Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Effects of Curcumin on High Glucose-Induced Epithelial-to-Mesenchymal Transition in Renal Tubular Epithelial Cells Through the TLR4-NF-κB Signaling Pathway
Authors Liu X, Zhang X, Cai X, Dong J, Chi Y, Chi Z, Gu HF
Received 21 December 2020
Accepted for publication 3 February 2021
Published 2 March 2021 Volume 2021:14 Pages 929—940
DOI https://doi.org/10.2147/DMSO.S296990
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Xinhui Liu,1 Xiuli Zhang,2,3 Xiaoyi Cai,2 Jiqiu Dong,2 Yinmao Chi,4 Zhihong Chi,3 Harvest F Gu5
1Traditional Chinese Medicine, Liaoning University of Traditional Chinese Medicine, Shenyang, Liaoning Province, 110847, People’s Republic of China; 2Department of Nephrology, Second People’s Hospital, The First Affiliated Hospital of Shenzhen University, Shenzhen, Guangdong Province, 518000, People’s Republic of China; 3Department of Pathophysiology, China Medical University, Shenyang, Liaoning Province, 110001, People’s Republic of China; 4Department of Physiology, China Medical University, Shenyang, Liaoning Province, 110001, People’s Republic of China; 5Center for Pathophysiology, School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, Jiangsu Province, 210009, People’s Republic of China
Correspondence: Xiuli Zhang
Department of Nephrology, Second People’s Hospital, The First Affiliated Hospital of Shenzhen University, Shenzhen, Guangdong Province, 518000, People’s Republic of China
Email [email protected]
Harvest F Gu
Center for Pathophysiology, School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, Jiangsu Province, 210009, People’s Republic of China
Email [email protected]
Objective: Diabetic kidney disease (DKD) is a microvascular complication in diabetes mellitus, while tubuloepithelial to mesenchymal transition (EMT) of mature tubular epithelial cells is a key point in the early development and progression of renal interstitial fibrosis. The present study aimed to investigate the protective effects of Curcumin on EMT and fibrosis in cultured normal rat kidney tubular epithelial cell line (NRK-52E).
Methods: By using immunofluorescence staining and Western blot protocols, in vitro experiments were designed to analyze EMT markers, including collagen I and E-cadherin in high glucose (HG) exposed NRK-52E cells and to detect the expression levels of phosphorylated-NF-κB, TLR4 and reactive oxygen species (ROS) after Curcumin pre-treatment. With co-treatment with TAK242, these molecules in the TLR4-NF-κB signaling pathway were further evaluated.
Results: Curcumin decreased the HG-induced EMT levels and ROS production in NRK-52E cells. Furthermore, Curcumin was found to inhibit the TLR4-NF-κB signaling activation in HG-induced EMT of NRK-52E cells.
Conclusion: The present study provides evidence suggesting a novel mechanism that Curcumin exerts the anti-fibrosis effects via inhibiting activation of the TLR4-NF-κB signal pathway and consequently protecting the HG-induced EMT in renal tubular epithelial cells. Thereby, TLR4-NF-κB may be a useful target for therapeutic intervention in DKD.
Keywords: diabetic kidney disease, tubuloepithelial to mesenchymal transition, toll-like receptor 4, curcumin, inflammation
Introduction
Chronic kidney disease (CKD) is a health crisis worldwide, while diabetic kidney disease (DKD, previously termed as diabetic nephropathy) has become a growing cause of end-stage kidney disease (ESKD). DKD is a major microvascular complication associated with diabetes mellitus and occurs in 30–40% of diabetes patients.1 DKD is characterized by a distinct histopathological pattern of glomerular basement membrane thickening, nodular glomerulosclerosis, mesangial matrix expansion, and arteriolar hyalinosis. Tubulointerstitial fibrosis is widely suggested to be the final common pathway for loss of renal function in DKD. Long-term exposure to hyperglycaemia and other metabolic disturbances associated with diabetes lead to progressive and cumulative atrophy of tubular epithelial cells.2 In DKD, up to 50% of the glomeruli are associated with dilated and atrophic tubules, and about 17% of glomeruli may be a tubular that also contributes to albuminuria.3 In DKD, renal function and prognosis may correlate better with tubulointerstitial fibrosis than with classic and early glomerular changes.4 Increasing evidence suggested that the accumulation of activated myofibroblasts, in terms of tubuloepithelial to mesenchymal trans-differentiation (EMT), is the major contributor to progressive renal scarring in diabetes.5 Importantly, evidence from the studies with preclinical models has demonstrated that fibrosis in the tubulointerstitium is the most important predictor of progression to ESKD in DKD.6,7
