Back to Journals » Clinical Ophthalmology » Volume 12
Effects of different sleeping positions on intraocular pressure in secondary open-angle glaucoma and glaucoma suspect patients
Authors Sedgewick JH , Sedgewick JA, Sedgewick BA, Ekmekci B
Received 25 January 2018
Accepted for publication 5 May 2018
Published 1 August 2018 Volume 2018:12 Pages 1347—1357
DOI https://doi.org/10.2147/OPTH.S163319
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jeffrey H Sedgewick,1 Justin A Sedgewick,2 Brandon A Sedgewick,2 Berk Ekmekci3
1Private Practice, Ashburn, Virginia, 2Department of Psychology, University of Virginia, 3Department of Statistics, University of Virginia, Charlottesville, VA, USA
Purpose: The aim of this study was to investigate the effects of different recumbent sleeping positions of the head and body on intraocular pressure (IOP) in secondary open-angle glaucoma and glaucoma suspect patients, specifically pigmentary dispersion (PD) as measured using the ICare rebound tonometer.
Patients and methods: A total of 44 eyes of 24 patients with PD were selected in this study. The IOP of 44 eyes was measured in the initial seated position, in the 4 recumbent positions, and again in the sitting position between each of the recumbent positions.
Results: The IOP of the right eyes and left eyes was higher in each of the 4 recumbent positions compared to its initial sitting position (all P<0.001). Dependent (D) vs nondependent (ND) comparisons failed to show a significant difference. All lateral vs prone comparisons showed a higher average IOP in the prone position than in the lateral position regardless of D vs ND status. The range of recumbent IOP changes was -4 to +17 mmHg or -17% to +142%. A total of 64% had at least a ≥33% IOP increase with 43% having a ≥50% increase.
Conclusion: Lateral and prone sleeping positions usually do result in significant elevations of IOP in PD patients. Dependency status did not make a difference. A significantly larger IOP increase was seen in the prone position than in the lateral position. The presence of 3 clinical variables (disk hemorrhage [DH], notches, and BV changes) might increase the chances of developing a large recumbent increase in IOP. These patients and possibly all PD syndrome (PDS) or PD glaucoma (PDG) patients should consider sleeping in a bed that allows a head elevation of 30°.
Keywords: recumbent position intraocular pressure changes, secondary open angle glaucoma, pigmentary dispersion, intraocular pressure, recumbent intraocular pressure changes
Introduction
Intraocular pressure (IOP) is a well-established risk factor for glaucoma1–3 with a lowering of the IOP reducing the risk of progressive glaucoma damage in both ocular hypertensive and normal-tension open-angle glaucoma (OAG) patients.4–7,62 Searching for the etiology of glaucoma damage in normal-tension glaucoma (NTG), while potentially different than pigmentary dispersion (PD), has given rise to examine the effect of calcium channel blockers, ocular perfusion pressure (OPP), central corneal thickness (CCT), corneal hysteresis (CH), diurnal IOP changes, the differences between central cerebral spinal fluid (CSF) pressure and IOP, and IOP changes during recumbent positioning of the body.
Studies over many decades have looked at short-term IOP changes (generally <30 minutes) in sitting position vs various recumbent sleeping positions.1–29 These studies assume that a significant IOP increase over the short-term period in the study translates to an increase in IOP over the entire sleeping period and hence an increase in the risk of developing glaucoma damage. Approximately one-third of our life is spent in various sleeping positions, and IOP measurements are typically not performed during these nonclinical hours. Soft contact lenses with inserted IOP-measuring devices have been developed to obtain this information,51 especially in NTG patients.8 Until wide spread use of such devices is a part of clinical practice, IOP elevations in recumbent sleeping positions remain an important unknown parameter in assessing a patient’s risk for developing glaucoma damage, especially in NTG patients. Prior studies have shown that these increases can be significant, up to 11 mmHg, compared to sitting IOP measurements.10–29 IOP elevations, over many years of sleeping in these recumbent positions, can potentially increase a patient’s risk of developing glaucomatous damage. Recommended initial IOP reduction goals in primary OAG (POAG) are between 20% and 30%.9 The prostaglandin analogs typically reduce IOP by 25%–33%.9 Increases in IOP of this magnitude while sleeping can potentially be clinically significant. An avoidance or reduction in recumbent positioning has not entered wide spread clinical use, probably due to a lack of commercially available beds that can elevate the head without whole body depression in a cost-effective manner.
Recumbent position increases in IOP have perhaps been an underappreciated clinical tool. There is no mention of altering recumbent sleeping positioning in reducing the risk of developing glaucoma or in the treatment of POAG in the current POAG Preferred Practice Pattern (PPP) 2015.9 Barring extensive replication of the many sitting POAG clinical studies, we can attempt to approximate the lessons learned in these studies to recumbent positioning concerns and assume that avoiding IOP elevation in recumbent positioning may reduce the risk of developing glaucomatous damage. The secondary OAGs (SOAGs), such as PD syndrome (PDS) and PD glaucoma (PDG), may require its own unique extrapolation of the OAG studies as well.
A number of studies have shown that a normal, nonglaucoma patient’s IOP can significantly increase in recumbent sleeping positions.10–18 All of the following studies have statistical significance at least to the P<0.01 level unless otherwise indicated.
