least one type of adverse birth outcome among HIV positive women was 35.2% whereas it was 24.3% among those without HIV. The incidence of LBW among HIV positive women was 24.7% whereas it was 17.8% among HIV negative women. The incidence of preterm birth among HIV positive women was 10.7% while it was 7.9% among HIV negative women. The incidence of stillbirth was 4.5%, and 3.7% among HIV negative and HIV-positive women, respectively. The multivariate Poisson regression model revealed that maternal HIV infection was independently associated with LBW and preterm birth, but not with stillbirth.
Women with HIV infection had a 45% higher risk of having at least one type of adverse birth outcome than those HIV negative women. This suggests that maternal HIV infection has a significant impact on adverse birth outcomes. This finding is consistent with previous studies in Lesotho,9 South Africa,24,63 Nigeria,23 Canada,22 Latin America and the Caribbean,64 USA,65 and China.27
The incidence of LBW was higher among HIV positive women than those HIV negative with 24.7% and 17.8%, respectively. This finding is consistent with studies conducted elsewhere in Ethiopia66 and South Africa24 but found to be lower than a meta-analysis study.10 The reason for this discrepancy could be due to time gap in that the meta-analysis study was conducted before seven years ago with articles published in 1986, the time when ART and other interventions were not available for most developing countries. However, huge efforts have been made for the last two to three decades to make ART and related interventions accessible and affordable for all individuals living with HIV/AIDS such as the implementation of PMTCT package, the WHO “treat all approach” and the Sustainable Development Goals (SDG) of ending HIV/AIDS by 2030.67,68 These interventions in turn have improved the health and wellbeing of women living with HIV since ART effectively suppresses HIV viral load and eventually decreases the risk of adverse birth outcomes among HIV positive women to a certain extent.69
The incidence of LBW in this study was higher than studies in other parts of Ethiopia31 Guinea-Bissau70 Uganda,71 Lesotho,9 South Africa,63 Amsterdam,72 China,12,27 and Canada.22 The difference in the estimated incidence might be due to differences in study sites in that almost all of these studies were conducted at a single study site while our study was a multi-centre study where most of the complicated pregnancies were referred for better management. Another reason could be due to a difference in the multifaceted factors that contribute to LBW that might vary in geography, socio-demographic, economic difference, lifestyle, nutritional status, disease burden and health care seeking behavior of the community.39
The multivariate Poisson regression model revealed maternal HIV infection was independently associated with LBW where the risk of having a LBW baby was higher among women living with HIV than those without by 47%. Similarly, other studies from other parts of Ethiopia,66,73 Lesotho,9 Nigeria,23 Multi Centre, Study in a Developing Country,74 Canada,22 Florida USA,75 China,12,27 and a meta-analysis study10 showed similar findings. The possible explanations for this might be related with improper functioning of the placenta. It is indicated that HIV can replicate and modify the cytokine profile in the placenta which impairs the proper function of the placenta eventually limiting foetal development which could cause LBW and preterm delivery.10,76,77 Another reason could be decreased immunity. Studies show that HIV-infected women become immune-compromised during pregnancy due to the destruction of CD4 cells, which may increases the risk of opportunistic infections, disease progression, and reproductive tract infections, with all these jointly contributing to the occurrence of LBW.12,76,78 Evidence also suggests that using combination ART during pregnancy increases the risk of LBW and preterm birth. The use of ART has been linked to changes in placental vasculatures and immune reconstitution which may cause changes in circulating cytokine levels resulting in a premature onset of labour.22,63,79 On the other hand, combination ART containing protease inhibitors can cause disruptions in progesterone synthesis, a hormone vital for foetal growth and development through regulating placental angiogenesis and vascular formation of the foetus.10,79,80 In addition, in HIV-infected women maternal weight loss due to low dietary intake related with loss of appetite, mouth ulcers, food insecurity, mal-absorption, and altered metabolism are common and these eventually lead them to have a LBW baby.81,82 HIV infection-causes vascular damage or ARV-triggered endothelial injury, which in turn may cause in-utero foetal hypoxia and nutrition insufficiency.83
The incidence of preterm birth was higher among the HIV positive women (10.7%) than those HIV negative (7.9%). This finding is consistent with studies in Lesotho,9 and China,12,27 but lower than studies in Uganda,71 South Africa,24,63 Abuja, Nigeria,84 North-Holland, Amsterdam,72 and Canada.22 The discrepancy could be due to methodological differences or differences in time. Most of the studies were conducted before six years ago, at the minimum. However, much has been planned and implemented in the last ten years to control and prevent HIV, prevent vertical transmission from mother to foetus, and improve the quality of life and wellbeing of people living with HIV. Another difference could be due to differences in study settings. Some of these studies, particularly the one conducted in Canada, were population-based studies which allowed researchers to include women who did not begin ART. Our study, on the other hand, was conducted at referral hospitals where participants were available while receiving care. Another difference could be explained by differences in socio-demographic status, lifestyle, economic status, and cultural as well as behavioral characteristics.
