Back to Journals » Drug Design, Development and Therapy » Volume 10
Effects of Dang-Gui-Bu-Xue-Tang, an herbal decoction, on iron uptake in iron-deficient anemia
Authors Huang G, Chen S, Tsai P , Ganzon JG, Lee C , Shiah H, Wang C
Received 12 August 2015
Accepted for publication 14 October 2015
Published 2 March 2016 Volume 2016:10 Pages 949—957
DOI https://doi.org/10.2147/DDDT.S94309
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Wei Duan
Guan-Cheng Huang,1–3 Shih-Yu Chen,4 Po-Wei Tsai,5 Jerome G Ganzon,5 Chia-Jung Lee,6 Her-Shyong Shiah,7 Ching-Chiung Wang4,5
1School of Medicine, College of Medicine, Taipei Medical University, Taipei, 2Division of Hematology and Oncology, Department of Internal Medicine, Yuan’s General Hospital, 3Department of Leisure and Recreation Industry Management, College of Humanities and Management, Fooyin University, Kaohsiung, 4Graduate Institute of Pharmacognosy, College of Pharmacy, 5School of Pharmacy, College of Pharmacy, 6PhD Program for Clinical Drug Discovery of Chinese Herbal Medicine, College of Pharmacy, Taipei Medical University, 7Division of Hematology and Oncology, Department of Internal Medicine, Taipei Medical University Hospital, Taipei, Taiwan
Abstract: Dang-Gui-Bu-Xue-Tang (DBT), a combination of Angelicae Sinensis Radix and Astragali Radix, is a widely used herbal decoction in traditional Chinese medicine primarily to promote or invigorate the “blood”. In this study, we explored this ancient formulation and provide evidence of its blood-toning properties. We used the improvement iron uptake as promote or invigorate the “blood” indicator. Ferritin formation of Caco-2 cells in vitro assay and diet-induced anemia (DIA) in rat model were used to prove its improvement iron uptake and ameliorating effects. Finally, the iron–DBT interactions were measured by iron-binding assay. We first demonstrated DBT increased uptake of ferrous iron through the biosynthesis of ferritin by Caco-2 cells and determined which complementary treatment would provide optimum results. Thereafter, effects of the treatment on improving the bioavailability of absorbed iron in the form of hemoglobin (Hb) were established using a DIA-animal model. The results showed that DBT slightly improved Hb levels compared with the baseline Hb and pretreatment with DBT for 2 hours prior to supplementation with ferrous sulfate provided the greatest gain in Hb levels in DIA rats. However, DBT and ferrous sulfate were co-treated with Caco-2 cell or DIA rats, the ferritin formation and Hb levels both were decreased. In iron-binding assay, the DBT extract influenced the free Fe(II) type in the FeSO4 solution. Therefore, we suggest that complementary treatment with DBT and iron supplementation can have a strong ameliorating effect on iron-deficiency anemia in clinical settings, but needs a 2-hour interval of DBT administration prior to ferrous sulfate treatment.
Keywords: Dang-Gui-Bu-Xue-Tang, ferulic acid, iron uptake, ferritin, iron-deficiency anemia, herb–drug interaction
Introduction
Dang-Gui-Bu-Xue-Tang (DBT), a popular traditional Chinese medicine, was described as early as 1247 AD during the Jin dynasty to have functions of tonifying the blood, and invigorating and replenishing “qi” (energy).1 The main components of this decoction are Angelicae sinensis Radix (dang-gui) and Astragali Radix (huang-qi) in a traditional ratio of 1:5.2 Studies showed that DBT improves hematopoiesis.3,4 It was also found that this traditional ratio of dang-gui and huang-qi was the most effective in promoting immune responses, such as by lymphocytes, macrophages, and interleukin-2.5 Immunomodulatory activities of DBT were also established in a tumor-bearing murine model of rats and were found to regulate CD4+/CD25+ and tumor necrosis factor-α.6 Another study revealed that DBT can suppress the progression of diabetic nephropathy in rats with streptozotocin-induced diabetes.7 The anti-inflammatory properties of DBT were reported to have decreased inflammatory circulating markers in rats.8 Moreover, orthogonal studies revealed that the traditional way of extracting components of DBT, by boiling them in water, provided a high concentration of ferulic acid (FA).9 However, the ability of DBT to prevent iron-deficiency anemia (IDA) has not been explored. Therefore, we used a ferritin formation assay of Caco-2 cells and an IDA rat model to evaluate the function of DBT in toning the blood.
