Back to Journals » Journal of Pain Research » Volume 15
Effects of Different 0.2% Ropivacaine Infusion Regimens for Continuous Interscalene Brachial Plexus Block on Postoperative Analgesia and Respiratory Function After Shoulder Arthroscopic Surgery: A Randomized Clinical Trial
Authors Meng Y, Wang S, Zhang W, Xie C, Chai X, Shu S, Zong Y
Received 14 February 2022
Accepted for publication 29 April 2022
Published 12 May 2022 Volume 2022:15 Pages 1389—1399
DOI https://doi.org/10.2147/JPR.S362360
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ellen M Soffin
Yan Meng,1,2 Sheng Wang,1 Wei Zhang,1 Chunlin Xie,1 Xiaoqing Chai,1 Shuhua Shu,1 Yu Zong1,2
1Department of Anesthesiology, The First Affiliated Hospital of University of Science and Technology of China (USTC)/Anhui Provincial Hospital, Hefei, People’s Republic of China; 2Core Facility Center, The First Affiliated Hospital of USTC/Anhui Provincial Hospital, Hefei, People’s Republic of China
Correspondence: Yu Zong, Department of Anesthesiology, The First Affiliated Hospital of USTC, 17 Lujiang Road, Hefei, Anhui, People’s Republic of China, Email [email protected]
Objective: Continuous interscalene brachial plexus block (cIBPB) is an effective perioperative analgesic therapy for shoulder arthroscopic surgery (SAS) patients. This trial aimed to compare the effect of different cIBPB infusion methods on postoperative analgesia and respiratory function in patients undergoing SAS.
Methods: After SAS, 88 patients were randomly assigned to four groups. Through interscalene catheter, all the patients received an initial dose of 10 mL 0.2% ropivacaine. The CI group received 0.2% ropivacaine 4 mL/h, and the PIBI1, PIBI2, and PIBI3 groups received intermittent 0.2% ropivacaine boluses at 4 mL/h, 8 mL/2 h, and 12 mL/3 h, respectively. The patients could also use a patient-controlled analgesia (PCA) pump to self-inject a tramadol bolus each time he/she felt pain. The primary outcome was the cumulative tramadol consumption over the 48 h after surgery. Secondary outcome measures included PCA frequency, pain (visual analogue scale, VAS) score, patient satisfaction, diaphragmatic excursion, pulmonary function, and adverse events.
Results: The cumulative tramadol consumption and PCA frequency over the 48 h after surgery in groups PIBI2 and PIBI3 were lower than in both the CI and PIBI1 groups (p< 0.001). The VAS scores (at rest and on movement) in groups PIBI2 and PIBI3 were lower than those in the CI and PIBI1 groups at 8 and 12 h after surgery (all p< 0.001). Patient satisfaction scores were significantly higher in the PIBI2 group than in the other three groups (all p< 0.001). Diaphragmatic excursion was significantly decreased in the PIBI3 group compared to the other three groups (p< 0.05). The incidence of adverse events over the 48 h after surgery was significantly higher in the PIBI3 group compared to the other three groups (p< 0.001).
Conclusion: Programmed intermittent bolus infusion with 0.2% ropivacaine 8 mL/2 h for cIBPB can achieve lower tramadol consumption, along with better analgesia after surgery, lower reduction in diaphragmatic excursion, lower incidence of adverse events, and higher patient satisfaction.
Keywords: continuous interscalene brachial plexus block, programmed intermittent bolus infusion, shoulder arthroscopic surgery, perioperative analgesic therapy, respiratory function
Introduction
Patients undergoing shoulder arthroscopic surgery (SAS) can often experience severe postsurgical pain,1 which is exacerbated by shoulder movement during postoperative rehabilitation and physical therapy. Severe postsurgical pain may increase the incidence of pulmonary complications, affect rehabilitation, prolong the length of the hospital stay, and increase the cost of hospitalization.2,3 Continuous interscalene brachial plexus block with a single or multiple catheter(s) has been found to provide effective and prolonged analgesia in patients after shoulder surgery.4–10 The continuous interscalene brachial plexus block technique offers many advantages, including early and aggressive mobilization, extension of analgesia for prolonged periods and use of smaller doses of local anaesthetics to prevent motor block and reduce opioid requirements and associated side effects.4
Recently, the use of programmed intermittent bolus infusion (PIBI) of local anaesthetics for continuous peripheral nerve block to provide postoperative analgesia has attracted the attention of researchers. It can be used in combination with patient-controlled analgesia (PCA) involving additional local anaesthetic boluses. PIBI has been shown to be superior to CI for epidural block11–14 and certain peripheral nerve blocks.15–20 Compared to CI, PIBI reduces local anaesthetic consumption and improves analgesia.