The multivariate Poisson regression model revealed maternal HIV infection was independently associated with preterm birth. The risk of preterm birth was higher among women infected with HIV than those without by 74%. Previous studies in Nigeria,23 United States of America,65,75 Canada,22 and China12,27 revealed similar findings. The possible explanation could be related to using ART for a longer period of time.72,85 This explanation could be supported by the findings from our study that 99.4% of the participants were on ART. Combination ART may counteract with the immune system’s natural shift during pregnancy by modulating it through a cytokine-mediated effect from ART which may contribute to an increased risk of preterm birth.79,86,87 Evidences revealed that using Nevirapine based (NVP-based) ART during pregnancy increases the risk of preterm birth.88,89 Furthermore, the use of protease inhibitor-based combination ART may disrupt angiogenesis by reducing progesterone synthesis, a vital hormone resulting in preterm birth.80,86
The incidence of stillbirth was slightly higher among HIV negative women at 4.5% than HIV-positive women at 3.7%. A similar finding was reported from a study conducted in South Africa.24 On the other hand, studies in southern Mozambique,30 Lesotho,9 and China12 revealed the incidence of stillbirth is higher among HIV positive than HIV negative women. The lower incidence of stillbirth among HIV positive women in our study could be due to the fact that HIV positive woman and their physicians taking extra precautions during ANC and intrapartum period. Moreover, HIV positive women strictly adhere to counseling and other services provided to them preconception and during pregnancy to get a favourable birth outcome. Evidences also show that stillbirth is primarily associated with inadequate prenatal care and inappropriate management of complications during pregnancy and delivery. In this regard, HIV positive women have an advantage over HIV negative women because early delivery planning is more common among HIV positive women, delivery planning being one of the components of PMTCT services.90 Finally, the multivariate Poisson regression model revealed that maternal HIV infection was not associated with stillbirth. Similar findings were reported from studies in Bissau, Guinea-Bissau,70 a study in Sub-Saharan Africa,91,92 and USA.93
Limitation and Strength of the Study
The study has several strengths. One is that we used a prospective cohort study design, one of the most effective observational study designs for identifying a cause-and-effect relationship between predictor and outcome variables. The other is that psychosocial variables, which are the most underappreciated but crucial determinant factors for adverse birth outcomes, were also included in our study to estimate the effect of the exposure variable while minimizing the effects of confounding variables. The third is that being a multisite study, it allowed us to generalize the results to the Amhara Regional State as well as the national level, Ethiopia. Despite the strengths, this study has a limitation related to institutional settings where the study included only pregnant women who had ANC follow up. However, there are some pregnant women in the community who did not have ANC follow-up as well as PMTCT services for a variety of reasons. Not including those pregnant women who did not have ANC follow-up as well as PMTCT services could result in an under or over report on the study’s findings.
Conclusion and Recommendation
Our findings illustrate that maternal HIV infection increases the risk of LBW, and preterm birth among HIV positive women. This necessitates due attention while providing maternal health services particularly antenatal care and delivery services primarily for women with HIV infection. Prevention strategies aimed at reducing these adverse pregnancy outcomes should be developed and implemented. Although HIV infection is not a modifiable factor, it is possible to mitigate its impact by ending new incidence of HIV infection which may reduce LBW by 32% and preterm birth by 47%. Premature rupture of membrane, household food insecurity, pregnancy-induced hypertension, intimate partner violence, and antepartum haemorrhage should be targeted in the prevention process of these adverse pregnancy outcomes.
