Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 11
Effects of coenzyme Q10 on cardiovascular and metabolic biomarkers in overweight and obese patients with type 2 diabetes mellitus: a pooled analysis
Authors Huang H, Chi H, Liao D, Zou Y
Received 17 August 2018
Accepted for publication 5 October 2018
Published 29 November 2018 Volume 2018:11 Pages 875—886
DOI https://doi.org/10.2147/DMSO.S184301
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Steven F. Abcouwer
Haohai Huang,1 Honggang Chi,2 Dan Liao,3 Ying Zou2,4
1Department of Clinical Pharmacy, Dongguan Third People’s Hospital, Affiliated Dongguan Shilong People’s Hospital of Southern Medical University, Dongguan, Guangdong, China; 2Department of Traditional Chinese Medicine, Scientific Research Platform, The Second Clinical Medical College, Guangdong Medical University, Dongguan, China; 3Department of Gynaecology & Obstetrics, Dongguan Third People’s Hospital, Affiliated Dongguan Shilong People’s Hospital of Southern Medical University, Dongguan, Guangdong, China; 4Key Laboratory for Medical Molecular Diagnostics of Guangdong Province, Guangdong Medical University, Dongguan, Guangdong, China
Background: The potential effects of coenzyme Q10 (CoQ10) supplementation in overweight/obese patients with type 2 diabetes mellitus are not fully established. In this article, we aimed to perform a pooled analysis to investigate the effects of CoQ10 intervention on cardiovascular disease (CVD) risk factors in overweight/obese patients with type 2 diabetes mellitus (T2DM).
Methods: MEDLINE, Embase, and Cochrane databases were searched for randomized controlled trials that evaluated the changes in CVD risk factors among overweight and obese patients with T2DM following CoQ10 supplementation. Two investigators independently assessed articles for inclusion, extracted data, and assessed risk of bias. Major endpoints were synthesized as weighted mean differences (WMDs) with 95% CIs. Subgroup analyses were performed to check the consistency of effect sizes across groups. Publication bias and sensitivity analysis were also performed.
Results: Fourteen eligible trials with 693 overweight/obese diabetic subjects were included for pooling. CoQ10 interventions significantly reduced fasting blood glucose (FBG; –0.59 mmol/L; 95% CI, −1.05 to –0.12; P=0.01), hemoglobin A1c (HbA1c; –0.28%; 95% CI−0.53 to –0.03; P=0.03), and triglyceride (TG) levels (0.17 mmol/L; 95% CI, −0.32 to –0.03; P=0.02). Subgroup analysis also showed that low-dose consumption of CoQ10 (<200 mg/d) effectively reduces the values of FBG, HbA1c, fasting blood insulin, homeostatic model assessment of insulin resistance, and TG. CoQ10 treatment was well tolerated, and no drug-related adverse reactions were reported.
Conclusion: Our findings provide substantial evidence that daily CoQ10 supplementation has beneficial effects on glucose control and lipid management in overweight and obese patients with T2DM.