This significant increase in IOP in recumbent positions is also seen in NTG patients,14,19 newly diagnosed and untreated OAG,20 treated OAG patients with new disk hemorrhage (DH) (P=0.03),21 treated OAG,12 OAG patients having had a trabeculectomy performed,22 ocular hypertension patients,14 and primary angle closure glaucoma patients.12 These IOP increases have been shown to be larger in OAG patients receiving treatment than in OAG suspects.23
Furthermore, a significantly larger recumbent IOP increase was found in the worse eye (measured by mean deviation [MD]) of bilateral, asymmetric POAG patients (P=0.0224 and P=0.017625) and in the worse eye of asymmetric, bilateral NTG patients.26 One study of NTG patients showed a significant correlation between a worsening Humphrey visual field (HVF) loss, as measured on the visual field index (VFI) MD slope, and a larger magnitude of recumbent IOP increase.19 Furthermore, there was no change in recumbent IOP increases in pretreating normal-tension patients with Timolol Maleate, then with Latanoprost, and then with Azopt (Brinzolamide) (in a random order).27 Having a trabeculectomy performed does not reduce the recumbent IOP increases22 nor does pretreatment with a beta blocker in normal patients.15
Recent studies have included the common sleeping positions of lateral decubitus and prone with head turn and have compared the IOP between the lower or dependent (D) and the higher or nondependent (ND) of the patient’s two eyes. A higher average recumbent IOP elevation has sometimes been found in the D position than in the ND position. In a recent study of bilateral, asymmetric NTG patients, the worse eye and better eye showed no difference in IOP in the sitting position but the worse eye had a significantly higher IOP increase than the better eye in its D lateral decubitus position and in the supine position.28 All eyes in the D position showed a significantly higher increase in IOP than ND eyes in healthy young patients in the lateral decubitus and in the prone with head turn positions10 and in untreated, bilateral, newly diagnosed OAG patients (P<0.05).20 NTG and OAG (P=0.013) patients with asymmetric bilateral glaucoma preferred the worse eye in the D lateral decubitus position on a questionnaire.29
The effort to understand the etiology of NTG despite having “normal” IOP during day time clinical examinations has been sought for decades. Recumbent positioning studies simulating various sleeping positions have been thought to be one etiological source. Furthermore, significant elevation of IOP, above normal IOP levels, in recumbent sleeping positions in NTG patients, calls into question the appropriateness of the diagnosis of normal tension. Despite the large amount of research supporting the importance of recumbent positing as listed earlier, a change in sleeping position in order to reduce the risk of glaucoma has not entered wide spread clinical use, probably due to a lack of appropriate bedding.
SOAG and glaucoma suspect patients, such as PDG and PDS, have not, to the best of our knowledge, been studied for IOP increases in various recumbent sleeping positions despite PDG/PDS patients having a propensity for severely elevated IOP after exercise,30 even though this is probably a rare event.31,32 This study is the first that we are aware of examining recumbent IOP changes in SOAG patients, specifically PD.
Patients and methods
This is a prospective, observational study. This study adhered to the World Medical Association Declaration of Helsinki. Written informed consent was obtained from all subjects before participating in the study. Institutional Review Board (IRB) approval from Aspire was obtained, and the study was Health Insurance Portability and Accountability Act (HIPAA) compliant. All PDS and PDG patients seen in a general ophthalmologist’s office (the main author) from 2014 to 2017 were asked to participate. Patients were excluded if gonioscopy did not reveal CBB and 3–4+ trabecular meshwork (TM) pigment continuously for 360°; pseudo exfoliation was present; optic disk drusen, optic pits, or other known sources of visual field abnormalities were present; other causes of transillumination (TI) defects were present; a history of ocular trauma was present; or orthostatic hypotension was in the past medical history.
The subjects had undergone a complete ophthalmology examination prior to the study. The same rebound tonometer was used in all participants with the same person taking all of the readings (second author). Studies of air puff, noncontact tonometers, similar to the ICare in which no topical anesthesia is used before testing, have shown no change in measurements after repeated NCT readings.33
Not all patients included in this study had Krukenberg’s spindles (KS), but all patients had to have 3–4+ continuous pigment in the TM consistent with PDS/PDG and either KS or radial TI defects consistent with PDS/PDG. An eye was included if it had 3–4+ TM pigment and did not have KS or TI, but the opposite eye had 3–4+ continuous TM pigment and either KS or radial TI consistent with PDS/PDG (8 eyes of 8 patients). We measured IOP between 9 am and 5 pm. The ICare rebound tonometer takes 6 IOP continuous measurements. The highest and lowest IOP measurements of the 6 are automatically discarded by the tonometer. The remaining 4 readings were averaged, and the reliability was assessed by the tonometer with “P” readout. Valid averages have no line, a line under, or a line half way into the P. Invalid readings are displayed with a line above the P. Invalid readings were discarded, and a new set of 6 readings was obtained. Readings were continued until a reliable set of IOP measurements was obtained for each position. The right eye was measured first in all positions. The patient was asked to look straight ahead and the IOP measurements were made to the center of the patient’s cornea. The ICare tonometer was rested on the patient’s forehead with the tonometer probe always parallel to the floor as per the manufacturer’s recommendations. Only the ICare Pro, which was not approved in the United States in the beginning of the study, can measure IOP in the supine position, and therefore, supine IOP was not measured in this study.