However, the evidence for different PIBI regimens is scarce and inconsistent. Fredrickson et al7 reported that ropivacaine PIBI with mandatory 6-h plus PCA boluses provided similar analgesia to CI with PCA boluses only, but with fewer adverse events (3% vs 21%, p=0.02). Hamdani et al21 reported that there were no significant differences in pain relief or local anaesthetic consumption between PIBI combined with PCA (involving local anaesthetic) and CI combined with PCA in shoulder surgery patients treated using cIBPB. Oxlund et al22 also demonstrated no difference in rescue analgesia or opioid consumption between PIBI of local anaesthetic (ropivacaine, 16 mg/2 h) combined with local anaesthetic PCA compared to CI (ropivacaine, 8 mg/h) combined with PCA, but the former led to higher hoarseness and lower cough intensity. In addition, due to the absorption of local anaesthetics, theoretically, PIBI with different local anaesthetics at different time intervals probably impacts the analgesic effect. Although a larger volume of local anaesthetic solution can provide effective analgesia, it may also spread widely and increase the frequency of adverse events. The prolonged dosing interval may increase the rescue pain medication usage due to insufficient pain relief. However, this was not considered in the above studies. Therefore, further studies are still necessary.
The primary aim of this prospective, randomized, blinded trial, was to test the hypothesis that the optimal infusion regimen for cIBPB will reduce the tramadol consumption after surgery compared with CI in patients undergoing an cIBPB for shoulder arthroscopic surgery. Our secondary aims were to assess for quality differences between groups related to the pain VAS scores, PCA (tramadol) frequency, patient satisfaction, diaphragmatic excursion, pulmonary function, and adverse events.
Methods
Recruitment
The study was approved by the First Affiliated Hospital of the University of Science and Technology of China (USTC) Ethics Committee (No: 2019 KY 108; date of approval: October 7, 2019), and written informed consent was obtained from all patients participating in the trial, in accordance with the code of the Declaration of Helsinki. The trial was registered before patient enrollment in the Chinese Clinical Trial Register at http://www.chictr.org.cn (ChiCTR2100043588; principal investigator: Yan Meng, date of registration: February 23, 2021). The study was conducted in the First Affiliated Hospital of USTC from March 2021 to September 2021.
Eighty-eight patients who were scheduled to undergo SAS (arthroscopic rotator cuff repair) and agreed to have an interscalene catheter placed for postoperative analgesia were enrolled. The inclusion criteria were aged >18 years, American Society of Anesthesiologists (ASA) grade I–III, and patients able to understand and cooperate with nerve block operation and postoperative evaluation. The exclusion criteria were contra-indication to peripheral nerve block (study drug allergy, coagulopathy or local infection), refused the nerve block procedure, with severe cardiovascular disease, unable to understand and cooperate with nerve block operation or postoperative evaluation, using chronic opioids recently, a history of phrenic paralysis or phrenic nerve injury, moderate or severe pulmonary disease, chest deformity (pectus carinatum or pectus excavatum), severe renal or hepatic failure.
Randomization and Blinding
The 88 patients who met the enrolment criteria were randomized into four groups. The randomization list was computer generated by an anaesthesiologist (A1) who was unknown to the other study anaesthesiologists (A2–A4). The CI group received 0.2% ropivacaine 4 mL/h. The PIB I1, PIBI2, and PIBI3 groups received intermittent 0.2% ropivacaine boluses at 4 mL/h, 8 mL/2 h, and 12 mL/3 h, respectively. All drugs were delivered using a PCA pump (ZZB-1; AiPeng Medical Technology, Jiangsu, China). The pump parameters were set by A1. The patients and other anaesthesiologists (A2–A4) were not informed about the infusion regimens.
Anaesthesia Management
None of the patients were premedicated. All patients were admitted for elective surgery after a fast ≥8 h. The patients were continuously monitored by electrocardiography, pulse oximetry, and invasive blood pressure measurement. General anaesthesia was mainly induced by bolus injection of 1.5–2.5 mg/kg propofol, 1.5–2.5 µg/kg remifentanil, and 0.6 mg/kg rocuronium. Sevoflurane, propofol, remifentanil, and cisatracurium besylate (for muscle relaxation) were applied as required to maintain general anaesthesia and keep the bispectral index (BIS) at 40–60. The patients were mechanically ventilated with an oxygen–air mixture, maintaining the end-tidal CO2 at 35–45 mmHg. The optimal time point and quality of tracheal extubation corresponded to when the patient was fully awake and recovery of respiratory muscle strength, respectively. An intravenous catheter was attached to a PCA pump to enable the patient to self-inject a tramadol bolus each time he/she felt pain. The pump had no basal rate, and the PCA dose was 10 mg tramadol, with a 10-min lockout, for 2 days.