Abbreviations
ANC, Antenatal Care; APH, Ante partum haemorrhage; ARR, Adjusted Relative Risk; ART, Antiretroviral Treatment; CI, Confidence interval; EPDS, Edinburgh postnatal depression scale; ETB, Ethiopian Birr; GLM, Generalized Linear Model; HIV, Human immunodeficiency virus; IRB, institutional review board; LBW, Low Birth Weight; LMP, Last menstrual period; IPV, Intimate partner violence; MSSS, Maternity Social Support Scale; MTCT, mother-to-child transmission; MUAC, Mid-upper arm circumference; NVP, nevirapine; PA, Population attributable risk; PIHTN, pregnancy induced hypertension; PMTCT, Prevention of mother-to-child transmission; PPH, Postpartum haemorrhage; PROM, Premature rupture of membranes; RR, Relative risk; SDG, Sustainable Development Goals; UNAIDS, The Joint United Nations Programme on HIV/AIDS; UNFPA, the United Nations Population Fund; WHO, World Health Organization; WHOQOL, World Health Organization quality of life.
Data Sharing Statement
We can share the data if there are reasonable requests. Data can be shared by the corresponding author.
Ethical Approval and Consent to Participate
Ethical approval was obtained from the institutional review board (IRB) of University of Gondar with identification number of (Ref.No;V/P/RCS/05/1977/2020). Letters of permission were granted from the three referral hospitals as well as from the ANC clinics’ focal persons of the facility prior to the study. Participants in the study were asked to participate completely voluntarily, and written informed consent was obtained from each participate after a thorough explanation of the study’s purpose and significance. Codes were used instead of personal identifiers. Face-to-face interviews were conducted in an enclosed area with only one participant present at a time. The data was stored in a secure, lockable cabin. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We are grateful to the three Amhara Regional State referral hospitals (University of Gondar Comprehensive Specialized Hospital, Felege Hiwot Referral Hospital, and Debre Tabor Referral Hospital) for allowing us to conduct this research. We would like to express our gratitude to the data collectors and supervisors for their dedication and cooperation throughout the data collection process. We would also like to thank everyone who participated in the tool validation and language translation process. Finally, we would like to express our appreciation to the study participants without whom the study could not be realized.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was obtained.
Disclosure
The authors report no conflicts of interest in relation to this work.
References
1. UNAIDS. Global HIV & AIDS statistics: fact sheet 2021; 2021. Available from: https://www.unaids.org/sites/default/files/media_asset/.
2. World Health Organization. World Health Organization: global progress report on HIV viral hepatitis and sexually transmitted infections, 2021. Accountability for the global health sector strategies 2016–2021: actions for impact. Geneva switzerland; 2021.
3. Assefa Y, Gilks CF. Ending the epidemic of HIV/AIDS by 2030: will there be an endgame to HIV, or an endemic HIV requiring an integrated health systems response in many countries? Int J Infect Dis. 2020;100:273–277. doi:10.1016/j.ijid.2020.09.011
4. Mirkuzie AH, Ali S, Abate E, Worku A, Misganaw A. Progress towards the 2020 fast track HIV/ AIDS reduction targets across ages in Ethiopia as compared to neighboring countries using global burden of diseases 2017 data. BMC Public Health. 2021;21(285). doi:10.1186/s12889-021-0269-y
5. Ethiopian Ministry of Health. HIV prevention in Ethiopia national road map 2018–2020, federal HIV/AIDS prevention and control office of Ethiopia Addis Ababa; 2018.
6. Ethiopian public health institute, HIV Related Estimates and Projections for Ethiopia–2017; 2017. Available from: http://repository.iifphc.org/bitstream/handle/123456789/465/HIV_estimation_and_projection_for_Ethiopia_2017%20.pdf?sequence=1&isAllowed=y.
7. UNAIDS. Ethiopia 2020 HIV and AIDS Estimates Country fact sheets: 2020; 2020. Available from: https://www.unaids.org/en/regionscountries/countries/Ethiopia.