IDA patients need to take iron supplements; therefore, we explored the interaction of DBT with iron-containing drugs. Caco-2 cells and IDA rats were treated only with DBT, an iron-containing drug only, DBT co-administered with an iron-containing drug, and DBT alternatively administered with iron. From these results, we are able to discuss how to take DBT in combination with iron.
Materials and methods
Preparation of the DBT
Angelicae sinensis Radix (dang-gui) and Astragali Radix (huang-qi) were purchased from a traditional Chinese medicinal store in Taipei, Taiwan. The medicinal materials were authenticated by the non-profit organization Brion Research Institute of Taiwan. Dang-gui is the dried root of Angelica sinensis (Oliv.) Diels (Umbelliferae), no: AS-001. Huang-qi is the dried root of Astragalus membranaceus (Fisch.) Bge. (Leguminosae), no: AM-001. Voucher specimens (no: AS-0001 and AM-0001) were deposited at the Herbarium of the College of Pharmacy, Taipei Medical University.
The prescription of DBT was based on the unified formula announced by the Department of Chinese Medicine and Pharmacy, Ministry of Health and Welfare, Taiwan. The prescription of DBT includes dang-gui and huang-qi in a ratio of 1:5. Specifically, DBT was immersed in a 20-fold amount of distilled water, and boiled in a herb-extracting machine until half of the original amount was left. The extract was then filtered and freeze-dried. The yield of the extract was approximately 34.09% (w/w). The freeze-dried DBT powder was stored at -20°C until use.
Phytochemical analysis of DBT
Total polyphenol analysis
The total polyphenol content was detected by the Folin–Ciocalteu method.10,11 The DBT extract was dissolved in double-distilled water (ddH2O). The diluted sample was mixed with Folin–Ciocalteu reagent and a 7.5% aqueous Na2CO3 solution. After standing for 5 minutes at 50°C, the absorbance was measured at 600 nm against water on an enzyme-linked immunosorbent assay (ELISA) reader. The amount of total polyphenol was expressed as gallic acid (GA) equivalents (mg GA/g sample) through a calibration curve prepared from standard amounts of GA (Sigma-Aldrich Co., St Louis, MO, USA, purity: 97.5%–102.5%).
Total flavonol analysis
The total flavonol content was determined by the vanillin assay.12 The vanillin reagent consisted of vanillin dissolved in 80% H2SO4. The DBT extract was dissolved in ddH2O. The diluted sample was mixed with the vanillin reagent. After incubation for 15 minutes at room temperature, the absorbance was measured at 530 nm against water on an ELISA reader. The amount of total flavonol was expressed as catechin equivalents (mg catechin/g sample) through a calibration curve prepared from standard amounts of catechin (Sigma-Aldrich Co., purity: >99%).
Total saponin analysis
The total saponin content was determined by the sulfuric acid assay.13 The DBT extract was dissolved in ddH2O, and the diluted sample was mixed with a 98% H2SO4 solution. After being allowed to stand for 15 minutes at room temperature, the absorbance was measured at 414 nm against water on an ELISA reader. The amount of total saponin was expressed as diosgenin equivalents (mg diosgenin/g sample) using a calibration curve prepared from standard amounts of diosgenin (Sigma-Aldrich Co., purity: >93%).