cIBPB Procedure
At the end of surgery, anesthesia was still sustained for the patients receiving cIBPB by using a continuous infusion of propofol (4 mg/kg/h) by an anaesthesiologist (A2). The anaesthesiologist (A2) is an experienced attending physician and is skilled in ultrasound-guided interscalene brachial plexus block. The patient was placed in the supine position, and the patient’s head was turned 45° to the opposite side. After a standard skin disinfection, an ultrasound probe (13–6 MHz, Wisonic Beidou Labat; Wisonic Medical Technology Co., Ltd., Shenzhen, China) was used to observe the brachial plexus over the interscalene region. The insertion point was infiltrated with 2% lidocaine. A catheter-over-needle (Contiplex; Braun, Frederiksberg, Denmark) approach was used, inserting a needle through the middle scalene muscle, and the final needle tip was placed in the middle of the C5 and C6 nerve roots.20 After injection of 2 mL saline, ultrasound was used to verify the correct catheter-over-needle position with regard to the nerve roots. The needle was withdrawn, and a loading dose of 0.2% ropivacaine 10 mL was administered via the catheter. Subsequently, a PCA pump containing 0.2% ropivacaine was connected to the interscalene catheter. When the patients awoke, they were instructed to rate their perioperative pain using visual analogue scale (VAS; 0–10, 0 = no pain and 10 = worst imaginable pain).
Functional Respiratory Outcomes
An anaesthesiologist (A3) used a convex array probe (5–2 MHz, Wisonic Beidou Labat; Wisonic Medical Technology Co., Ltd., Shenzhen, China) to measure hemidiaphragmatic excursion before surgery and at 24 h after surgery. All measurements were performed based on spontaneous tidal breathing at rest in patients lying in the semi-recumbent position. The hemidiaphragm was identified as a hyperechoic line with breathing-related movements, using the liver or spleen as an acoustic window. The hemidiaphragmatic excursion was measured as the difference in distance between resting expiration and inspiration, using real-time M-mode ultrasonography. Forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and peak expiratory flow (PEF) were measured three times and recorded only the highest of three reproducible measurements, using a bedside spirometer (S4, Saike Medical Equipment Company, Xiamen, China), with the patient seated upright.
Outcome Assessment
The primary outcome was tramadol consumption. To assess this, the cumulative tramadol consumption was recorded over the 48 h after surgery. The secondary outcomes were pain VAS scores, PCA (tramadol) frequency, patient satisfaction, diaphragmatic excursion, pulmonary function, and adverse events. Postoperative pain was assessed using a 10-point VAS at 2, 4, 8, 12, 24, and 48 h after surgery, at rest and on movement. PCA frequency was recorded over the 48 h after surgery. Patient satisfaction with the analgesia was evaluated by using a 5-point Likert scale (1 = very unsatisfactory; 3 = neutral; 5 = very satisfactory).23 Any adverse events, such as hoarseness, cough, or persistent upper limb paraesthesia or weakness, were recorded. The interscalene catheter was removed at 48 h after surgery. All data were collected by an anaesthesiologist (A4).
Sample Size Calculation
The sample size was determined according to tramadol consumption using Power Analysis and Sample Size (PASS) 15. In a preliminary study, 12 patients were assigned to CI, PIBI1, PIBI2, and PIBI3 groups (n = 3 per group), and the mean ± SD tramadol consumption at 48 h postoperatively was 183 ± 26, 167 ± 25, 143 ± 21, and 150 ± 30 mg, respectively. For a significance level of 0.05 and a power of 0.9, we required 16 patients in each group. We planned to recruit at least 22 patients per group considering the potential for catheter leakage or dislodgement.
Statistical Analysis
The data analysis was performed using SPSS 26 (IBM Corp, Armonk, NY, USA). The normality of the data was tested using the Shapiro–Wilk test. The normally distributed continuous variables are presented as mean ± SD, and they were compared among the four groups using a one-way analysis of variance. The non-normally distributed continuous variables are presented as median with interquartile range (IQR), and are further analyzed using the Mann–Whitney U or Kruskal–Wallis H-test. The categorical variables are expressed as frequency and percentage, and they were compared among groups using the χ2 test. A two-sided p value <0.05 was considered statistically significant.