8. Reis HLBD, Araujo K, Ribeiro LP, et al. Preterm birth and fetal growth restriction in HIV-infected Brazilian pregnant women. Revista do Instituto de Medicina Tropical de São Paulo. 2015;52(2):111–120. doi:10.1590/S0036-46652015000200003
9. Tukei VJ, Hoffman HJ, Greenberg L, et al. Adverse pregnancy outcomes among HIV-positive women in the era of universal antiretroviral therapy remain elevated compared with HIV-negative women. Pediatr Infect Dis J. 2021;40(9):821–826. doi:10.1097/INF.0000000000003174.
10. Xiao P-L, Zhou Y-B, Chen Y, et al. Association between maternal HIV infection and low birth weight and prematurity: a meta-analysis of cohort studies. BMC Pregnancy Childbirth. 2015;2015(15):246. doi:10.1186/s12884-015-0684-z
11. Chilaka VN, Konje JC. HIV in pregnancy: an update. Eur J Obstet Gynecol Reprod Biol. 2021;2021(256):484–491. doi:10.1016/j.ejogrb.2020.11.034
12. Li H, Liu J, Tan D, et al. Maternal HIV infection and risk of adverse pregnancy outcomes in Hunan province, China A prospective cohort study. Medicine. 2020;99(8):e19213. doi:10.1097/MD.0000000000019213
13. World Health Organization. Survive and thrive: transforming care for every small and sick newborn. Geneva: World Health Organization; 2019. Available from: https://apps.who.int/iris/bitstream/handle/10665/276655/WHO-FWC-MCA.
14. Degno S, Lencha B, Aman R, et al. Adverse birth outcomes and associated factors among mothers who delivered in Bale zone hospitals, Oromia Region, Southeast Ethiopia. J Int Med Res. 2021;49(5):1–12. doi:10.1177/03000605211013209
15. Kassahun EA, Mitku HD, Getu MA. Adverse birth outcomes and its associated factors among women who delivered in North Wollo zone, northeast Ethiopia: a facility based cross-sectional study. BMC Res Notes. 2019;2019(12):357. doi:10.1186/s13104-019-4387-9
16. Environment AsCat. Adverse birth outcomes america’s children and the environment; 2019:264–266. Available from: https://www.epa.gov/sites/default/files/2019-08/documents/ace3-birth-outcomes-updates.pdf.
17. Baskaradoss JK, Geevarghese A, Dosari AAFA. Causes of adverse pregnancy outcomes and the role of maternal periodontal status: a review of the literature. Open Dent J. 2012;6(1):79–84. doi:10.2174/1874210601206010079
18. Blencowe H, Krasevec J, Onis M, et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Global Health. 2015;7(7):e849–e60. doi:10.1016/S2214-109X(18)30565-5
19. Mulualem G, Wondim A, Woretaw A. The effect of pregnancy induced hypertension and multiple pregnancies on preterm birth in Ethiopia: a systematic review and meta-analysis. BMC Res Notes. 2019;2019(12):91. doi:10.1186/s13104-019-4128-0
20. World Health Organization. Global nutrition targets 2025: low birth weight policy brief Geneva World Health Organization; 2014. Available from: https://www.who.int/publications/i/item/WHO-NMH-NHD-14.5.
21. WHO/UNICEF. Discussion paper The extension of the 2025 Maternal, Infant and Young Child nutrition targets to 2030. Available from: https://www.who.int/nutrition/global-target-2025/discussion-paper-extension-targets-2030.pdf.
22. Macdonald EM, Ng R, Bayoumi AM, et al. Adverse neonatal outcomes among women living with HIV: a population-based study. J Obstet Gynaecol Can. 2015;37(4):302–309. doi:10.1016/S1701-2163(15)30279-6
23. Ezechi OC, DA Oladle, Kalejaiye OO, et al. Pregnancy, obstetric and neonatal outcomes in HIV positive Nigerian women. Afr J Reprod Health. 2013;17(3):160–168.
24. Santosaa WB, Staines-Uriasa E, Tshivuila-Matalaa OO, Norrisb SA, Hemelaar J. Perinatal outcomes associated with maternal HIV and antiretroviral therapy in pregnancies with accurate gestational age in South Africa. AIDS. 2019;33(10):1623–1633.