For each patient, the initial sitting IOP was obtained after sitting for 5 minutes. To randomize the order of the recumbent positions, the patient was asked to select one of the 4 cards with one of the following 4 different recumbent positions written on it: right lateral (laying on the right side facing left), left lateral (laying on the left side facing right), prone (laying on the stomach) with right head turn, and prone with left head turn. The patient selected each card individually, and this determined the order of recumbent positions for the study. The patient was placed in each recumbent position for 5 minutes before the IOP was measured for that position. After each recumbent position, the patient resumed the sitting position for additional 2 minutes. Then, each eye’s IOP was measured and the next recumbent position was configured. The sitting position IOP between recumbent positions was measured in order to assess whether there was any residual effect of the testing by comparing the sitting IOP after the third recumbent position with the initial sitting IOP. Sitting IOP was not measured after the last recumbent position. This resulted in 176 recumbent IOP readings (44 eyes in 4 different recumbent positions) and 44 initial sitting IOP measurements.
The room was kept in a semidarkened condition with soft music playing in the background. The door to the examination room was closed to further seclude the patient from external noise. No other patients were being examined in the office at the time of the testing. A total of 1–2 soft pillows were used below the head to keep the head level with the body or to help with comfort in the prone position. Hard vs soft pillows were not found to have a difference in the IOP increase in normal subjects between supine and lateral decubitus positioning.50 A Reliance ophthalmic chair was fully extended parallel to the floor for all recumbent positions. Care was taken to ensure that the patient was as comfortable as possible before any IOP measurements were obtained.
Charts were reviewed for age at first visit, precataract surgery glasses spherical equivalent prescription, HVF MD and slope on VFI, initial visit IOP, average pretreatment IOP, central corneal thickness intraocular pressure correction (CCT), age, cup-to-disk (C/D) ratio, and type of PD (PDG vs PDS). All charts and photos were reviewed for the presence of disk hemorrhages (DHs), nerve fiber layer defects (NFLDs), and notches. No patients had undergone LASIK. None of the patients were taking IOP-lowering drops at the time of the initial visit, and none had undergone laser peripheral iridectomy (LPI) or argon laser trabeculoplasty (ALT) or selective laser trabeculoplasty (SLT) treatments prior to their initial visit to the primary author’s clinic. One patient had taken “drops” years before but had stopped on their own accord.
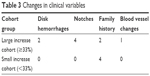
The cohort of eyes that responded in any of the 4 recumbent positions with a rise of ≥33% (28 of 44 eyes, termed the “large increase cohort”) in any of the trials were compared with the cohort of eyes that responded with a <33% IOP rise (16 of 44 eyes, termed the “low increase cohort”) in an effort to determine if a baseline clinical risk factor resulting in a large IOP increase could be identified.
The D position for the right eye is right lateral and prone with left head turn. The D position for the left eye is left lateral and prone with right head turn. The ND position for the right eye is left lateral and prone with right head turn. The ND position for the left eye is right lateral and prone with left head turn.
An earlier study of normal patients concluded that, using an α=0.05, a sample size of 40 eyes of 20 patients would be required to detect a >2 mmHg rise in recumbent IOP with a power of 80%.10 A study of untreated, newly diagnosed POAG patients calculated that 44 eyes of 22 patients would be required to detect a difference of ≥2 mmHg in D vs ND eyes with a power of 80%.20 Another recumbent IOP change study calculated that 19 eyes would be needed to detect an IOP difference of 2 mmHg at a power of 80%.11 We obtained 44 eyes of 24 consecutive, consenting pigment dispersion patients for this study.
The paired Student’s t-test was used for the same eye and the opposite eye IOP comparisons listed in Table 1. All monocular patients were excluded from opposite eye comparisons in order to ensure proper pairing of eyes for the test and prevent differences in individual eyes from confounding the results of changes in eye position. It should be noted, however, that opposite eye comparison, though paired by individual, do necessitate comparison of unique eyes against one another and introduce a second variable into the statistical test. The average IOP for each individual group was calculated and used for the comparison analysis.
Table 2 illustrates comparisons between the high-responding and low-responding groups for each eye, right and left. Given that these comparisons did not involve eyes from each individual in the two compared groups, the Welch two sample t-tests were used. The average of all pretreatment IOPs was calculated and used for the “pretreatment” IOP comparison analysis.
Results
Forty-four eyes of 24 patients comprised the subject body. Sixteen subjects were male and 8 subjects were female with an average age of 55 years. The average refractive error of precataract surgery was −2.96 diopters with only 3 eyes having a >−7.00 D refractive error (−7.50, −9.50, and −9.50). Thirty-five eyes of 20 patients had PDS, and 9 eyes of 5 patients were diagnosed with PDG (1 patient had PDS in one eye and PDG in the other eye). By definition, all PDG patients received IOP-lowering treatment and none of the PDS patients did. An LPI was performed in 1 PDS eye and in 5 eyes with PDG. PDG was diagnosed by the main author using standard clinical guidelines9 including reproducible HVF 24-2 defects consistent with glaucoma in the face of repeated IOP measurements >21 (corrected or uncorrected for CCT), a C/D of ≥0.5, and/or other risk factors for developing glaucomatous damage such as the presence of DHs, NFLDs, blood vessel changes, a family history of glaucoma, age >60 years, and a significant asymmetry of the C/D or IOP between the two eyes.
PDG was defined as having had any IOP-lowering intervention performed including SLT and/or the use of chronic glaucoma drops, but not whether an LPI was performed. An LPI was offered for those eyes with PDS and queer configuration on gonioscopy and deemed to be at high risk for conversion to PDG such as IOPs >21 or a C/D of ≥0.5 in any meridian. One PDS patient elected to have an LPI performed in each eye without the need for IOP-lowering treatments before or afterward.