There is now clear evidence to suggest that the primary disorder is inflammation leading to irreversible fibrosis and organ failure. The innate immune system is also involved in the inflammatory process of diabetic complications, including DKD.8,9 Toll-like receptor (TLRs) is a group of receptors of the innate immune system and plays a vital role to protect the host to sense danger and detect the presence of invading pathogens in the host immune system.10 The genome encodes 10 TLRs (TLR1–10) in human. TLRs are type 1 transmembrane receptors and all belong to the interleukin-1 receptor (IL-1R) superfamily, which share significant homology in their cytoplasmic regions, eg, the Toll/IL-1R (TIR) domain.11,12 Although TLR 1, 2, 3, 4 and 6 are found in the kidney proximal tubule cells, TLR2 and TLR4 are the most expressed and extensively studied.13 TLR2 and TLR4 are involved in the development of DKD under a sterile environment.14 Recent studies in vitro and in vivo have demonstrated that the innate immune system-driven inflammatory processes result in cell apoptosis, tissue fibrosis and renal failure.9,13,15 TLRs and their associated cytokine responses may be indicated in the kidneys of the patients with DKD.14 Furthermore, TLR4 mRNA and protein were overexpressed in glomeruli and tubules, which were associated with microalbuminuria and overt DKD.16 In addition, TLR4 signal is obviously expressed throughout the brush border of proximal tubule cells in patients with overt DKD, which suggests the increased accessibility of renal TLRs to systemically generated molecules.16 Our previous studies have reported a strong association between TLRs (TLR2 and TLR6) and pro-inflammatory factors (TNF-α and IL-L6) in the injury of rat peritoneum in peritoneal dialysis associated peritonitis.17,18 Based upon these studies, TLRs may play a vital role in the pathogenesis of DKD.
Curcumin, a polyphenol extracted from curcuma longa L. commonly used as a spice and a natural food pigment as well as a component of many herbal medicines has been reported to possess extensive pharmacological activities, such as anti-inflammation, anti-oxidation, anti-fibrosis, anti-cancer, and anti-atherosclerosis.19–21 Evidence has demonstrated that mechanisms such as mitochondrial overproduction of reactive oxygen species (ROS), oxidative stress, generation of pro-fibrotic and fibrotic cytokine, lipid disorders, glomerular hemodynamic and structural alterations have been proved to be involved in the development and progression of DKD.22 Curcumin plays beneficial effects on renal fibrosis, the crucial process underlying the progression of DKD to ESRD.23,24 A previous study has indicated that Curcumin exhibits protective effects in DKD by regulating inflammatory cytokines, ameliorating oxidative stress and podocyte apoptosis through related pathways or molecules mediated by specific molecular targets.25 In DKD, Curcumin also increases insulin sensitivity and decreases blood sugar and cholesterol in diabetes. With the researches of Curcumin nanoformulations may address the limitation of low bioavailability and provides a promising therapeutic strategy, there is the potential of Curcumin being a therapeutic agent to suppress renal fibrosis and delay the development of DN.26 Previously, we have reported that Curcumin plays an important role in cellular antioxidant defense via the activation of Nrf2 and HO-1, thereby protecting HG-induced EMT in the NRK-52E cells.27 However, whether Curcumin has anti-fibrosis effects in DKD, especially in TLR4-NF-κB during EMT of kidney tubular epithelial cell is still unknown.
In the present study, we have designed the experiments to investigate whether Curcumin plays a protective role against DKD by restraining HG-induced EMT in renal tubular cells via the inhibition of TLR4-NF-κB signaling activation because better understanding the role of Curcumin in EMT may provide a potential novel strategy for the prevention and treatment of EMT-tubulointerstitial fibrosis in DKD.
Materials and Methods
Cell Culture
Normal rat kidney tubular epithelial cell line (NRK-52E) was purchased from the American Type Culture Collection (Rockville, MD, USA). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with10% fetal calf serum (FCS, Sigma) as described previously.27 In the control group, the cells were treated with serum free DMEM medium only. In HG group, the cells were treated with 30 mM HG for 48 h with 0, 5, 10, or 20 μM Curcumin for 24 h, respectively.
Assessment of Cell Viability
Cell viability was texted by quantitative colorimetric assay with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) in 96-well plates. The cells were added 10 μL MTT (final concentration, 500 μg/mL) to the medium and incubated at 37°C for 3 h at the indicated time after treatment. Next, the MTT solution was removed, then 100 μL dimethyl sulfoxide (DMSO) was added to dissolve the colored formazan crystals for 15 min. The absorbance at 490 nm of each aliquot was measured using a Sunrise RC microplate reader (TECAN, Männedorf, Switzerland).
Immunofluorescence Staining
NRK-52E cells that had undergone various treatments were fixed in 4% paraformaldehyde and per-mobilized in 0.1% Triton X-100, before they were, respectively, treated with primary mouse monoclonal anti-E-cadherin (E-ca) antibody (Abcam ab1416, 1:100), mouse monoclonal collagen I antibody (Abcam ab260043, 1:100), TLR4 (Abcam ab22048, 1:200), NF-κB (Abcam ab194726, 1:200). After three washes with PBS, the sections were incubated for 2 h with DAR-FITC (1:50) and Texas Red-DAM (1:50) at RT. The fluorescent images were visualized with a Fluoview 300 fluorescence microscope (Olympus, Tokyo, Japan).