Ferulic acid analysis
A high-performance liquid chromatography (HPLC) system consisted of a Shimadzu (Kyoto, Japan) LC-10ATvp liquid chromatograph equipped with a DGU-14A degasser, an FCV-10ALvp low-pressure gradient flow control valve, an SIL-10ADvp auto injector, an SPD-M10Avp diode array detector, and an SCL-10Avp system controller. Peak areas were calculated with Shimadzu Class-VP software (version 6.12 sp5, Shimadzu, Kyoto, Japan). The mobile phase was composed of 0.05% trifluoroacetic acid–acetonitrile (v/v) at 80:20. A Purospher STAR RP-18e reversed-phase column (5 μm, 250×4 mm ID, Merck, Darmstadt, Germany) and a Purospher STAR RP-18e guard column (4×4 mm ID) (Merck, Darmstadt, Germany) were used. The flow rate was 1.0 mL/min, with UV absorbance detection at 320 nm. The analysis involved a 10 μL of sample solution. The operation was carried out at an oven temperature of 40°C. FA (Sigma-Aldrich Co., purity: >99%) was accurately weighed, dissolved, and double-diluted in HPLC-grade methanol to give serial concentrations in the range of 0.488–500 mg/mL. Calibration curves were plotted after a linear regression of the peak areas.
Ferritin formation assay of Caco-2 cells
Caco-2 cells were obtained from the American Type Culture Collection (Rockville, MD, USA) and were cultured in Dulbecco’s Modified Eagle’s Medium containing 10% heat-inactivated fetal bovine serum, and 50 units/mL penicillin–streptomycin. The culture dishes were incubated at 37°C in a humidified incubator containing 5% CO2, and the medium was changed every 2 days. Cells were seeded in six-well plates, with each well containing 106 cells, and cultured for 2 days. When cell growth covered 80% of the area of each well, the test samples were added (day 0). The sample design for ferritin formation in Caco-2 cells is described in Figure 1. Samples were allocated into three groups as following. Group A: single treatment of FeSO4, DBT,, or FeSO4 + DBT co-treatment on day 0 and ferritin content analysis on day 1; group B: FeSO4 pretreatment on day 0, then ddH2O/DBT added on day 1, and ferritin content analysis on day 2; group C: ddH2O/DBT pretreatment on day 0, then FeSO4 added on day 1 and ferritin content analysis on day 2. Briefly, cells were harvested and lysed using radioimmunoprecipitation assay buffer, after which cells were frozen at -20°C until being analyzed. The total protein content of the cell lysate was measured with a Coomassie Brilliant Blue G-250 binding assay (Bio-Rad DC Protein assay, Bio-Rad, Hercules, CA, USA). The ferritin amount was assessed with a commercially available enzyme immunoassay system (Amersham Pharmacia Biotech, Buckinghamshire, UK). Ferritin formation by Caco-2 cells is dependent on the intracellular iron concentration. The index of cellular iron uptake is expressed as the ratio of ferritin (ng)/protein (mg). The ethics committee/institutional review board of Taipei Medical University deemed it is not necessary to review and provide approval for commercial cell lines.
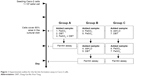  | Figure 1 Experimental outline for the ferritin formation assay in Caco-2 cells. |
Rats of the DIA model
Male Sprague-Dawley (SD) rats weighing 65±5 g were obtained from the BioLASCO Taiwan Co., Ltd. (Yilan City, Taiwan), and maintained in plastic cages at 21°C±2°C with free access to pellet food and water. They were kept on a 12-hour light–dark cycle. All rats used in this experiment were cared for according to the Ethical Regulations on Animal Research of Taipei Medical University. All animal experiments were approved by the Animal Research of Taipei Medical University (permit no: LAC-97-0075). Test animals used in this study had body weights (BWs) of <70 g. SD rats were conditioned and fed the AIG-93G diet (an iron-deficient diet, the formulation of which is given in Table 1) for 21 days. Blood was collected after 21 days from a tail vein into heparinized microcapillary tubes, and hemoglobin (Hb) levels were measured using a KX-21N Hematology Analyzer (Sysmex, Kobe, Japan). SD rats with Hb levels of <6 g/dL were treated with test materials. The sample design for the diet-induced anemia (DIA) model is described in Figure 2. Test animals were grouped and treated with the following materials: group 1 (ddH2O only), group 2 (FeSO4 only), group 3 (DBT 250 mg/kg BW), group 4 (DBT 500 mg/kg BW), group 5 (DBT 500 mg/kg BW co-treated with 1 mg FeSO4/kg BW), and group 6 (pretreated with DBT 500 mg/kg BW followed at a 2-hour interval by 1 mg FeSO4/kg BW). Six rats were assigned to each group, and materials were administered once a day for 28 days. After treatment for 28 days, blood samples from each test animals were collected, and Hb levels were measured. The gain in Hb was expressed as the difference in Hb levels from the initial treatment (day 21) and the last treatment (day 49).