One hundred fifty-four of 176 (88%) trials had an increase in IOP in any of the recumbent positions compared to its initial sitting position. 8 of 176 (4.5%) trials had no change, and 14 of 176 (8%) trials in 10 eyes had a decrease in IOP, usually mild with the average decrease being −1.8 mmHg, or an 8.7% drop. Two of the 10 eyes with a decrease in recumbent IOP had PDG. Only 2 of the 10 eyes with a decrease in IOP had a ≥33% increase (large increase cohort) in 1 or more of the other recumbent trials, 1 with PDS and 1 with PDG. Of the 6 eyes with no change in IOP when going from initial sitting to recumbent positioning, 1 eye had PDG. Four of the 6 eyes with no change had a negative value in a different recumbent position, supporting the idea that eyes with no or negative changes were similar in their response. Only 1 of the 6 eyes with no change had a ≥33% increase in 1 or more of the other recumbent positions.
Twenty eight of the 44 (64%) eyes had a ≥33% IOP increase (large increase cohort) from the initial sitting IOP in at least 1 of its 4 recumbent positions. Twenty-one of these eyes had PDS with 7 eyes having PDG. Sixteen of 44 (36%) eyes had a <33%, or small, increase. Nineteen of 44 (43%) eyes had a ≥50% IOP increase in at least 1 of its 4 recumbent trials.
All right eyes showed a significant elevation of IOP in all 4 of its recumbent positions compared to the average initial baseline IOP (P<0.001). The same was true for the left eye (P<0.001) (Table 1). There was no difference between the initial sitting and the final sitting average IOP for both the right (P=0.21) and left (P=0.86) eyes. There was also no difference between the right eye initial sitting average IOP and the left eye initial sitting average IOP (P=0.99). This last result helps increase the validity of comparing right eye cohorts with left eye cohorts.
On comparing same eye D with ND cohorts in the same recumbent position, no significant difference was observed in any of the 4 same eye comparisons (P-values ranged from 0.36 to 0.94). This is a direct comparison of the same eye in D vs ND status and in the same recumbent positioning without a significant difference being observed. There was also no difference between D and ND status comparing opposite eyes in the same recumbent position in all 4 permutations (Table 1).
On comparing same eye average IOP in lateral vs prone positioning, the prone position average IOP was significantly higher than the lateral position, regardless of D or ND status, for 9 of the 10 permutations at the P<0.01 level with 1 trial significant at the P=0.05 level. These are same eye comparisons of lateral vs prone cohorts showing a significantly higher average for prone positioning in all 8 comparisons, regardless of D or ND status.
On comparing the right eye large increase cohort with the right eye small increase cohort and the same for the left eye, age, precataract surgery glasses spherical equivalent prescription, HVF MD, HVF slope, first IOP (found to be the most significant risk factor for conversion from PDS to PDG in a large general population),60 CCT correction factor in mmHg, and C/D ratio were not found to be significantly different between the two cohorts for either the right eye or the left eye. The first visit IOP was lower for the left and right eye large increase cohorts than the left and right eye small increase cohorts, for unknown reasons (Table 2). Table 3 summarizes the presence of DHs, notches, family history, and optic nerve blood vessel changes for the two cohorts. While a P-value is not reliable for bimodal data, Table 3 shows a much higher presence of DHs, notches, and BV changes for the large increase cohort and may represent the only clinical risk factor for identifying a large increase IOP response in recumbent positioning. Of the 28 eyes that had a large IOP increase in any recumbent trial, 7 (25%) eyes had PDG and 21 (75%) eyes had PDS. PDG constituted 20% and PDS constituted 80% of the overall population of eyes. Although statistical analysis is not possible on ratios, the percentage of PDS (80) and PDG (20) eyes in the total population is about the same ratio as in the large increase cohort group (75% PDS and 25% PDG) so having PDG vs PDS did not appear to increase the risk of developing a large increase in IOP in a recumbent position.
  | Table 3 Changes in clinical variables |
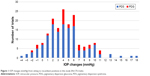
The mean of the IOP increases for all of the 176 trials was (+)3.994±3.363 mmHg (standard deviation). The range of recumbent IOP changes was −4 to +17 mmHg for all 176 recumbent trials compared to the initial sitting IOP, with +17 being the largest increase in any horizontal (lying flat) study to date,45–47 although 1 case report also had a postexercise 17 mmHg increase in a PDS patient, representing only a 46% increase from baseline.30 Our percentage range of change was −17 to +142, which represent the largest IOP increases in any flat recumbent study or report to date. Figures 1 and 2 present these data in a bar graph for increases in mmHg and the percentage of IOP changes, respectively.
The Ocular Hypertensive Treatment Study (OHTS) found that DHs were missed in 86% of clinical examinations by academic glaucoma specialists but were found upon photo review.37 No DHs, NFLDs, or notches were found upon photo review that was not documented in the charts.
Discussion
This study confirms many earlier reports of significant elevation in IOP when going from a sitting position to a flat recumbent position, including the common sleeping positions of lateral decubitus and prone with head turn. We found the highest average sitting to recumbent position IOP increases published so far using patients with PD, either PDG or PDS. All 8 cohorts of the same eye initial sitting to recumbent position had a higher average IOP in the recumbent position. This is consistent with many earlier studies for a number of ocular conditions.10–29 We found prone positioning having a statistically higher increase than lateral decubitus in all 10 same eye permutations analyzed.