Results
This study was conducted between February and September 2021 at the First Affiliated Hospital of USTC, China. A total of 125 patients were screened for recruitment, of which 24 declined to participate and 13 did not meet the eligibility criteria. A total of 88 patients were randomly allocated to four groups (n = 22 per group), and primary outcome data were obtained for 80 patients. Figure 1 depicts the study flow chart. Demographic features and surgical procedures were similar between the four groups (p>0.05) (Table 1).
 |
Table 1 Demographic and Clinical Characteristics of the Patients |
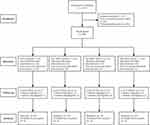 |
Figure 1 Flow diagram of the study. |
PCA
The cumulative tramadol consumption and PCA frequency were assessed over the 48 h after surgery. Tramadol consumption and PCA frequency were significantly lower in the PIBI2 and PIBI3 groups than in groups CI and PIBI1 (p<0.05). There were no differences between groups PIBI2 and PIBI3, nor groups CI and PIBI1 (p>0.05) (Table 2).
 |
Table 2 PCA and Patient Satisfaction by Group |
Postoperative Pain VAS Scores
Figure 2 shows the change in VAS scores postoperatively. At 8 h and 12 h postoperatively, VAS scores (at rest and on movement) were lower in the PIBI2 and PIBI3 groups than those in the CI and PIBI1 groups (all p<0.001), with no significant differences between groups PIBI2 and PIBI3 groups, nor groups CI and PIBI1 (all p>0.05). At ≤4 h and ≥24 h, there were no significant differences in VAS scores (at rest or on movement) among the four groups (p>0.05). Figure 2 Continued.
Patient Satisfaction
Patient satisfaction scores were significantly higher in the PIBI2 group than in the other three groups (all p<0.001). There were no significant differences among the other three groups (all p>0.05) (Table 2).
Functional Respiratory Outcomes
Pulmonary function and diaphragmatic excursion values are shown in Table 3. The baseline values (before the block) were similar among the four groups. At 24 h after block placement, all values decreased compared to baseline (p<0.05). Diaphragmatic excursion was significantly decreased in the PIBI3 group compared to the CI group (p<0.05), but there was no difference between either the PIBI2 or PIBI1 group and the CI group (p>0.05). No significant differences in FVC, FEV1 and PEF were observed among the four groups (p>0.05). No patients had dyspnoea after block placement.
 |
Table 3 Functional Respiratory Outcomes by Group |
Adverse Events
No patients in the CI or PIBI1 groups developed adverse reactions within 48 h after the procedure. One patient in the PIBI2 group and four in the PIBI3 group had upper limb weakness, and two patients in the PIBI3 group developed Horner’s syndrome. The incidence of adverse events over the 48 h after the procedure was significantly higher in the PIBI3 group than in the other three groups (p<0.001). All these adverse reactions disappeared after withdrawal of the intervention.
Discussion
This double-blind, randomized, controlled trial indicated that the method of PIBI1 did not reduce additional rescue analgesia consumption compared with CI. The results are consistent with the findings of Hamadi et al.21 The infusion modes PIBI2 and PIBI3 provided significant analgesic effect and reduced tramadol consumption over 48 hours after SAS. Pronounced movement in different directions and loose skin at the neck makes catheter fixation difficult and catheter displacement much more likely.24 This helps to explain our finding that the PIBI2 and PIBI3 groups had better analgesia. A larger volume of the local anaesthetic in a bolus generates higher injection pressures, thereby potentially overcoming the anatomical distances regarding the catheter tip and the nerve distribution. This makes it easier for the local anaesthetic to reach the nerve fascicles and thus provide effective analgesia.20,22 To avoid the influence of the potential confounding factor of residual analgesic drugs that were used intraoperatively, only the short-acting μ-opioid receptor agonist remifentanil was used to induce and maintain anaesthesia in our study. Additionally, avoidance of long-acting opioids also reduces the occurrence of postoperative respiratory complications, as well as postoperative nausea and vomiting.25 Surgical site and techniques are independent risk factors that affect the severity and duration of postoperative pain.26 To ensure a homogenous group for analysis, the type of surgery in our study was restricted to arthroscopic rotator cuff surgery.
At 8–12 h after the surgery, VAS scores (at rest and on movement) were lower in the PIBI2 and PIBI3 groups than those in the CI and PIBI1 groups. At other time periods, VAS scores of the four groups did not hold obvious differences. Oxlund et al22 compared the effect of PIBI and CI in cIBPB with ropivacaine for shoulder surgery analgesia. They found that the highest median VAS pain score (ie breakthrough pain) was recorded at 16 h postoperatively. This was similar to our results (after SAS at 8 and 12 h). Our target postoperative pain VAS score was <3, to ensure good prognosis and patient satisfaction. The regimens of CI and PIBI1 groups were unable to provide effective analgesia against breakthrough pain at 8 and 12 h in our study. In groups PIBI2 and PIBI3, sufficient local anaesthetic and higher injection pressures could block the interscalene brachial plexus adequately, leading to effective analgesia while reducing local anaesthetic consumption.20,22 Before the analgesic effect produced by remifentanil had begun to wear off, 10 mL of 0.2% ropivacaine was used for the initial bolus in all groups to provide adequate analgesia.18,24 We then initiated the infusion regimens immediately after the initial bolus injection to examine the difference in analgesic effects between the four groups under the same conditions. The combined effect of the initial bolus injection and the subsequent infusion may have confounded the postoperative outcomes, particularly at the early time points. This is indicated by the fact that there was no significant difference in pain VAS score between the four groups after SAS at ≤4 h.