25. Lopez M, Figueras F, Hernandez S, et al. Association of HIV infection with spontaneous and iatrogenic preterm delivery: effect of HAART. AIDS Behav. 2012;26(1):37–43. doi:10.1097/QAD.0b013e32834db300
26. Ethiopia profile of preterm and low birth weight prevention and care. American college of nurse-midwives; 2015. Available from: http://www.everypreemie.org/wpcontent/uploads/2015/11/Ethiopia.
27. Yang M, Wang Y, Chen Y, Zhou Y, Jiang Q. Impact of maternal HIV infection on pregnancy outcomes in southwestern China - a hospital registry based study. Epidemiol Infect. 2019;147(e124):1–9. doi:10.1017/S0950268818003345
28. Mulatu T, Debella A, Feto T, Dessie Y. Determinants of stillbirth among women who gave birth at Hiwot Fana Specialized University Hospital, Eastern Ethiopia: a facility-based cross-sectional study. SAGE Open Med. 2022;2022(10):1–7. doi:10.1177/20503121221076370
29. EDHS. Ethiopia demographic and health survey 2016, Central Statistical Agency (CSA), Addis Ababa, Ethiopia, and Rockville, Maryland, USA: CSA and ICF; 2016; 2016.
30. González R, Rupérez M, Sevene E, et al. Effects of HIV infection on maternal and neonatal health in southern Mozambique: a prospective cohort study after a decade of antiretroviral drugs roll out. PLoS One. 2017;12(6):e0178134. doi:10.1371/journal.pone.0178134
31. FentieID EA, Yeshita HY, Bokie MM. Low birth weight and associated factors among HIV positive and negative mothers delivered in northwest Amhara region referral hospitals, Ethiopia,2020 a comparative cross sectional study. PLoS One. 2020;17(2):e0263812. doi:10.1371/journal.pone
32. Kebede B, Andargie G, Gebeyehu A. Birth outcome and correlates of low birth weight and preterm delivery among infants born to HIV-infected women in public hospitals of Northwest Ethiopia. Sci Res. 2013;5(7):25–34. doi:10.4236/health.2013.57A4004
33. Rahmana M, Nasrina SO, Rahmana M, et al. Maternal pregnancy intention and its association with low birthweight and pregnancy complications in Bangladesh: findings from a hospital-based study Md. Int Health. 2019;11(6):447–454. doi:10.1093/inthealth/ihz010
34. Alemu A, Abageda M, Assefa B, Melaku G. Low birth weight: prevalence and associated factors among newborns at hospitals in Kambata-Tembaro zone, southern Ethiopia 2018. Pan Afr Med J. 2019;34:68. doi:10.11604/pamj.2019.34.68.18234
35. Butali A, Ezeaka C, Ekhaguere O, et al. Characteristics and risk factors of preterm births in a tertiary center in Lagos, Nigeria. Pan Afr Med J. 2016;24(1). doi:10.11604/pamj.2016.24.1.8382
36. Tamirat KS, Sisay MM, Tesema GA, Tessema ZT. Determinants of adverse birth outcome in Sub-Saharan Africa: analysis of recent demographic and health surveys. BMC Public Health. 2021;21:1092. doi:10.1186/s12889-021-11113-z
37. Abadiga M, Mosisa G, Tsegaye R, Oluma A, Abdisa E, Bekele T. Determinants of adverse birth outcomes among women delivered in public hospitals of Ethiopia. Archiv Public Health. 2022;80:12. doi:10.1186/s13690-021-00776-0
38. Gebreslasie KZ, Weldemariam S, Gebre G, Mehari M-A. Intimate partner violence during pregnancy and risk of still birth in hospitals of Tigray region Ethiopia. Ital J Pediatr. 2020;46:107. doi:10.1186/s13052-020-00857-w
39. Chowdhury M, Dibley MJ, Alam A, Huda TM, Raynes-Greenow C. Household food security and birth size of infants: analysis of the Bangladesh Demographic and Health Survey 2011. Curr Dev Nutr. 2018;2(3):nzy003–nzy004. doi:10.1093/cdn/nzy003
40. Sahlu D, Deyessa N, Firdu N, Asfaw S. Food insecurity and other possible factors contributing to low birth weight: a case control study in Addis Ababa, Ethiopia. Asian Pac J Reprod. 2020;9(4):174–181. doi:10.4103/2305-0500.288585
41. Patil S, Bhosale R, Sambarey P, et al. Impact of maternal human immunodeficiency virus infection on pregnancy and birth outcomes in Pune, India. AIDS Care. 2011;23(12):1562–1569.