Prior studies in normal patients have shown a significantly higher recumbent IOP elevation in D eyes vs ND eyes in lateral decubitus and prone with head turn positioning when the initial sitting and supine IOP between the two eyes were statistically same.10,11,20,28,29 Furthermore, that same study showed that the magnitude of IOP increase in all eyes in the D position was greater in prone with head turn than in the lateral decubitus position (P<0.001). Another study in the newly diagnosed and untreated OAG patients showed a significantly (P<0.05) higher IOP elevation in the D position than in the ND position in lateral decubitus positioning.20 These findings may be due to changes in the position of the internal carotid artery and the internal jugular vein, which have been shown to significantly change with head turning39 and which may increase the venous pressure and hence the episcleral pressure. We did not find a significant difference between D and ND status in any of our comparisons. These comparisons include 4 same eye and 4 opposite eye comparisons (Table 1).
We measured IOP 5 minutes after assuming a recumbent position and 2 minutes after a sitting position. Prior studies have used various times to measure IOP once a sitting position or a recumbent position has been attained. Some studies have used 2 minutes while other studies have used 5 minutes. One study looked at IOP immediately after obtaining a recumbent position and 15 minutes later and found no significant difference.34 Another study looked at IOP in a prone position immediately after assuming the position and 30 minutes later and concluded no change for normal and NTG patients but did find a further increase in OHTN patients.14 Another study looked at a 6° body inversion with IOP measured within 1 minute and for every 30 minutes after that for 90 minutes. It found that IOP elevation occurred within 1 minute and was sustained for 60 minutes and then decreased slightly.18 Another study measured IOP in prone flat and 7° Trendelenburg position 1 minute and every 10 minutes with no significant difference between 10 and 60 minutes.35 Another study completely inverted patients and measured IOP 30 seconds, 60 seconds, and 5 minutes after inversion. Their conclusion was that IOP was steady after 30 seconds and returned to the baseline IOP within 15 seconds after resuming the sitting posture.36 It is fairly clear that IOP changes quickly in different recumbent positions and remains steady for prolonged periods of time.
We chose a ≥33% IOP increase as our large increase cohort based on the 25%–30%9 reduction abilities of our strongest IOP-lowering drug, the prostaglandin analogs, and the results of The Collaborative Normal-Tension Glaucoma Study Group, which found that if IOP is reduced 30%, the probability of the visual fields “surviving” loss is increased from 60% with no treatment to 80% at 4 years of follow up and increased from 30% with no treatment to 60% at 6 years of follow up.5 An avoidance of an IOP increase of this magnitude while sleeping may be significant in halting further glaucoma damage in some patients.
We elected not to look at OPP in this study. Recent similar studies have concluded that OPP does not vary or increases in the short time period (5–30 minutes) that recumbent IOP changes are typically studied.10,21 Longo et al38 measured the theoretical OPP in standing position vs supine position and found an increase in the OPP. A decrease in OPP is thought to be potentially clinically significant. It is still possible that long-term recumbent positioning or other cardiovascular states may reveal OPP to be a significant risk factor worthy of specialized treatment.
PDS/PDG may have a different reaction to recumbent positioning than normal or POAG patients, and therefore, our results may not be applicable to other types of patients or study results. Our results show that in PDS or PDG, the type of recumbent position is important while the dependency status is not.
The most likely mechanism for IOP increases in recumbent positions is a rise in orbital venous pressure and hence increased episcleral venous pressure.40–42 Friberg et al40 also listed causes of the rapid increase in IOP in recumbent/inversion positions as mechanical compression of the orbit and increasing orbital venous pressure made worse due to a lack of venous valves in the orbits and increases in orbital arterial pressure. Friberg also found in this study a strong correlation (P=0.003, R=0.80) between increases in IOP and episcleral pressures as independent parameters in supine vs 90° inversion. Other studies suggest that the more the venous drainage system of the head is congested the more elevated the IOP can become.16 Patients developed a significantly higher IOP with neck flexion and extension than in a neck neutral position, which the authors also attributed to venous compression and increased episcleral venous pressure.16 Aqueous humor formation showed no change when comparing 15° head up to 15° head down positioning over 6 hours (P>0.05 for all 6 hours) with a slowing of aqueous formation in 50° head down positioning compared to 50° head up positioning (P<0.05), the opposite of what should occur if aqueous formation contributes to a higher IOP in recumbency.43
Both Carlson et al43 and Linder et al18 found a significant and steady increase in IOP with increasing complete body inversion. Complete inversion of the body produced some of the largest IOP increases in studies to date, up to 3-fold with 90° inversions in some individuals.18 A number of studies have shown that the more inverted the body, the higher the IOP increase will be. Viewing the raw data from 3 separate inversion studies18,43,48 in Figure 3 shows an increasing IOP change with increasing body inversion, with average complete body inversion increases up to 96%,36 94%,40 and 153%52 in normal subjects. This rise in IOP seems to accelerate with higher inversion. All of these studies support the notion that the cardiovascular system is involved in recumbent IOP increases.
  | Figure 3 Average IOP values in head up (negative) and down (positive) positions in 3 prior studies. |
Diurnal elevations in IOP can be due to other factors besides recumbent sleeping positions. The 24-hour sleep studies in sitting and supine positions measured every 2 hours in normal patients have shown significant elevations in IOP in both positions (P<0.01), suggesting that factors other than recumbent positioning may be present in nocturnal elevation of IOP.44 Increasing blood cortisol levels have been shown to be very closely related and precede nocturnal IOP increases by 3 hours in 24-hour sleep studies.56 In short, the cardiovascular system and other unknown mechanisms are present in recumbent IOP increases.