Western Blot Analysis
Western blot analysis was conducted as published previously.18,27 All the cells were washed twice with cold PBS and resuspended in five volumes of ice-cold extract buffer (20 mM Western blotting analysis Hepes-KOH, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, and 0.1 mM phenylmethanesulfonyl fluoride, pH 7.5 for 15 min at 4°C). Lysates were centrifuged at 25,000 ×g for 15 min. Protein concentrations were quantified using a Bio-Rad protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal amounts of protein samples (10 μg proteins) from NRK-52E cells were subjected to SDS-PAGE using 10% gradient Tris/glycine gels. Then, the proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Temecula, CA, USA). After blocking with 5% fat-free milk for 1 h, the blots were incubated with the following primary antibodies. The primary antibodies used in the present study were as follows: collagen I antibody (Abcam ab270993, 1:400), anti-E-ca antibody (1:400, Abcam ab1416), TLR4 (Abcam ab13867, 1:400), NF-κB (Abcam ab239882, 1:400), and mouse monoclonal anti-GAPDH (Abcam ab8245, 1:400). Following extensive washing in TBS-0.1% Tween 20, the membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies, including rabbit anti-goat IgG (1:400) and rabbit anti-mouse IgG (1:400) overnight at 4°C. Then, the membranes were incubated with secondary antibody in TBST solution at 37°C for 30 min after four washes with TBST. Finally, the membranes were incubated in ECL (Pierce, Thermo Co. Ltd, Waltham, MA, USA) reagent for HRP (30 s) and exposed to autoradiography film for visualization of the bands. Following the development, the band intensities were quantified using Image-Pro plus 6.0 analysis software (Media Cybernetics, Inc., Rockville, MD, USA). The blots were repeated at least three times for each condition.
Measurements of Superoxide Generation
ROS production in chondrocytes was measured by 2′,7′-DCF diacetate (DCFH-DA; Sigma-Aldrich; EMD Millipore) as previously described.28 After exposure to the various treatments, the cells in the six-well plate were stained with 20 μM DCFH for 30 min at 37°C and washed with PBS 3 times in order to remove residual probes. The mean fluorescence intensity was calculated by NIH ImageJ software and was expressed relative to the control.
Statistical Analysis
Data are presented as mean ± SEM. Statistical analysis was performed using SPSS (Version 18, IBM Corporation, Armonk, NY, USA). Variance was homogenous for use of standard ANOVA methodology. Individual comparisons were made using Tukey’s multiple comparison tests after statistical significance was established by ANOVA. All P values were two-sided, and statistical significance was set at P<0.05.
Results
Effects of Curcumin on Cell Viability of NRK-52E Cells
The cell viability of NRK-52E cells was assessed under HG (30 mM) and Curcumin (5 μM, 10 μM, 20 μM) conditions. NRK-52E cell viability was significantly inhibited in HG conditions compared to the control group (5 mM glucose and 0 µM Curcumin-treated cells) (Figure 1). However, when the cells were co-treated with HG and 10 or 20 µM Curcumin, respectively, the viability of the cells was elevated. Therefore, these results suggest that Curcumin has a protective role in NRK-52E cells under HG conditions.
Effects of Curcumin on EMT Markers Collagen I and E-Ca in NRK-52E Cells
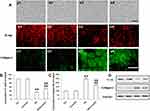
First, normally, the tubular epithelial cell exhibited typical cobblestone morphology under magnification. As shown in Figure 2A a1 and a2 above, a typical epithelial cuboidal shape was observed in the control and Curcumin group, with the characteristic cobblestone morphology. The cells morphological change to a fibroblast-like shape after HG treatment for 48 h (Figure 2A, a3 above) and Curcumin treatment reduced the morphological change (Figure 2A, a4 above). Meanwhile, expression of E-ca was down-regulated (Figure 2A, b3, b4 above), while collagen I was up-regulated (Figure 2A, c3, c4 above), whereas this HG-induced EMT can be attenuated by Curcumin in NRK-52E cells. Next, the EMT markers protein expression of collagen I and E-ca analysis by Western blot represented under the conditions of control, Curcumin, HG and HG plus Curcumin, respectively. The results suggested that Curcumin significantly decreased HG-induced EMT in NRK-52E cells, as evidenced by the decreased up-regulation of collagen I and ameliorated expression of E-ca (Figure 2B–D below).