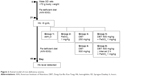  | Figure 2 Animal model of iron-deficiency anemia. |
Iron-binding analysis
Ferrous ion (Fe2+) was detected by a colorimetric reagent. The colorimetric reagent contained 0.025% bathophenanthroline disulfonic acid and 2 M sodium acetate. FeSO4 was accurately weighed, dissolved in ddH2O, and serially diluted to provide a concentration range of 0.375–12 mg/mL. Each diluted solution of FeSO4 was combined with 500 μg/mL DBT and 30 μg/mL of FA (the principal component in DBT). The colorimetric reagent was added and incubated at 37°C for 16 hours, after which the absorbance was measured at 530 nm against water in an ELISA reader.
Statistical analysis
Results are presented as the mean ± standard deviation from three independent experiments. A one-way analysis of variance in SPSS version 12 software (SPSS Inc., Chicago, IL, USA) was used to analyze the results. Results were considered statistically significant when P<0.05.
Results
Phytochemical contents of DBT
The phytochemical analysis of the water extract of DBT (Table 2) by the Folin–Ciocalteu method showed that it contained 6.51±0.29 mg/g total polyphenol compounds. Total flavonoids were found to be 2.31±0.05 mg/g using the vanillin test method. The innate iron II content of the DBT water extract was determined using the inductively coupled plasma mass spectrometry (ICP-MS) method, and provided a total ferrous content of 14.5 μg/g. FA, which was determined to be the principal component of DBT, was found to be 30.83±0.29 mg/g using the HPLC-UV method. Interestingly, the concentration of total saponin in the DBT water extract (34.48±3.99 mg/g) was comparable to the FA content. Total polyphenol, total flavonoids, Fe2+ content, and total saponin are respectively expressed as GA, catechin, FeSO4, and diosgenin equivalents. All calibration curves exhibited good linearity (0.9970–1.0000).
Stimulated ferritin formation effects of DBT in Caco-2 cells
Following the protocol as outlined in Figure 1, we measured the formation of ferritin in Caco-2 cells. Results are shown in Figure 3, where a significant dose-dependent increase in ferritin (hollow bars) was observed with DBT even when no FeSO4 was added. When Caco-2 cells were cultured with the DBT extract combined with FeSO4, FeSO4 promoted ferritin formation that the DBT extract inhibited (Figure 3). Related to this (results in gray bars), with low-concentration FeSO4 (25 ng/mL) co-treatment with DBT, a dose-dependent increase in ferritin formation was also observed, but this was only significant at 500 μg/mL. However, none of the DBT-FeSO4 (25 ng/mL) groups exhibited an enhanced compartment with the DBT groups. A dramatic increase in ferritin was observed with FeSO4 treatment at 25 μg/mL FeSO4 (black bars), which implies increased iron uptake could also be achieved at this high FeSO4 dosage. However, with high-concentration FeSO4 (25 μg/mL) co-treatment with DBT, ferritin formation began to decrease in a dose-dependent manner. These results show that the inhibitory effects of the DBT extract increased when co-treated with higher concentrations of FeSO4.