Although a larger volume of local anaesthetic solution can provide effective analgesia, it may also spread widely and increase the frequency of adverse events. Therefore, balancing analgesia and adverse events should be considered when selecting the dosage. In our study, compared to the other three groups, the adverse events (such as hoarseness, cough, and arm paraesthesia or weakness) were significantly increased and the diaphragmatic excursion was significantly decreased in the PIBI3 group, which is consistent with the results of similar studies.4,6 There were no significant differences in adverse reactions and diaphragmatic excursion between the remaining three groups. There were varying decreases in postoperative pulmonary function in all four groups, but the differences were not significant.
The most common adverse event during cIBPB was phrenic nerve block. The distance between the brachial plexus and the phrenic nerve is 0.18 cm at the C5–C6 level27 and the C3–C5 roots are the origin for the phrenic nerve.28 The diaphragm is the main breathing muscle and is innervated by the phrenic nerve. The incidence of phrenic nerve palsy is strongly associated with the volume of local anaesthetics used.28–31 Stundner et al32 reported that a larger volume of local anaesthetic in a bolus is more likely to cause phrenic nerve palsy. Riazi et al33 reported that reducing the volume of 0.5% ropivacaine for interscalene brachial plexus block from 20 to 5 mL considerably reduced hemidiaphragmatic paralysis caused by phrenic nerve block (45% vs 100%). Hemidiaphragmatic paralysis may be asymptomatic.29,34,35 This is because the compensation by the contralateral diaphragm, intercostal, and abdominal muscles can counteract the functional impact of ipsilateral hemidiaphragmatic paresis.27,30–32 Additionally, hemidiaphragmatic contraction reportedly accounts for only up to 30% of total minute ventilation at rest.34 In our study, most of the patients did not experience a feeling of tachypnoea.
Adequate analgesia is very important for early rehabilitation exercise, functional recovery, and postoperative satisfaction of SAS patients. cIBPB provides effective anaesthesia and analgesia for SAS. The advantages include use smaller doses of local anaesthetics to prevent motor block and reduce opioid requirements and associated side effects.4 CI and PIBI are the most frequently used methods of cIBPB. Many studies demonstrating the enhanced benefits of PIBI compared to CI regarding local/regional analgesia have been reported in recent years. As mentioned above, in various clinical settings, including epidural analgesia for labor,11–14 popliteal sciatic nerve block,19,20 paravertebral block,16–18 and femoral nerve block,1 PIBI has been shown to result in better analgesia, higher patient satisfaction scores, and lower doses of supplemental analgesics and local anaesthetic compared to CI. However, Hamdani et al21 showed that in patients undergoing cIBPB after shoulder surgery, PIBI of 0.2% ropivacaine at 4 mL/h combined with PCA involving 0.2% ropivacaine did not reduce local anaesthetic consumption or rescue analgesia compared to CI combined with PCA. Similarly, Oxlund et al22 also concluded that PIBI did not show obvious advantages over CI in reducing pain or opioid consumption. On the contrary, adverse reactions including hoarseness and reduced cough force were more frequent in the former group. However, the research subjects of Hamdani et al included SAS and open shoulder surgery patients. The two operations have markedly different pain profiles, which may influence pain scores and local anaesthetic use. Additionally, they did not investigate the effects of different PIBI administration intervals on postoperative analgesic efficacy. Moreover, PCA involving local anaesthetics on demand was used in both these studies. After local anaesthetic PCA administration, the PIBI bolus would be locked for 30 min to avoid administration of a large number of local anaesthetics within a short time. The pump setup might reduce local anaesthetic use in the PIBI group due to the lockout time and may have masked the potential benefits of PIBI regimen in the above study.