Influence of Curcumin on HG-Induced TLR4 in NRK-52E Cells
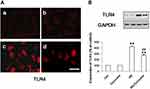
We initially performed immunofluorescence staining for text the expression of TLR4 in NRK-52E cells. Under the controlled conditions, Curcumin did not enhance the expression of TLR4 (Figure 3A). The expression of TLR4 was significantly increased in HG group compared with the control group (Figure 3A). Conversely, Curcumin significantly inhibited HG-induced levels of TLR4 (Figure 3A). The protein expression of TLR4 was also analyzed by Western blot represented under the same conditions. The results suggested that Curcumin decreased HG-induced up-regulation TLR4 significantly (Figure 3B).
Influence of Curcumin on HG-Induced NF-κB in NRK-52E Cells
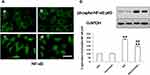
Previous studies demonstrated that NF-κB, as a downstream nuclear transcription factor of TLR4 signaling pathway activity, plays an important role in EMT of the various kinds of epithelial cells.29 In the present study, the activation of NF-κB by immunofluorescence staining analysis was significantly increased in HG-treated group compared with the control group (Figure 4A). Conversely, Curcumin significantly inhibited HG-induced phosphorylation of NF-κB p65. Moreover, we also tested phospho-NF-κB p65 level by Western blot. Like immunofluorescence staining, Curcumin treatment decreased the NF-κB p65 phosphorylation (Figure 4B).
Effect of Curcumin on the ROS Production in the HG-Treated NRK-52E Cells
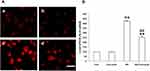
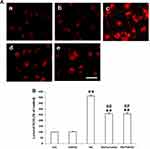
As ROS plays important role in the HG-induced EMT of the NRK-52E cells, we, therefore, examine the effect of Curcumin on the HG-induced ROS induction in the cells by measuring the intracellular ROS with DCF-DA staining. The result showed that HG-treatment resulted in a substantial increase in ROS production in the NRK-52E cells (Figure 5A and B). In contrast, Curcumin pretreatment significantly attenuated HG-induced ROS production in the NRK-52E cells (Figure 5A and B).
TLR4-NF-κB Signaling Pathway Involves in Protective Effect of Curcumin on EMT in HG-Treated Cells
To confirm the involvement of TLR4-NF-κB signaling pathway in the protective effect of Curcumin on HG-induced EMT in NRK-52E cells, 5 μM TAK242 (a molecularly targeted clinical TLR4 antagonist) was added to the medium of NRK-52E cells for 2 h.30 Subsequently, the cells were exposed to HG for 48 h after the treatment with Curcumin 20 μM for 24 h. The results showed that the HG group increased the expression of TLR4, activated NF-κB p65 phosphorylation and HG-induced EMT in the NRK-52E cells, namely the up-regulation of collagen I was reduced, while the ameliorated expression of epithelial protein E-ca increased (Figure 6A–E). In addition, the co-treatment with TAK242 also effectively inhibited expression of TLR4, activated NF-κB p65 phosphorylation and HG-induced EMT (Figure 6A–E), and there are no significant differences between HG/Curcumin and HG/TAK242 group.
Curcumin via Inhibition of TLR4/NF-κB Signaling Reversed the HG-Induced ROS Production in NRK-52E Cells
Previous studies provided evidence to support a novel role for oxidative stress in the pathogenesis of DKD and in other diabetic complications.22 To further understand the mechanism of TLR4-NF-κB signaling involvement in the protective effects of Curcumin via anti-oxidant stress effects on HG-induced EMT in NRK-52E cells. Thus, we measured the HG-induced intracellular ROS levels in NRK-52E cells with DCF-DA staining. HG increased the intracellular ROS levels, while pre-treatment with Curcumin intracellular ROS levels was decreased. Moreover, the co-treatment with TAK242 inhibited intracellular ROS levels effectively (Figure 7A and B). The results suggest that the anti-fibrosis role of Curcumin may be related to inhibition of TLR4-NF-κB signaling reversed the HG-induced ROS production in NRK-52E Cells.
Discussion
We have investigated the protective effects of Curcumin on EMT and fibrosis in the cultured NRK-52E cell line. Our findings mainly include: first, the expression level of EMT markers, including collagen I was increased, and E-cadherin decreased in HG exposed NRK-52E cells, while Curcumin reduced the HG-induced EMT. Second, expressions of phosphorylated-NF-κB, TLR4, as well as ROS, were up-regulated after HG stimulation but down-regulated after Curcumin pre-treatment. Third, the co-treatment with TAK242 also effectively inhibited the inactivation of the TLR4-NF-κB signaling pathway led to the reduction of EMT in HG-treated NRK-52E cells, which effect similar to the Curcumin group.