To establish whether interval administration of DBT and FeSO4 could improve iron uptake by Caco-2 cells by monitoring ferritin formation, alternate treatments with DBT–FeSO4 and FeSO4–DBT were performed (Figure 4). Baseline ferritin formation (ng/mg protein) was established for non-pretreated (hollow bars), DBT-pretreated (gray bars), and FeSO4-pretreated (black bars) Caco-2 cells. DBT concentrations of 125, 250, and 500 μg/mL were administered either as pre- or post-treatment with respect to ferrous sulfate which was administered at a fixed concentration of 25 μg/mL. A 1-day interval was applied in either case. Both pretreatment and post-treatment groups showed high iron uptake as reflected by ferritin formation. Results in Figure 4 suggest that DBT enhanced FeSO4 to promote ferritin formation. The promoting effects of FeSO4 were more enhanced when DBT was added before FeSO4.
The ameliorating effects of DBT in diet-induced anemia in rat
Further investigation of the bioavailability effects of pretreatment versus co-treatment by determining the gain in Hb (g/dL) in the DIA-animal model is shown in Figure 5. The baseline Hb level induced by the iron-deficient diet (AIN-93G) in rats was recorded. It can clearly be seen that treatment only with DBT (either 250 or 500 mg/kg) slightly improved Hb levels compared with the baseline Hb. On the other hand, co-treatment of FeSO4 (1 mg/kg) with DBT (500 mg/kg) showed no significant improvement in Hb levels of the test animals, and Hb levels even decreased compared with those of rats treated with FeSO4 only. This result supports the observed decrease in ferritin formation for the high-concentration FeSO4-co-treated group (black bars) in Figure 3, due to induction of a drug–herb interaction. However, the 2-hour interval prior of FeSO4 administration prior to DBT treatment provided the greatest improvement in Hb levels in DIA rats. This was also found to be significant compared with rats treated only with FeSO4. Therefore, we used an iron-binding analysis to explore the mechanism of FeSO4–DBT extract interactions.
Ferulic acid as the bioactive marker of DBT, stimulated ferritin formation in Caco-2 cells
The DBT extract contained 30.8 mg FA/g (Table 2). Therefore, we used 15 μg/mL FA which was equivalent to 500 μg FA/mL DBT extract. Investigations of the effects of increased concentrations of FA on ferritin formation of Caco-2 cells were performed, and results are summarized in Figure 6. A dose-dependent increase in ferritin formation was well established by increasing the FA concentration from 7.5 to 30 μg/mL in the presence of 25 μg/mL FeSO4, but only 30 μg/mL FA significantly promoted ferritin formation compared with FeSO4 only (Figure 6). However, there was no apparent change in ferritin production in the absence of FeSO4 even though FA had increased as depicted by the hollow bars. The above-mentioned results argue that FA could be a bioactive marker, but it was not the only active component.
The interaction effects between iron II and DBT or ferulic acid
On the other hand, a possible interaction of DBT with iron II was explored in Figure 7. Here one can see that the baseline calibration curve for the increasing concentration of FeSO4 only ( ) was lower than the calibration curve drawn from the solution with the combination of FeSO4 and 30 μg/mL FA (
) was lower than the calibration curve drawn from the solution with the combination of FeSO4 and 30 μg/mL FA ( ). This result suggests that the presence of FA, the relatively low pH value condition, would help the dissociation of FeSO4 to Fe2+ and result in increment of iron II detection. Surprisingly, the presence of 500 mg/mL of DBT significantly decreased the calibration curve of FeSO4 (
). This result suggests that the presence of FA, the relatively low pH value condition, would help the dissociation of FeSO4 to Fe2+ and result in increment of iron II detection. Surprisingly, the presence of 500 mg/mL of DBT significantly decreased the calibration curve of FeSO4 ( ). In summary, our results suggest that the DBT extract influenced the free Fe(II) type in the FeSO4 solution.
). In summary, our results suggest that the DBT extract influenced the free Fe(II) type in the FeSO4 solution.