In our study, in contrast, cIBPB combined with intravenous PCA involving the analgesic tramadol (10 mg, with a 10-min lockout, for 2 days) as rescue analgesia was used postoperatively. The cumulative local anaesthetic dose and PIBI administration intervals remained unchanged in each group. In the study by Hamdani et al,21 PIBI (4 mL/h) did not show any advantages compared to CI, which may be related to the low bolus volume of ropivacaine. Although nerve block adverse events can be reduced by limiting the volume of ropivacaine, Vandepitte et al24 reported that a minimum of 7 mL of 0.75% ropivacaine via a catheter was required for successful surgical anaesthesia involving interscalene brachial plexus block. They found that the proportion of patients with successful blockade increased sharply from approximately 57% at 6 mL to 100% by 7 mL, indicating that a small change in the volume of local anaesthetics led to obvious clinical effects. Although a larger volume of local anaesthetic can provide effective analgesia, it may also spread along the interscalene widely and increase the frequency of adverse events. This is consistent with the results of our study.
There are several limitations associated with our study. First, although we checked the catheter tip position and the local anaesthetic distribution around the C5/C6 roots, we did not reconfirm the catheter tip position (with regard to the nerve roots) in the postoperative period. The initial catheter tip was at the correct location to ensure sufficient diffusion of the local anaesthetic. Although catheter tip position variation may affect the results, in order to observe the catheter tip after surgery, we would have had to remove the adhesive wound dressing. This increases the risk of dislodging the catheter tip and developing an infection. Second, our study lacked a detailed grouping method to determine the optimal PIBI regimen. We used a mean injection volume of 4 mL/h 0.2% ropivacaine, changing the bolus volume over different time intervals. Changing other PIBI variables, such as using the same time interval with different injections volume or the same injection volume with different time intervals, may lead to different results. However, with new groups, the sample size calculation and statistical analysis would have been more complex. Third, in our study, we did not examine the detailed effects of cIBPB on sensation and motor function. Patients who were still not awake were placed for cIBPB performance in operation room after the end of the procedure. And, the skin incisions and the surrounding skin had already been fully covered with wound dressings. To avoid the risk of infection, we did not remove the adhesive wound dressing to assess the scope of interscalene brachial plexus block. Although we did not examine the detailed effects of cIBPB on sensation and motor function, we included significant upper limb paresthesia, dysaesthesia and motor dysfunction in postoperative adverse events. In our research, we documented 5 patients with upper limb weakness and 2 patients with Horner’s syndrome within 48 h after surgery. Fourth, the surgeon generally used a splint to immobilize the affected upper limb and asked the patients to restrict movement in the early postoperative period after SAS. The observation times of the included studies were relatively short, which were only 48 h. We were unable to assess the outcomes related to shoulder physiotherapy and mobilisation during the follow-up period. Next, in our subsequent study, we will prolong the observation time and examine the effect of infusion regimens for continuous interscalene brachial plexus block on shoulder physiotherapy and mobilisation.
Conclusion
Compared with CI (continuous infusion of 0.2% ropivacaine at 4 mL/h) and PIB1 (intermittent boluses of 0.2% ropivacaine at 4 mL/h), PIB2 (intermittent boluses of 0.2% ropivacaine at 8 mL/2 h) for cIBPB can produce better analgesia with lower tramadol consumption. Compared with PIB3 (intermittent boluses of 0.2% ropivacaine at 12 mL/3 h), PIB2 for cIBPB can produce a similar analgesic effect with lower reduction in diaphragmatic excursion, lower incidence of adverse events, and higher patient satisfaction. In conclusion, the PIBI2 regimen may be a better choice for postoperative analgesia for SAS patients compared to the other three regimens investigated in this study.
Data Sharing Statement
The authors state that all data in the manuscript are accessible if requested (contact e-mail address [email protected]). The authors verify that all data intended for sharing are de-identified.
Funding
The authors have no sources of funding to declare for this manuscript.
Disclosure
The authors declare no conflicts of interest.
References
1. Lee SU, Lee HJ, Kim YS. The effectiveness of ramosetron and ondansetron for preventing postoperative nausea and vomiting after arthroscopic rotator cuff repair: a randomized controlled trial. J Orthop Surg Res. 2020;15(1):523. doi:10.1186/s13018-020-02060-3
2. Quan L, Zhang L. The effects of positive psychological intervention on obstetric surgery patients’ mental states, pain levels, and quality of life. Am J Transl Res. 2021;13(4):3819–3825.