Conclusion
Our study has the largest increases in sitting vs flat recumbent IOP observed with 19/44 of the eyes (43%) having a ≥50% increase on at least one recumbent trial with a range of −4 to +17 mmHg or −17% to +142%, in any of the 176 trials.19,23,30–32,34,45,46,49 Only one case report30 of a PDS patient had an IOP increase of 17 mmHg after exercise in the sitting position for both readings. This patient’s IOP went from 37 to 54 mmHg, a 17 mmHg difference, but this represents only a 46% increase from baseline IOP, not the 142% increase observed in our study. Our data showing such a high recumbent IOP elevation may be due to PDS/PDG being a unique subset of SOAG. Care should therefore be taken to extrapolate our results to all glaucoma/glaucoma suspect patients. More studies should include the percentage of increase in IOP in addition to the actual value since so much of what we do in treatment involves reducing IOP by a given percentage.
A 30% elevation of head only has been shown to significantly reduce nocturnal IOP from the recumbent position. In comparing supine flat with supine 30° head up positioning in well-controlled NTG or POAG eyes with a new DH, a significant night time mean reduction in IOP of 3.2 mmHg was found (P=0.03).21 This study showed a ≥20% IOP reduction in >35% of the eyes with no changes in the OPP values between the two positions. The head elevation of 30° from the supine position was also shown to reduce nocturnal IOP significantly in normal subjects, but the same study did not find an IOP reduction when 2 pillows were used to elevate the head a similar amount (P=0.061 OD, P=0.089 OS).53 A 20% head only up position utilizing only a wedge pillow in OAG and normal patients found an almost significant reduction in nocturnal IOP in either group (95% confidence interval 0.99–2.04).54 While a 3.2 mmHg IOP reduction overnight may not seem clinically significant, a millimeter of IOP reduction has been shown to reduce the risk of progression in OAG patients 10%55 and one-third of our life is spent in a sleeping position. Head elevation is also used in gastro-esophageal reflux disease (GERD) treatment.
Recent motorized beds now offer the option of head elevation, and we believe that this should become a part of the treatment discussion between glaucoma patients and their ophthalmologists. Most prior studies utilize whole bed tilting, which is not feasible for people in routine, nonstrapped sleeping. A bed that raises the upper body only has been advertised on the media and will stop people slipping off the bed. DHs, notches, and optic nerve blood vessel changes may dispose PDS/PDG patients to a large increase in recumbent IOP, and it may be prudent to advise glaucoma patients, especially PDS/PDG patients, to sleep in a 30° head only up position until 24-hour IOP-measuring devices can show no significant recumbent IOP increase in an individual patient. Prata et al45 have also advised this. A total of 75% of peak IOP increases in progressively worsening OAG patients (despite adequate treatment) occur during nonclinical hours, as found when 24-hour contact lens sensor was used to measure IOP.51 That study did not list what sleeping position was used.
This is the first study, that we know of, that has looked at IOP increases in recumbent positions in PDS/PDG patients. We have shown the increases to be very significant and of the same magnitude as initial treatment in the PPP POAG guidelines in 64% of the eyes.9 We believe that current glaucoma guidelines underestimate the importance of modifying recumbent sleeping positions as this topic is not mentioned in the risk factors, searches, or treatment sections of the most recent PPP for POAG9 or in an extensive 2006 think tank review of glaucoma.61 All PD patients should avoid the prone sleeping position. Until recently, it has been difficult to adjust the incline of only the head in commercially available beds.
A review of peer-reviewed journals reveals that most authors and the patient populations in their studies are associated with academic centers, despite the possibility that their patient population can be significantly different from that of a private practice general ophthalmologist. This can be due to academic centers receiving referrals of the more severe and difficult diseases sent to them from a general ophthalmologist’s offices. The difference in corneal ulcer treatment between academic centers and a general ophthalmologists’ practice is a recent example of this kind.57–59 Academic-based peer review studies therefore may not apply to general ophthalmology patients, and more importance should be placed on research using private practice general ophthalmology patients. Obtaining an IRB approval represents a major hurdle to general ophthalmologists but is overcome with any of the private, nonacademic IRB firms available.
Disclosure
The authors report no conflicts of interest in this work.