Evidence has demonstrated that EMT plays an important role in the genesis of fibroblasts during organ fibrosis.31 Further study of renal fibrosis has demonstrated that myofibroblasts are derived from tubular epithelial cells through the process of EMT, which is closely related to a decline in renal function in progressive renal disease.32 Loss of epithelial cell characteristics and gain of ECM-producing myofibroblast characteristics is an important mechanism involved in tubulointerstitial fibrosis.33 In DKD, EMT of mature tubular epithelial cells of the kidney has been considered closely associated with the progression of tubulointerstitial fibrosis.4,5 Indeed, high levels of glucose in diabetes-induced EMT of tubular cells can lead to matrix accumulation and deposition, which was usually regarded as the initial factor and a key mechanism of renal tubulointerstitial fibrosis in DKD.34 Thus, targeting EMT has been as a potential therapeutic method to attenuate the progression of renal fibrogenesis in the diabetic kidney. A recent study demonstrated that Curcumin significantly inhibits hyperglycemia-induced fibrosis in rat renal epithelial cells and cardiomyoblast-like cells in vitro, and in the heart and kidneys of Streptozotocin (STZ)-induced diabetic mice in vivo.35 A clinical study has shown that Curcumin, as an active turmeric metabolite, has an effective adjuvant therapy for reducing macroscopic proteinuria in type 2 diabetic patients. This effect may appear after 2 months of therapy and even in patients with a mild decrease in eGFR. Further studies with a larger sample size and longer duration are recommended.36 In the present study, we have confirmed that HG can induce the changes of EMT markers, namely decreasing the epithelial marker E-ca and increasing collagen I, and BBR pretreatment exerts the potential protective effect against HG-induced EMT in NRK-52E cells.
Inflammation converge on key transcription factors, such as NF-κB, to induce expression of inflammatory cytokines, adhesion molecules, or cause changes to cell growth and differentiation.37,38 Previous studies have described that TLR4 is involved in hyperglycemia-induced inflammatory state of renal tubules in vitro and in vivo.39,40 TLR4 signaling pathway to be critical for the activation of NF-κB and subsequent production of proinflammatory cytokines induce inflammation, which contributes to the pathogenesis of inflammation-associated renal injury and tubulointerstitial inflammation in DKD.29,41 The previous study has showed that HG-stimulated activation of TLR4 signaling leads to the activation of NF-κB signaling and upregulation of inflammatory and fibro-genic factors in both podocytes and tubular epithelial cells. Importantly, depletion of TLR4 lessened renal hypertrophy, alleviated renal injury, and inhibited inflammatory and fibrosis.42 A recent study has demonstrated that Curcumin effectively prevented EMT and renal interstitial fibrosis via inhibiting the activation of TLR4/NF-κB and PI3K/AKT signaling pathways using unilateral ureteral obstruction mice and TGF-β1-induced HK-2 cell models.43 In addition, Curcumin treatment significantly reduced the proliferation and migration of non-small cell lung cancer cell lines, possibly via TLR4/MyD88-EGFR-mediated of decrease in AP-1 protein expression and inhibition of the EMT process.44 Meanwhile, Sun et al have reported that Curcumin treatment ameliorates DKD via inhibition of inflammatory gene expression by reversing caveolin-1 Tyr(14) phosphorylation that influenced TLR4 activation in STZ-induced diabetic rats.45 But little is known regarding the role of Curcumin in the TLR4-NF-κB pathways in the pathogenesis of early tubulointerstitial fibrosis during DKD. In this study, we have demonstrated that Curcumin inhibited the activation of the TLR4-NF-κB signal pathway in HG-treated NRK-52E cells. In addition, the inactivation of the TLR4 signaling pathway by its inhibitor TAK242 led to the reduction of EMT in HG-treated NRK-52E cells, as similar to the Curcumin group.
Accumulating evidence in both experimental and clinical studies has suggested that there is a close link among hyperglycemia, oxidative stress, and inflammation in DKD. DKD patients are mainly caused by EMT in the tubular epithelial cells, which are usually regarded to be the result of hyperglycemia-induced oxidative stress, while the symptom of EMT events can be reversed by anti-oxidants effect in tubular epithelial cells.46 Normally, ROS was produced in minute amounts that are necessary to maintain cellular homeostasis, but their levels increase dramatically in states of hyperglycemia leading to damage to various target organs.47,48 Previous studies demonstrated that the activities of ROS are interrelated and that the latter may view as reciprocal inducers and amplifiers of the signaling cellular events that occur under HG conditions.19,22,27 In addition, in vitro and in vivo studies suggesting that overproduction of ROS by HG concentrations lowers the antioxidant defense mechanisms in DKD, such as reduced levels of mitochondrial-specific manganese superoxide dismutase that further aggravates oxidative stress.27,49 TLR4, as a vital regulator of the NF-κB signaling pathway, has been shown to regulate cell apoptosis, inflammatory response and oxidative damage to renal tubular epithelial cell under high glucose conditions.30,50 Previous studies indicated that Curcumin has a protective role to prevent HG-induced functional and structural changes at both the organ and cellular levels.51,52 Specifically, the application of Curcumin is in reducing the degree of HG-induced oxidative stress effectively in the heart of diabetic animals and endothelial cells.51,53 Moreover, a recent study has supported the potential therapy of Curcumin in diabetes, which controlled oxidative stress and regulated inflammatory cytokines.19,22 The protective role of Curcumin against renal damage, mediating free radicals, was also demonstrated by STZ-induced diabetic models.22 These data demonstrated that Curcumin is a promising therapeutic target by mitigating oxidative stress-induced tissue injury associated wit diabetes. Data from the present study have indicated that Curcumin can effectively attenuate cellular oxidative stress under HG conditions. Notably, Curcumin inhibits EMT and protects against oxidative damage might via a TLR4-NF-κB signaling pathway-dependent mechanism in NRK-52E cells.