3. Haager B, Schmid D, Eschbach J, Passlick B, Loop T. Regional versus systemic analgesia in video-assisted thoracoscopic lobectomy: a retrospective analysis. BMC Anesthesiol. 2019;19(1):183. doi:10.1186/s12871-019-0851-2
4. Ullah H, Samad K, Khan FA. Continuous interscalene brachial plexus block versus parenteral analgesia for postoperative pain relief after major shoulder surgery. Cochrane Database Syst Rev. 2014;2014(2):CD007080. doi:10.1002/14651858.CD007080.pub2
5. Ilfeld BM, Morey TE, Wright TW, Chidgey LK, Enneking FK. Interscalene perineural ropivacaine infusion: a comparison of two dosing regimens for postoperative analgesia. Reg Anesth Pain Med. 2004;29(1):9–16. doi:10.1016/j.rapm.2003.08.016
6. Falcão LF, Perez MV, de Castro I, Yamashita AM, Tardelli MA, Amaral JL. Minimum effective volume of 0.5% bupivacaine with epinephrine in ultrasound-guided interscalene brachial plexus block. Br J Anaesth. 2013;110(3):450–455. doi:10.1093/bja/aes419
7. Fredrickson MJ, Abeysekera A, Price DJ, Wong AC. Patient-initiated mandatory boluses for ambulatory continuous interscalene analgesia: an effective strategy for optimizing analgesia and minimizing side-effects. Br J Anaesth. 2011;106(2):239–245. doi:10.1093/bja/aeq32
8. Mariano ER, Afra R, Loland VJ, et al. Continuous interscalene brachial plexus block via an ultrasound-guided posterior approach: a randomized, triple-masked, placebo-controlled study. Anesth Analg. 2009;108(5):1688–1694. doi:10.1213/ane.0b013e318199dc86
9. Ilfeld BM, Morey TE, Wright TW, et al. Continuous interscalene brachial plexus block for postoperative pain control at home: a randomized, double-blinded, placebo-controlled study. Anesth Analg. 2003;96(4):1089–1095. doi:10.1213/01.ane.0000049824.51036.ef
10. Fredrickson MJ, Price DJ. Analgesic effectiveness of ropivacaine 0.2% vs 0.4% via an ultrasound-guided C5-6 root/superior trunk perineural ambulatory catheter. Br J Anaesth. 2009;103(3):434–439. doi:10.1093/bja/aep195
11. Bullingham A, Liang S, Edmonds E, Mathur S, Sharma S. Continuous epidural infusion vs programmed intermittent epidural bolus for labour analgesia: a prospective, controlled, before-and-after cohort study of labour outcomes. Br J Anaesth. 2018;121(2):432–437. doi:10.1016/j.bja.2018.03.038
12. Sia AT, Lim Y, Ocampo C. A comparison of a basal infusion with automated mandatory boluses in parturient-controlled epidural analgesia during labor. Anesth Analg. 2007;104(3):673–678. doi:10.1213/01.ane.0000253236.89376.60
13. Wong CA, Ratliff JT, Sullivan JT, Scavone BM, Toledo P, McCarthy RJ. A randomized comparison of programmed intermittent epidural bolus with continuous epidural infusion for labor analgesia. Anesth Analg. 2006;102(3):904–909. doi:10.1213/01.ane.0000197778.57615.1a
14. Leo S, Ocampo CE, Lim Y, Sia AT. A randomized comparison of automated intermittent mandatory boluses with a basal infusion in combination with patient-controlled epidural analgesia for labor and delivery. Int J Obstet Anesth. 2010;19(4):357–364. doi:10.1016/j.ijoa.2010.07.006
15. Hillegass MG, Field LC, Stewart SR, et al. The efficacy of automated intermittent boluses for continuous femoral nerve block: a prospective, randomized comparison to continuous infusions. J Clin Anesth. 2013;25(4):281–288. doi:10.1016/j.jclinane.2012.11.015
16. Chen L, Wu Y, Cai Y, et al. Comparison of programmed intermittent bolus infusion and continuous infusion for postoperative patient-controlled analgesia with thoracic paravertebral block catheter: a randomized, double-blind, controlled trial. Reg Anesth Pain Med. 2019;44(2):240–245. doi:10.1136/rapm-2018-000031
17. Hida K, Murata H, Ichinomiya T, Inoue H, Sato S, Hara T. Effects of programmed intermittent thoracic paravertebral bolus of levobupivacaine on the spread of sensory block: a randomized, controlled, double-blind study [published correction appears in Reg Anesth Pain Med. 2020;45(8):e1]. Reg Anesth Pain Med. 2019;44(3):326–332. doi:10.1136/rapm-2018-100021
18. Taketa Y, Irisawa Y, Fujitani T. Programmed intermittent bolus infusion versus continuous infusion of 0.2% levobupivacaine after ultrasound-guided thoracic paravertebral block for video-assisted thoracoscopic surgery: a randomised controlled trial. Eur J Anaesthesiol. 2019;36(4):272–278. doi:10.1097/EJA.0000000000000945