References
Francis BA, Varma R, Chopra V, et al; Los Angeles Latino Eye Study Group. Intraocular pressure, central corneal thickness, and prevalence of open angle glaucoma: the Los Angles Latino Eye Study. Am J Ophthalmol. 2008;146(5):741–746. | ||
Nemesure B, Honkanen R, Hennis A, Wu SY, Leske MC; Barbados Eye Studies Group. Incident open-angle glaucoma and intraocular pressure. Ophthalmology. 2007;114(10):1810–1815. | ||
Jiang X, Varma R, Wu S, et al. Baseline risk factors that predict the development of open-angle glaucoma in a population. Ophthalmology. 2012;119(11):2245–2253. | ||
Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713. | ||
Collaborative Normal Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressure. Am J Ophthalmol. 1998;126(4):487–497. | ||
Heijl A, Bengtsson B, Hyman L, Leske MC; Early Manifest Glaucoma Trial Group. Natural history of open-angle glaucoma. Ophthalmology. 2009;116(12):2271–2276. | ||
Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114(11):1965–1972. | ||
De Moraes CG, Jasien JV, Simon-Zoula S, Liebmann JM, Ritch R. Visual field change and 24-hour IOP-related profile with a contact lens sensor in treated glaucoma patients. Ophthalmology. 2016;123(4):744–753. | ||
American Academy of Ophthalmology. Preferred Practice Patterns: Primary Open Angle Glaucoma. San Francisco, CA: American Academy of Ophthalmology; 2015. | ||
Lee TE, Yoo C, Kim YY. Effects of different sleeping postures on intraocular pressure and ocular perfusion pressure in healthy young subjects. Ophthalmology. 2013;120(8):1565–1570. | ||
Lee JY, Yoo C, Jung JH, Hwang YH, Kim YY. The effect of lateral decubitus position on intraocular pressure in healthy young subjects. Acta Opthalmol. 2012;90(1):e68–e72. | ||
Sawada A, Yamamoto T. Posture-induced intraocular pressure changes in eyes with open-Angle glaucoma, primary angle closure with or without glaucoma medications, and control eyes. Invest Ophthalmol Vis Sci. 2012;53(12):7631–7635. | ||
Vaz F, Gupta AK. The influence of posture on intraocular pressure. Indian J Ophthalmol. 1981;29(1):1–3. | ||
Yamabayashi S, Aguilar RN, Hosoda M, Tsukahara S. Postural change of intraocular and blood pressure in ocular hypertension and low tension glaucoma. Br J Ophthalmol. 1991;75(11):652–655. | ||
Smith DA, Trope GE. Effect of a beta blocker on altered body position: induced ocular hypertension. Br J Ophthalmol. 1990;74(10):605–606. | ||
Malihi M, Sit AJ. Effect of head and body position on intraocular pressure. Ophthalmology. 2012;119(5):987–991. | ||
Chiquet C, Custaud MA, Le Traon AP, et al. Changes in intraocular pressure during prolonged (7 day) head down tilt bedrest. J Glaucoma. 2003;12(3):204–208. | ||
Linder BJ, Trick GL, Wolf ML. Altering body position affects intraocular pressure and visual function. Invest Ophthalmol Vis Sci. 1988;29(10):1492–1497. | ||
Kiuchi T, Motoyama Y, Oshika T, et al. Relationship of progression of visual field damage to postural changes in intraocular pressure in patients with normal tension glaucoma. Ophthalmology. 2006;113(12):2150–2155. | ||
Lee JY, Yoo C, Kim YY. The effect of lateral decubitus position on intraocular pressure in patient with untreated open angle pressure. Am J Ophthalmol. 2013;155:329–335e2. | ||
Buys YM, Alasbali T, Jin YP, et al. Effect of sleeping in a head-up position on intraocular pressure in patients with glaucoma. Ophthalmology. 2010;117(7):1348–1351. | ||
Parsley J, Powell RG, Keightley SJ, Elkington AR. Postural response of intraocular pressure in chronic open angle glaucoma following trabeculectomy. Br J Ophthalmol. 1987;71(7):494–496. | ||
Anderson DR, Grant WM. The influence of position on intraocular pressure. Invest Ophthalmol. 1973;12:204–212. | ||
Hirooka H, Shiraga F. Relationship between postural change of the intraocular pressure and visual filed loss in primary open-angle glaucoma. J Glaucoma. 2003;12(4):379–382. | ||
Postural changes in intraocular pressure are associated with asymmetric retinal fiber thinning in treated patients with primary open angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2011;249(6):879–885. | ||
Kiuchi T, Motoyama Y, Oshika T. Postural changes of intraocular pressure and visual field damage in patients with untreated normal tension glaucoma. J Glaucoma. 2010;19(3):191–193. | ||
Kiuchi T, Motoyama Y, Oshika T. Influence of ocular hypotensive eye drops on intraocular pressure fluctuations with postural change in eyes with normal tension glaucoma. Am J Ophthalmol. 2007;143(4):693–695. | ||
Kim KN, Jeoung JW, Park KH, Lee DS, Kim DM. Effect of lateral decubitus position on intraocular pressure in glaucoma patients with asymmetric visual field loss. Ophthalmology. 2013;120(4):731–735. | ||
Kim KN, Jeoung JW, Park KH, Kim DM, Ritch R. Relationship between preferred sleeping position and asymmetric visual field loss in open angle glaucoma patients. Am J Ophthalmol. 2014;157(6):739–745. | ||
Schenker HI, Luntz MH, Kels B, Podos SM. Exercise-induced increase of intraocular pressure in the pigmentary dispersion syndrome. Am J Ophthalmol. 1980;89(4):598–600. | ||
Haynes WL, Johnson AT, Alward WL. Effects of jogging exercise on patients with the pigmentary dispersion syndrome and pigmentary glaucoma. Ophthalmology. 1992;99(7):1096–1103. | ||