Discussion
Caco-2 cells are a popular and widely used cell line to investigate in vitro iron uptake.14 It was also established that monitoring ferritin production by Caco-2 cells provides comparable results to intrinsic radiolabeling of food and food products in iron uptake studies.14,15 Thus, this method was used in our study. As outlined in Figure 1, it was necessary to determine the response of Caco-2 cells in each of the components of this experiment, such as with ddH2O, FeSO4, DBT, and the latter two in combination to establish which components have effects on iron uptake by this cell line. However, it was found that co-treatment with a low concentration of FeSO4 (25 ng/mL) and DBT produced no significant difference in ferritin formation compared with DBT only (Figure 3). The addition of FeSO4 (25 ng/mL) in combination with DBT (gray bars) produced the same level of ferritin as did treating cells with DBT only (hollow bars). We also found that the ferritin formation response of Caco-2 cells with DBT only was due to the innate iron content of the DBT formulation as depicted by the total Fe2+ concentration analysis by ICP-MS (Table 2). However, a dose-dependent increase in ferritin formation was observed at these low levels of innate iron and with the addition of iron to the cells. In contrast, at a high concentration (25 μg/mL) of FeSO4 co-treated with increasing doses of DBT, a dose-dependent decline in ferritin formation was observed (black bars). This decrease in iron uptake by Caco-2 cells was thought to be due to the high innate saponin content of the DBT formulation (Table 2). Previous studies established that complexation of various naturally occurring saponins has a negative effect on iron availability for absorption and uptake in in vitro and in vivo experiments.16,17 According to the study from Milgate and Roberts,18 the insoluble saponin-iron complex would affect the iron availability for absorption and uptake, while it is the possible mechanism of this phenomenon.
To further improve iron uptake by Caco-2 cells, an alternate pretreatment with DBT-FeSO4 was designed, in which 1-day pretreatment was carried out before the second drug was administered (Figure 4). It was observed that if the cell lines were first pretreated with DBT then after 1 day 25 μg/mL of FeSO4 was added, a dose-dependent increase in ferritin formation occurred. The obvious dose-dependent decrease observed at this concentration of added FeSO4 in Figure 3 was eliminated by the pretreatment process. It was thought that pretreatment was necessary to prevent the saponins from complexing with the added iron. Moreover, polyphenol being good antioxidants may prevent the oxidation of Fe2+ to the unabsorbable ferric form. Furthermore, the interval may be a necessary procedure to allow production of free phenols in the gastrointestinal tract.
Because pretreatment was established to prevent complexation of DBT components with ferrous ion, it was necessary to develop a treatment process that would be feasible if developed in clinical settings. Thus, an interval of 2 hours was applied to DIA rats for DBT-FeSO4 administration. It can clearly be seen in Figure 5 that among other treatments, animals treated with the 2-hour interval exhibited the most significant gain in Hb. This strongly suggests that the designed program of administration greatly improved the bioavailability of iron. Pretreatment also ensured that saponins were eliminated through the intestines, and thus complexation with the added iron was prevented, because it was found to be unabsorbed in the small intestine.19
To further validate that FA, the major component of DBT, contributes to the observed increased iron absorption, we returned to ferritin formation by Caco-2 cells. Figure 6 clearly shows that only FA assisted the absorption of added FeSO4 at 25 μg/mL which produced a significant dose-dependent increase in ferritin formation. This was further supported by results provided in Figure 7, where the highest colorimetric concentration of FeSO4 was due to FeSO4 + FA. Moreover, the large decline in available Fe2+ observed in the FeSO4 + DBT curve strongly suggests that indeed DBT-saponins interfered with the absorption of ferrous ions. Therefore, in conclusion, this study has established the ameliorating effects of the 2-hour interval between the administration of DBT prior to FeSO4 on ferritin formation in vitro by Caco-2 cells and in vivo on the gain of Hb by DIA rats. We strongly suggest that incorporating DBT as a complementary drug to FeSO4 treatment can improve Hb levels of iron-deficient patients in clinical settings.
Acknowledgments
This work was supported by grants from Division of Hemato-oncology, Department of Internal Medicine, Yuan’s General Hospital, and Taipei Medical University (103YGH-TMU-02-1).
Disclosure
The authors report no conflicts of interest in this work.