19. Taboada M, Rodríguez J, Bermudez M, et al. A “new” automated bolus technique for continuous popliteal block: a prospective, randomized comparison with a continuous infusion technique. Anesth Analg. 2008;107(4):1433–1437. doi:10.1213/ane.0b013e3181824164
20. Taboada M, Rodríguez J, Bermudez M, et al. Comparison of continuous infusion versus automated bolus for postoperative patient-controlled analgesia with popliteal sciatic nerve catheters. Anesthesiology. 2009;110(1):150–154. doi:10.1097/ALN.0b013e318191693a
21. Hamdani M, Chassot O, Fournier R. Ultrasound-guided continuous interscalene block: the influence of local anesthetic background delivery method on postoperative analgesia after shoulder surgery: a randomized trial. Reg Anesth Pain Med. 2014;39(5):387–393. doi:10.1097/AAP.0000000000000112
22. Oxlund J, Clausen AH, Venø S, et al. A randomized trial of automated intermittent ropivacaine administration vs. continuous infusion in an interscalene catheter. Acta Anaesthesiol Scand. 2018;62(1):85–93. doi:10.1111/aas.13011
23. Jebb AT, Ng V, Tay L. A review of key likert scale development advances: 1995–2019. Front Psychol. 2021;12:637547. doi:10.3389/fpsyg.2021.637547
24. Vandepitte C, Gautier P, Xu D, Salviz EA, Hadzic A. Effective volume of ropivacaine 0.75% through a catheter required for interscalene brachial plexus blockade. Anesthesiology. 2013;118(4):863–867. doi:10.1097/ALN.0b013e3182850dc7
25. Brown J, Setnik B, Lee K, et al. Effectiveness and safety of morphine sulfate extended-release capsules in patients with chronic, moderate-to-severe pain in a primary care setting. J Pain Res. 2011;4:373–384. doi:10.2147/JPR.S23024
26. Xu J, Bian F. Pain-related risk factors after arthroscopic minimally invasive treatment of meniscus injury of knee joints. Exp Ther Med. 2020;20(3):2317–2324. doi:10.3892/etm.2020.8953
27. Choromanski DW, Patel PS, Frederick JM, Lemos SE, Chidiac EJ. The effect of continuous interscalene brachial plexus block with 0.125% bupivacaine vs 0.2% ropivacaine on pain relief, diaphragmatic motility, and ventilatory function. J Clin Anesth. 2015;27(8):619–626. doi:10.1016/j.jclinane.2015.03.006
28. Renes SH, Rettig HC, Gielen MJ, Wilder-Smith OH, van Geffen GJ. Ultrasound-guided low-dose interscalene brachial plexus block reduces the incidence of hemidiaphragmatic paresis. Reg Anesth Pain Med. 2009;34(5):498–502. doi:10.1097/AAP.0b013e3181b49256
29. Ricoy J, Rodríguez-Núñez N, Álvarez-Dobaño JM, Toubes ME, Riveiro V, Valdés L. Diaphragmatic dysfunction. Pulmonology. 2019;25(4):223–235. doi:10.1016/j.pulmoe.2018.10.008
30. Katagiri M, Young RN, Platt RS, Kieser TM, Easton PA. Respiratory muscle compensation for unilateral or bilateral hemidiaphragm paralysis in awake canines. J Appl Physiol. 1994;77(4):1972–1982. doi:10.1152/jappl.1994.77.4.1972
31. Fujimura N, Namba H, Tsunoda K, et al. Effect of hemidiaphragmatic paresis caused by interscalene brachial plexus block on breathing pattern, chest wall mechanics, and arterial blood gases. Anesth Analg. 1995;81(5):962–966. doi:10.1097/00000539-199511000-00012
32. Stundner O, Meissnitzer M, Brummett CM, et al. Comparison of tissue distribution, phrenic nerve involvement, and epidural spread in standard- vs low-volume ultrasound-guided interscalene plexus block using contrast magnetic resonance imaging: a randomized, controlled trial. Br J Anaesth. 2016;116(3):405–412. doi:10.1093/bja/aev550
33. Riazi S, Carmichael N, Awad I, et al. Effect of local anaesthetic volume (20 vs 5 mL) on the efficacy and respiratory consequences of ultrasound-guided interscalene brachial plexus block. Br J Anaesth. 2008;101(4):549–556. doi:10.1093/bja/aen229
34. Albrecht E, Bathory I, Fournier N, Jacot-Guillarmod A, Farron A, Brull R. Reduced hemidiaphragmatic paresis with extrafascial compared with conventional intrafascial tip placement for continuous interscalene brachial plexus block: a randomized, controlled, double-blind trial. Br J Anaesth. 2017;118(4):586–592. doi:10.1093/bja/aex050
35. Ridyard JB, Stewart RM. Regional lung function in unilateral diaphragmatic paralysis. Thorax. 1976;31(4):438–442. doi:10.1136/thx.31.4.438
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