Epstein DL, Boger WP 3rd, Grant WM. Phenylephrine provocative testing on the pigmentary dispersion syndrome. Am J Ophthalmol. 1978;85(1):43–50. | ||
Almubrad TM, Ogbuehi KC. On repeated corneal applination with the goldmann and two non-contact tonometers. Clin Exp Optom. 2010;93(2):77–82. | ||
Leonard TJK, Muir MGK, Kirkby GR, Hitchings RA. Ocular hypertension and posture. Br J Ophthalmol. 1983;67(6):362–366. | ||
Walick KS, Kragh JE Jr, Ward JA, Crawford JJ. Changes in intraocular pressure due to surgical positioning: studying potential risk for postoperative vision loss. Spine. 2007;32(23):2591–2595. | ||
Weinreb RN, Cook J, Friberg TR. Effect of inverted body position on intraocular pressure. Am J Ophthalmol. 1984;98(6):784–787. | ||
Budenz DL, Anderson DR, Feuer WJ, et al; Ocular Hypertension Treatment Study Group. Detection and prognostic significance of optic disc hemorrhages during the ocular hypertension treatment study. Ophthalmology. 2006;113(12):2137–2143. | ||
Longo A, Geiser MH, Riva CE. Posture changes and subfoveal choroidal blood flow. Invest Ophthalmol Vis Sci. 2004;45(2):546–551. | ||
Sulek CA, Gravenstein D, Blackshear RH, Weiss L. Head rotation during internal jugular vein cannulation and the risk of carotid artery puncture. Anaesth Analg. 1996;82(1):125–128. | ||
Friberg TR, Sanborn G, Weinreb RN. Intraocular and episcleral pressure increase during inverted posture. Am J Ophthalmol. 1987;103(4):523–526. | ||
Blondeau P, Tétrault JP, Papamarkakis C. Diurnal variation of episcleral venous pressure in healthy patients: a pilot study. J Glaucoma. 2001;10(1):18–24. | ||
Krieglstein GK, Waller WK, et al. The vascular basis of the positional influence on the intraocular pressure. Albrecht V. Graefes Arch Clin Exp Ophthalmol. 1978;206:99–106. | ||
Carlson KH, McLaren JW, Topper JE, Brubaker RF. Effect of body position on intraocular pressure and aqueous flow. Invest Ophthalmol Vis Sci. 1987;28(8):1346–1352. | ||
Liu JHK, Bouligny RP, Kripke DF, Weinreb RN. Nocturnal elevation on intraocular pressure is detectable in the sitting position. Invest Ophthalmol Vis Sci. 2003;44(10):4439–4442. | ||
Prata TS, De Moraes CG, Kanadani FN, Ritch R, Paranhos A Jr. Posture-induced intraocular pressure changes: considerations regarding body position in glaucoma patients. Surv Ophthalmol. 2010;55(5):445–453. | ||
Galin MA, McIvor JW, Magruder GB, et al. Influence of position on intraocular pressure. Am J Ophthalmol. 1963;55:720–723. | ||
Tsukahara S, Sasaki T. Postural change of IOP in normal persons and in patients with primary open angle glaucoma and low tension glaucoma. Br J Ophthalmol. 1984;68(6):389–392. | ||
Tarrkanen A, Leikola J. Postural variations of the intraocular pressure as measured with the Mackay-Marg Tonometer. Acta Ophthalmol. 1967;45(4):569–575. | ||
Jain MR, Marmion VJ. Rapid pneumatic and Markay-Marg applination tonometry to evaluate the postural effect on intraocular pressure. Br J Ophthalmol. 1976;60(10):687–693. | ||
Wong MH, Lai AH, Singh M, Chew PT. Sleeping posture and intraocular pressure. Singapore Med J. 2013;54(3):146–148. | ||
Masouri K, Shaarawy T. Continuous intraocular pressure monitoring with a wireless ocular telemetry sensor: initial clinical experience in patients with open angle glaucoma. Br J Ophthalmol. 2011;95(5):627–629. | ||
Friberg TR, Weinreb RN. Ocular manifestations of gravity inversion. JAMA. 1985;253(12):1755–1757. | ||
Yeon DY, Yoo C, Lee TE, Park JH, Kim YY. Effects of head elevation on intraocular pressure in healthy subjects: raising bed head vs using multiple pillows. Eye. 2014;28(11):1328–1333. | ||
Lazzaro EC, Mallick A, Singh M, et al. The effect of positional changes on intraocular pressure during sleep in patients with and without glaucoma. J Glaucoma. 2014;23(5):282–287. | ||
Leske MC, Heijl A, Hussein M, et al; Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121(1):48–56. | ||
Weitzman ED, Henkind P, Leitman M, Hellman L. Correlative 24-hour relationships between intraocular pressure and plasma cortisol in normal subjects and patients with glaucoma. Br J Ophthalmol. 1975;59(10):566–572. | ||
McDonnell P, Nobe J, Gauderman WJ, Lee P, Aiello A, Trousdale M. Community care of corneal ulcers. Am J Ophthalmol. 1992;114(5):531–538. | ||
Rodman RC, Spisak S, Sugar A, Meyer RF, Soong HK, Musch DC. The utility of culturing corneal ulcers in a tertiary referral center versus a general ophthalmology clinic. Ophthalmology. 1997;104(11):1897–1901. | ||
Amescua G, Miller D, Alfonso EC. What is causing the Corneal Ulcer? Management strategies for unresponsive corneal ulceration. Eye. 2011;26(2):228–236. | ||
Siddiqui Y, Ten Hulzen RD, Cameron JD, Hodge DO, Johnson DH. What is the risk of developing pigmentary glaucoma from pigment dispersion syndrome? Am J Ophthalmol. 2003;135(6):794–799. | ||
Caprioli J, Garway-Heath DF; International Glaucoma Think Tank. A critical reevaluation of current glaucoma management. Ophthalmology. 2007;114(11 suppl):S1–S41. | ||
Heijl A, Leske MC, Bengtsson B, et al; Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression, results from the early manifest glaucoma trial. Arch Ophthalmol. 2002;120(10):1268–1279. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.