References
Wang P, Liang YZ. Chemical composition and inhibitory effect on hepatic fibrosis of Danggui Buxue decoction. Fitoterapia. 2010;81:793–798. | ||
Liu IM, Tzeng TF, Liou SS. A Chinese herbal decoction, Dang Gui Bu Xue Tang, prepared from Radix Astragali and Radix Angelicae sinensis, ameliorates insulin resistance induced by a high-fructose diet in rats. Evid Based Complement Alternat Med. 2011;2011:248231. | ||
Wang XL, Wang T, Wang YN. Effect of danggui buxue decoction on the hemopoiesis reconstruction of mouse transplanted by the muscle satellite cell receptor. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2011;31:1093–1096. | ||
Zheng KY, Choi RC, Xie HQ, et al. The expression of erythropoietin triggered by danggui buxue tang, a Chinese herbal decoction prepared from Radix Astragali and Radix Angelicae Sinensis, is mediated by the hypoxia-inducible factor in cultured HEK293T cells. J Ethnopharmacol. 2010;132:259–267. | ||
Gao QT, Cheung JK, Li J, et al. A Chinese herbal decoction, Danggui Buxue Tang, activates extracellular signal-regulated kinase in cultured T-lymphocytes. FEBS Letters. 2007;581:5087–5093. | ||
Hsieh CC, Lin WC, Lee MR, et al. Dang-Gui-Bu-Xai-Tang modulated the immunity of tumor bearing mice. Immunopharmacol Immunotoxicol. 2003;25:259–271. | ||
Zhang YW, Xie D, Xia B, Zhen RT, Liu IM, Cheng JT. Suppression of transforming growth factor-beta1 gene expression by Danggui buxue tang, a traditional Chinese herbal preparation, in retarding the progress of renal damage in streptozotocin-induced diabetic rats. Horm Metab Res. 2006;38:82–88. | ||
Zhang H, Chen S, Deng X, Yang X, Huang X. Danggui-Buxue-Tang decoction has an anti-inflammatory effect in diabetic atherosclerosis rat model. Diabetes Res Clin Pract. 2006;74:194–196. | ||
Song ZH, Ji ZN, Lo CK, et al. Chemical and biological assessment of a traditional Chinese herbal decoction prepared from Radix Astragali and Radix Angelicae Sinensis: orthogonal array design to optimize the extraction of chemical constituents. Planta Med. 2004;70:1222–1227. | ||
Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. | ||
Everette JD, Bryant QM, Green AM, Abbey YA, Wangila GW, Walker RB. Thorough study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. J Agric Food Chem. 2010;58:8139–8144. | ||
He X, Liu D, Liu RH. Sodium borohydride/chloranil-based assay for quantifying total flavonoids. J Agric Food Chem. 2008;56:9337–9344. | ||
Hiai S, Oura H, Nakajima T. Color reaction of some sapogenins and saponins with vanillin and sulfuric acid. Planta Med. 1976;29:116–122. | ||
Glahn RP, Lee OA, Yeung A, Goldman MI, Miller DD. Caco-2 cell ferritin formation predicts nonradiolabeled food iron availability in an in vitro digestion/Caco-2 cell culture model. J Nutr. 1998;128:1555–1561. | ||
Viadel B, Perales S, Barberá R, Lagarda MJ, Farré R. Ferritin synthesis by Caco-2 cells as an indicator of iron bioavailability: application to milk-based infant formulas. Food Chem. 2007;102:925–931. | ||
Southon S, Johnson IT, Gee JM, Price KR. The effect of Gypsophila saponins in the diet on mineral status and plasma cholesterol concentration in the rat. Br J Nutr. 1988;59:49–55. | ||
West LG, Greger JL, White A, Nonnamaker BJ. In vitro studies on saponin-mineral complexation. J Food Sci. 1978;43:1342–1343. | ||
Milgate J, Roberts DCK. The nutritional & biological significance of saponins. Nutr Res. 1995;15:1223–1249. | ||
Gestetner B, Birk Y, Tencer Y. Soybean saponins. Fate of ingested soybean saponins and the physiological aspect of their hemolytic activity. J Agric Food Chem. 1968;16:1031–1035. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