42. Health Sector Development Program IV 2010/11 – 2014/15. Ethiopia Ministry of Health, Addis Ababa. Available from: https://www.healthynewbornnetwork.org/hnn-content/uploads/HSDP-IV-Final-Draft-October-2010-2.pdf.
43. Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013;35(2):121–126. doi:10.4103/0253-7176.116232
44. Dean AG, Sullivan KM, Soe MM. Epi Info and OpenEpi in epidemiology and clinical medicine: health applications of free software: createSpace; 2010. Available from: https://www.epiinformatics.com/DownloadFiles/EpiBook.pdf.
45. Wardlaw T, Blanc A, Zupan J, Åhman E; World Health Organization, UNICEF. Low birthweight: country regional and global estimates. Geneva: New York: WHO; UNICEF; 2004. Available from: https://apps.who.int/iris/bitstream/handle/10665/43184/9280638327.
46. Laopaiboon M, Lumbiganon P, Rattanakanokchai S, et al. An outcome-based definition of low birthweight for births in low- and middleincome countries: a secondary analysis of the WHO global survey on maternal and perinatal health. BMC Pediatr. 2019;19:166. doi:10.1186/s12887-019-1546-z
47. World Health Organization. Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization; 2012.
48. Silvaa FTD, Gonik B, McMillanc M, et al. Stillbirth: case definition and guidelines for data collection, analysis, and presentation of maternal immunization safety data The Brighton Collaboration Stillbirth Working Group. Vaccine. 2016;34(49):6057–6068. doi:10.1016/j.vaccine.2016.03.044
49. World Health Organization. Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-Related Disease in Adults and Children. Geneva: World Health Organization WHO; 2007.
50. Federal HIV/AIDS Prevention and Control Office Ethiopian Ministry of Health, FHAPCO/FMOHE. Guidelines for HIV counseling and testing in Ethiopia; 2007. Available from: https://www.ilo.org/wcmsp5/groups/public/—ed_protect/—protrav/—ilo_aids/documents/legaldocument/wcms_125389.pdf.
51. Bisetegn TA, Mihretie G, Muche T. Prevalence and predictors of depression among pregnant women in Debre Tabor Town, Northwest Ethiopia. PLoS One. 2016;11(9):e0161108. doi:10.1371/journal.pone
52. DadiID AF, MillerID ER, Woodman RJ, Azale T, Mwanri L. Effect of antenatal depression on adverse birth outcomes in Gondar town, Ethiopia: a community-based cohort study. PLoS One. 2020;15(6):e0234728. doi:10.1371/journal.pone
53. World Health Organization. The World Health Organization Quality of Life (WHOQOL)-BREF. Geneva 27, Switzerland: World Health Organization; 2004.
54. Webster J, Linnane JW, Dibley LM, Hinson JK, Starrenburg SE, Roberts JA. Measuring social support in pregnancy: can it be simple and meaningful? Birth. 2000;27(2):97–101. doi:10.1046/j.1523-536x.2000.00097.x
55. Ballard T, Coates J, Swindale A, Deitchler M. Household hunger scale: indicator definition and measurement guide. Washington, DC: Food and Nutrition Technical Assistance II Project FHI 360; 2011. Available from: www.fantaproject.org.
56. Schraiber LB, MdRDO L, Jr IF, Segri NJ, D’Oliveira AFP. Validity of the WHO VAW study instrument for estimating gender-based violence against women. Rev Saúde Pública. 2010;44(4):658–666. doi:10.1590/s0034-89102010000400009
57. García-Moreno C, Jansen HAF, Ellsberg M, Heise L, Watts C. WHO multi-country study on women’s health and domestic violence against women: initial results on prevalence, health outcomes and women’s responses World Health Organization. World Health Organization; 2005. Available from: https://apps.who.int/iris/handle/10665/43309.