Taking together, the present study provides evidence that Curcumin inhibits activation of the TLR4-NF-κB signal pathway and subsequently plays the protective effects on HG-induced EMT of NRK-52E cells and suggests that Curcumin may be an agent useful for therapy in DKD.
Abbreviations
ACR, albumin to creatinine ratio; cAMP, adenosine 3ʹ, 5ʹ-cyclic monophosphate; CKD, chronic kidney disease; DKD, diabetic kidney disease; DMSO, dimethyl sulfoxide; DN, diabetic nephropathy; ECM, accumulation of extracellular matrix; ECL, enhanced chemiluminescence; EMT, tubuloepithelial to mesenchymal trans-differentiation; ESKD, end-stage kidney disease; FSH, follicle-stimulating hormone; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HG, high glucose; HO-1, heme oxygenase-1; IDF, international Diabetes Federation; IL-6, interleukin-6; MTT, 3-(4,5-dimethylthiazol-2-yl)-2 and 5-diphenyltetrazolium bromide; Nrf2, NF-E2-related factor 2; PB, phosphate buffer; PI3K, phosphatidylinositol 3-kinase; PVDF, polyvinylidene difluoride; ROS, reactive oxygen species; STZ, Streptozotocin; TGF-β1, Transforming growth factor beta 1; TLRs, toll-like receptor; TNF-α, tumor necrosis factor-alpha.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the Natural Science Foundation of China (81670670), Postdoctoral Science Foundation of China (2014MM551144), Shenzhen Key Medical Discipline Construction Fund (SZXK009), and Start grant of China Pharmaceutical University (20180705).
Disclosure
All the authors declared no competing interests.
References
1. Adler S, Nast C, Artishevsky A. Diabetic nephropathy: pathogenesis and treatment. Annu Rev Med. 1993;44:303–315. doi:10.1146/annurev.me.44.020193.001511.
2. Najafian B, Kim Y, Crosson JT, Mauer M. Atubular glomeruli and glomerulotubular junction abnormalities in diabetic nephropathy. J Am Soc Nephrol. 2003;14(4):908–917. doi:10.1097/01.asn.0000057854.32413.81.
3. Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D. Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol. 2009;20(3):489–494. doi:10.1681/ASN.2008050503.
4. Katz A, Caramori ML, Sisson-Ross S, Groppoli T, Basgen JM, Mauer M. An increase in the cell component of the cortical interstitium antedates interstitial fibrosis in type 1 diabetic patients. Kidney Int. 2002;61(6):2058–2066. doi:10.1046/j.1523-1755.2002.00370.x.
5. Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1:15018. doi:10.1038/nrdp.2015.18.
6. Bohle A, Wehrmann M, Bogenschütz O, Batz C, Müller CA, Müller GA. The pathogenesis of chronic renal failure in diabetic nephropathy. Investigation of 488 cases of diabetic glomerulosclerosis. Pathol Res Pract. 1991;187(2–3):251–259. doi:10.1016/s0344-0338(11)80780-6.
7. Forbes JM, Thorburn DR. Mitochondrial dysfunction in diabetic kidney disease. Nat Rev Nephrol. 2018;14(5):291–312. doi:10.1038/nrneph.2018.9.
8. Wada J, Makino H. Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol. 2016;12(1):13–26. doi:10.1038/nrneph.2015.175.
9. Wang YH, Zhang YG. Kidney and innate immunity. Immunol Lett. 2017;183:73–78. doi:10.1016/j.imlet.2017.01.011.
10. Garibotto G, Carta A, Picciotto D, Viazzi F, Verzola D. Toll-like receptor-4 signaling mediates inflammation and tissue injury in diabetic nephropathy. J Nephrol. 2017;30(6):719–727. doi:10.1007/s40620-017-0432-8.
11. Medzhitov R, Preston-Hurlburt P, Janeway CAJ. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388(6640):394–397. doi:10.1038/41131.
12. Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci U S A. 1998;95(2):588–593. doi:10.1073/pnas.95.2.588.
13. Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol. 2002;168(3):1286–1293. doi:10.4049/jimmunol.168.3.1286.
14. Panchapakesan U, Pollock C. The role of toll-like receptors in diabetic kidney disease. Curr Opin Nephrol Hypertens. 2018;27(1):30–34. doi:10.1097/MNH.0000000000000377.
15. McLaughlin CN, Perry-Richardson JJ, Coutinho-Budd JC, Broihier HT. Dying Neurons Utilize Innate Immune Signaling to Prime Glia for Phagocytosis during Development. Dev Cell. 2019;48(4):506–522. doi:10.1016/j.devcel.2018.12.019.
16. Verzola D, Cappuccino L, D’Amato E, et al. Enhanced glomerular Toll-like receptor 4 expression and signaling in patients with type 2 diabetic nephropathy and microalbuminuria. Kidney Int. 2014;86(6):1229–1243. doi:10.1038/ki.2014.116.
17. Zhang X, Sun L, Fan Y, et al. Effects of curcumine on expressions of toll-like receptor 2 and 6 in rat peritoneum during acute peritonitis. Chin J Nephrology Dialysis Transplant. 2012;21(3):244–248.
18. Zhang X, Ma J, Wu Y, et al. TNF-α Expression in the Peritoneum of Curcumin e Pretreated Acute Peritonitis Rat. J China Med Univ. 2009;38(10):749–750.
19. Kant V, Gopal A, Pathak NN, Kumar P, Tandan SK, Kumar D. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int Immunopharmacol. 2014;20(2):322–330. doi:10.1016/j.intimp.2014.03.009.
20. Yu Y, Sun J, Wang R, Liu J, Wang P, Wang C. Curcumin Management of Myocardial Fibrosis and its Mechanisms of Action: a Review. Am J Chin Med. 2019;47(8):1675–1710. doi:10.1142/S0192415X19500861.
21. Mbese Z, Khwaza V, Aderibigbe BA. Curcumin and Its Derivatives as Potential Therapeutic Agents in Prostate, Colon and Breast Cancers. Molecules. 2019;24(23):4386. doi:10.3390/molecules24234386.
22. Lin K, Chen H, Chen X, Qian J, Huang S, Huang W. Efficacy of Curcumin on Aortic Atherosclerosis: a Systematic Review and Meta-Analysis in Mouse Studies and Insights into Possible Mechanisms. Oxid Med Cell Longev. 2020;2020:1520747. doi:10.1155/2020/1520747.
23. Parsamanesh N, Moossavi M, Bahrami A, Butler AE, Sahebkar A. Therapeutic potential of curcumin in diabetic complications. Pharmacol Res. 2018;136:181–193. doi:10.1016/j.phrs.2018.09.012.
24. Zeng LF, Xiao Y, Sun LA. Glimpse of the Mechanisms Related to Renal Fibrosis in Diabetic Nephropathy. Adv Exp Med Biol. 2019;1165:49–79. doi:10.1007/978-981-13-8871-2_4.
25. Sun X, Liu Y, Li C, et al. Recent Advances of Curcumin in the Prevention and Treatment of Renal Fibrosis. Biomed Res Int. 2017;2017:2418671. doi:10.1155/2017/2418671.
26. Chen Y, Lu Y, Lee RJ, Xiang G. Nano Encapsulated Curcumin: and Its Potential for Biomedical Applications. Int J Nanomedicine. 2020;15:3099–3120. doi:10.2147/IJN.S210320.
27. Zhang X, Liang D, Guo L, et al. Curcumin protects renal tubular epithelial cells from high glucose-induced epithelial-to-mesenchymal transition through Nrf2-mediated upregulation of heme oxygenase-1. Mol Med Rep. 2015;12(1):1347–1355. doi:10.3892/mmr.2015.3556.
28. Tang Y, Vater C, Jacobi A, Liebers C, Zou X, Stiehler M. Salidroside exerts angiogenic and cytoprotective effects on human bone marrow-derived endothelial progenitor cells via Akt/mTOR/p70S6K and MAPK signalling pathways. Br J Pharmacol. 2014;171(9):2440–2456. doi:10.1111/bph.12611.
29. Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi:10.1038/nri1391.
30. Yuan S, Liu X, Zhu X, et al. The Role of TLR4 on PGC-1α-mediated oxidative stress in tubular cell in diabetic kidney disease. Oxid Med Cell Longev. 2018;2018:6296802. doi:10.1155/2018/6296802.
31. Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112(12):1776–1784. doi:10.1172/JCI20530.
32. Burns WC, Twigg SM, Forbes JM, et al. Connective tissue growth factor plays an important role in advanced glycation end product-induced tubular epithelial-to-mesenchymal transition: implications for diabetic renal disease. J Am Soc Nephrol. 2006;17(9):2484–2494. doi:10.1681/ASN.2006050525.
33. Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159(4):1465–1475. doi:10.1016/S0002-9440(10)62533-3.
34. Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15(1):1–12. doi:10.1097/01.asn.0000106015.29070.e7.
35. Chen H, Yang X, Lu K, et al. Inhibition of high glucose-induced inflammation and fibrosis by a novel curcumin derivative prevents renal and heart injury in diabetic mice. Toxicol Lett. 2017;278:48–58. doi:10.1016/j.toxlet.2017.07.212.
36. Vanaie A, Shahidi S, Iraj B, et al. Curcumin as a major active component of turmeric attenuates proteinuria in patients with overt diabetic nephropathy. J Res Med Sci. 2019;24:77. doi:10.4103/jrms.JRMS_1055_18.
37. Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336(15):1066–1071. doi:10.1056/NEJM199704103361506.
38. Flohé L, Brigelius-Flohé R, Saliou C, Traber MG, Packer L. Redox regulation of NF-kappa B activation. Free Radic Biol Med. 1997;22(6):1115–1126. doi:10.1016/s0891-5849(96)00501-1.
39. Lin M, Tang SC. Toll-like receptors: sensing and reacting to diabetic injury in the kidney. Nephrol Dial Transplant. 2014;29(4):746–754. doi:10.1093/ndt/gft446.
40. Lin M, Yiu WH, Li RX, et al. The TLR4 antagonist CRX-526 protects against advanced diabetic nephropathy. Kidney Int. 2013;83(5):887–900. doi:10.1038/ki.2013.11.
41. Pulskens WP, Rampanelli E, Teske GJ, et al. TLR4 promotes fibrosis but attenuates tubular damage in progressive renal injury. J Am Soc Nephrol. 2010;21(8):1299–1308. doi:10.1681/ASN.2009070722.
42. Ma J, Chadban SJ, Zhao CY, et al. TLR4 activation promotes podocyte injury and interstitial fibrosis in diabetic nephropathy. PLoS One. 2014;9(5):e97985. doi:10.1371/journal.pone.0097985.
43. Wang Z, Chen Z, Li B, et al. Curcumin attenuates renal interstitial fibrosis of obstructive nephropathy by suppressing epithelial-mesenchymal transition through inhibition of the TLR4/NF-кB and PI3K/AKT signalling pathways. Pharm Biol. 2020;58(1):828–837. doi:10.1080/13880209.2020.1809462.
44. Zhang L, Tao X, Fu Q, et al. Curcumin inhibits cell proliferation and migration in NSCLC through a synergistic effect on the TLR4/MyD88 and EGFR pathways. Oncol Rep. 2019;42(5):1843–1855. doi:10.3892/or.2019.7278.
45. Sun LN, Yang ZY, Lv SS, Liu XC, Guan GJ, Liu G. Curcumin prevents diabetic nephropathy against inflammatory response via reversing caveolin-1 Tyr14 phosphorylation influenced TLR4 activation. Int Immunopharmacol. 2014;23(1):236–246. doi:10.1016/j.intimp.2014.08.023.
46. Rhyu DY, Yang Y, Ha H, et al. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. 2005;16(3):667–675. doi:10.1681/ASN.2004050425.
47. Lee HB, Yu MR, Yang Y, Jiang Z, Ha H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol. 2003;14(8 Suppl 3):S241–5. doi:10.1097/01.asn.0000077410.66390.0f.
48. Werner E. GTPases and reactive oxygen species: switches for killing and signaling. J Cell Sci. 2004;117(Pt 2):143–153. doi:10.1242/jcs.00937.
49. Al-Kafaji G, Golbahar J. High glucose-induced oxidative stress increases the copy number of mitochondrial DNA in human mesangial cells. Biomed Res Int. 2013;2013:754946. doi:10.1155/2013/754946.
50. Shen J, Liu L, Zhang F, Gu J, Pan G. LncRNA TapSAKI promotes inflammation injury in HK-2 cells and urine derived sepsis-induced kidney injury. J Pharm Pharmacol. 2019;71(5):839–848. doi:10.1111/jphp.13049.
51. Farhangkhoee H, Khan ZA, Chen S, Chakrabarti S. Differential effects of curcumin on vasoactive factors in the diabetic rat heart. Nutr Metab. 2006;3:27. doi:10.1186/1743-7075-3-27.
52. Chiu J, Khan ZA, Farhangkhoee H, Chakrabarti S. Curcumin prevents diabetes-associated abnormalities in the kidneys by inhibiting p300 and nuclear factor-kappaB. Nutrition. 2009;25(9):964–972. doi:10.1016/j.nut.2008.12.007.
53. Zeng C, Zhong P, Zhao Y, et al. Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-κB both in vitro and in vivo. J Mol Cell Cardiol. 2015;79:1–12. doi:10.1016/j.yjmcc.2014.10.002.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.