58. McNutt L-A, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–943. doi:10.1093/aje/kwg074
59. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi:10.1093/aje/kwh090
60. Yelland LN, Salter AB, Ryan P. Performance of the modified poisson regression approach for estimating relative risks from clustered prospective data. Am J Epidemiol. 2011;174(8):984–992. doi:10.1093/aje/kwr183
61. Zou G, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22(6):661–670. doi:10.1177/0962280211427759
62. Jena BH, Biks GA, Gete YK, Gelaye KA. The effect of inter-pregnancy interval on stillbirth in urban South Ethiopia: a community-based prospective cohort study. BMC Pregnancy Childbirth. 2021;21:847. doi:10.1186/s12884-021-04325-z
63. Malaba TR, Phillips T, Roux SL, et al. Antiretroviral therapy use during pregnancy and adverse birth outcomes in South African women. Int J Epidemiol. 2017;2017:1678–1689. doi:10.1093/ije/dyx136
64. Kreitchmann R, Li SX, Melo VH, et al. Predictors of adverse pregnancy outcomes in HIV infected women in latin america and the Caribbean: a cohort study. BJOG. 2014;121(12):1501–1508.
65. Arab K, Spence AR, Czuzoj-Shulman N, Abenhaim HA. Pregnancy outcomes in HIV-positive women: a retrospective cohort study. Arch Gynecol Obstet. 2017;295(3):599–606. doi:10.1007/s00404-016-4271-y
66. Zenebe A, Eshetu B, Gebremedhin S. Association between maternal HIV infection and birthweight in a tertiary hospital in southern Ethiopia: retrospective cohort study. Ital J Pediatr. 2020;46:70. doi:10.1186/s13052-020-00834-3
67. World Health Organization. World Health Organization guidelines for PMTCT, global PMTCT targets, progress in the prevention of mother-to-child-transmission, barriers to the uptake of PMTCT programmes and the future of PMTCT programming. World Health Organization; 2020. Available from; https://www.avert.org/printpdf/node/375.
68. UNAIDS. Understanding FastTrack; accelerating action to end the AIDS epidemic by 2030. Geneva; 2014. Available from: http://wwwunaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf.
69. Rollins NC, Essajee SM, Bellare N, Doherty M, Hirnschall GO. Improving retention in care among pregnant women and mothers living with HIV: lessons from INSPIRE and implications for future WHO guidance and monitoring. J Acquir Immune Defic Syndr. 2017;75(2):S111–S114. doi:10.1097/QAI.0000000000001366
70. Rasmussen DN, NoelVieira BL, SilvaTé D, et al. HIV 1 and HIV 2 prevalence, risk factors and birth outcomes among pregnant women in Bissau, Guinea Bissau: a retrospective cross sectional hospital study. Sci Rep Nature Res. 2020;2020:12174. doi:10.1038/s41598-020-68806-5
71. BaterID J, Lauer JM, Ghosh S, et al. Predictors of low birth weight and preterm birth in rural Uganda: findings from a birth cohort study. PLoS One. 2020;15(7):e0235626. doi:10.1371/journal.pone.0235626
72. Boer K, Nellen JF, Patel D, et al. Pregnancy outcome in HIV-1-infected pregnant women under effective highly active antiretroviral therapy and a policy of vaginal delivery. Int J Gynaecol Obstet. 2007;2007(114):148–155. doi:10.1111/j.1471-0528.2006.01183.x
73. Zeleke BM, Zelalem M, Mohammed N. Incidence and correlates of low birth weight at a referral hospital in Northwest Ethiopia. Pan Afr Med J. 2012;12(4):1937–8688.
74. Ndu IK, Edelu BO, Uwaezuoke SN, et al. Maternal risk factors associated with low birth weight neonates: a multicentre, cross-sectional study in a developing country. J Neonatal Biol. 2015;4(3). doi:10.4172/2167-0897.1000190
75. Salihu HM, Stanley KM, August EM, Weldeselasse H, Mbah AK, Whiteman VE. The association between HIV/AIDS during pregnancy and fetal growth parameters in Florida: a population based study. Curr HIV Res. 2012;10(6):539–545. doi:10.2174/157016212802429839
76. Kumar SB, Handelman SK, Voronkin I, et al. Different regions of HIV-1 subtype C env are associated with placental localization and in utero mother-to-child transmission. J Virol. 2011;85(14):7142–7152. doi:10.1128/JVI.01955-10
77. Moussa M, Roques P, Fievet N. Placental cytokine and chemokine production in HIV-1-infected women: trophoblast cells show a different pattern compared to cells from HIV-negative women. Clun Exp Immunol. 2001;125(3):455–464. doi:10.1046/j.365-2249.001.01629.x
78. Magiorkinis G, Angelis K, Mamais I, et al. The global spread of HIV-1 subtype B epidemic. Infect Genet Evol. 2016;2016(46):169–179. doi:10.1016/j.meegid.2016.05.041
79. Mohammadi H, Papp E, Cahill L, et al. HIV antiretroviral exposure in pregnancy induces detrimental placenta vascular changes that are rescued by progesterone supplementation. Nature Sci Report. 2018;8(1):6552. doi:10.1038/s41598-018-24680-w
80. Papp E, Mohammadi H, Loutfy MR, et al. HIV protease inhibitor use during pregnancy is associated with decreased progesterone levels, suggesting a potential mechanism contributing to fetal growth restriction. J Infect Dis Epidemiol. 2015;211(1):10–18.
81. Pee S, Semba RD. Role of nutrition in HIV infection: review of evidence for more effective programming in resource-limited settings. Food Nutr Bull. 2010;31(4):S313–44. doi:10.1177/15648265100314S403
82. World Health Organization. Comprehensive implementation plan on maternal, infant and young child nutrition. WHA 65/6. Geneva: World Health Organization; 2012. Available from: https://apps.who.int/iris/bitstream/handle/10665/113048/WHO_NMH_NHD_14.1_eng.pdf.
83. Snijdewind IJM, Smit C, Godfried MH, et al. Preconception use of cART by HIV-positive pregnant women increases the risk of infants being born small for gestational age. PLoS One. 2018;13(1):e0191389. doi:10.1371/journal.pone
84. EON Samuels, AY Isah, RA Offlong, Ba E. Foeto-maternal outcome of HIV-positive pregnant women on Highly Active Antiretroviral Therapy. Int J Med Biomed Res. 2014;3(3):202–208. doi:10.14194/ijmbr.3.3.8
85. Li N, Sando MM, Spiegelman D, et al. Antiretroviral therapy in relation to birth outcomes among HIV-infected women: a cohort study. J Infect Dis Epidemiol. 2016;213(7):1057–1064.
86. Fiore S, Newell M-L, Trabattoni D, et al. Antiretroviral therapy-associated modulation of Th1 and Th2 immune responses in HIV-infected pregnant women. J Reprod Immunol. 2006;70(1–2):143–150. doi:10.1016/j.jri.2005.12.001
87. Chen JY, Ribaudo HJ, Souda S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. 2012;206(11):1695–16705. doi:10.1093/infdis/jis553
88. Wang L, Zhao H, Cai W, et al. Risk factors associated with preterm delivery and low delivery weight among HIV-exposed neonates in China. Int J Gynecol Obstet. 2018;142(3):300–307. doi:10.1002/ijgo.12532
89. Ejigu Y, Magnus JH, Sundby J, Magnus MC. pregnancy outcome among HIVinfected women on different antiretroviral therapies in Ethiopia: a cohort study. BMJ Open. 2019;9(8):e027344. doi:10.1136/bmjopen-2018-027344
90. World Health Organization. Neonatal and perinatal mortality: country, regional and global estimates. World Health Organization; 2006. Available from: https://apps.who.int/iris/handle/10665/43444.
91. Chi BH, Wang L, Read JS, et al. Predictors of stillbirth in sub-saharan Africa. Obstet Gynecol Clin North Am. 2007;110(5):989–997. doi:10.1097/01.AOG.0000281667.35113.a5
92. Ball H. HIV may not increase risk of stillbirth among women in Sub-Saharan Africa. Int Perspect Sex Reprod Health. 2008;34(1):53.
93. Thompson KD, Meyers DJ, Lee Y, Cu-Uvin S, Wilson IB. HIV-positive and HIV-negative women with medicaid have similar rates of stillbirth and preterm birth. Women Health Report. 2022;3(1):1–9. doi:10.1089/whr.2021.0068
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