Keywords: coenzyme Q10, type 2 diabetes mellitus, cardiovascular risk factors, lipids, glucose, obesity
Introduction
Diabetes is a chronic disease with high rates of disability and mortality. In 2013, it was estimated that globally there were 382 million diabetic adults and is expected to increase to almost 592 million by 2035.1 Early onset of diabetes leads to longer lifetime exposure to hyperglycemia and consequently a greater propensity to long-term complications.2 Poor glycemic control causes long-term adverse outcomes in subjects with diabetes, including microvascular and macrovascular complications.3,4 Some researchers have suggested that up to two of every three cases of type 2 diabetes mellitus (T2DM) can be attributed to obesity.5 Being overweight and obese increases the risks of diabetes and cardiovascular disease (CVD).6 Obesity accentuates the metabolic and CVD complications in patients with T2DM by increasing insulin resistance, causing a progressive decline of β-cell functions, and subsequently increasing the difficulty in achieving the glycemic targets.7,8 The management of diabetes and its CVD-related complications impose enormous medical and economic burdens. Therefore, primary and secondary prevention of diabetes and delaying the onset or progression of diabetes-related complications have become a public health imperative. Treatment strategies for patients with T2DM who are obese should focus equally on glycemic control, weight loss, and the comprehensive management of CVD comorbidities or risk factors. In recent decades, large meta-analyses of randomized controlled trials (RCTs) have suggested that lifestyle modifications, such as dietary micronutrients or functional food supplementations, are generally used to improve glycemic management and clinically relevant metabolic biomarkers in patients with diabetes.9–11
Accumulating evidence indicates that mitochondrial dysfunction, inflammation, and oxidative stress contribute to the pathogenesis of diabetes and associated complications.12,13 Coenzyme Q10 (CoQ10; also called ubiquinone), a lipid-soluble benzoquinone with 10 isoprenyl units in the side chain, can be synthesized endogenously or obtained naturally from the diet. In clinical applications, oral CoQ10 treatment is a frequent mitochondrial energizer and antioxidant strategy in many diseases that may provide a significant symptomatic benefit. CoQ10 has been used to prevent and treat a wide range of diseases, including primary and secondary CoQ10 deficiencies, mitochondrial diseases, fibromyalgia, CVD, neurodegenerative diseases, cancer, diabetes mellitus, hypertension, and periodontal disease.14–16 The plasma levels of CoQ10 in patients with diabetes are significantly lower when compared with the healthy individuals.17,18 The deficiency of CoQ10 may further impair the body’s defensive mechanisms against oxidative stress induced by hyperglycemia in diabetes.19 Several studies have demonstrated that the restoration of CoQ10 levels in patients with diabetes by the supplemental use of exogenous CoQ10 could potentially preserve mitochondrial function, alleviate oxidative stress, and eventually lead to improvement of glycemic control.20–24 However, the results of whether the supplementation of CoQ10 helps to improve glucose and insulin levels, to modify lipid profiles, and to reduce blood pressure in overweight diabetic patients are inconsistent and have not been fully understood. Therefore, we undertook a comprehensive meta-analysis of RCTs to investigate the effects and safety of CoQ10 supplementation on multiple markers of cardiovascular health in overweight and obese patients with established T2DM.
Materials and methods
Search strategy
The protocol for this pooled analysis was conducted following PRISMA guidelines.25 Two independent investigators performed an electronic literature search of MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) from their inception to December 31, 2017, restricting the search to publications in English and clinical trials that investigated the effects of CoQ10 intervention in patients with established T2DM. We used the following search terms: “Coenzyme Q10” OR “Co-enzyme Q10” OR “CoQ10” OR “ubiquinone” OR “ubiquinol” and combined with “diabetes mellitus” OR “type 2 diabetes” OR “type 2 diabetes mellitus” OR “diabetes” OR “noninsulin-dependent diabetics” OR “NIDDM” OR “T2DM”. To identify any other missing eligible trials, we also performed a manual search of reference citations in relevant review articles and original articles that were selected for full-text retrieval.
Study inclusion criteria
To be included, an original study had to meet the following criteria: 1) population: target population (adults aged ≥18 years) with a body mass index (BMI) of ≥25 kg/m2 and diagnosed with T2DM; 2) intervention: the patients ingested a determined amount of CoQ10 or ubiquinol intervention for ≥4 weeks; 3) comparison: placebo or conventional antidiabetic agents were used; 4) outcomes of interest: an assessment of at least one of the following outcome markers, namely fasting blood glucose (FBG), hemoglobin A1c (HbA1c), homeostatic model assessment of insulin resistance (HOMA-IR), fasting blood insulin (FBI), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TGs), SBP, DBP, and BMI; and 5) study design: study was an RCT conducted in human subjects with either a parallel or crossover design.
Data extraction
Data extraction included information regarding study characteristics (first author’s name, year of publication, sample size, details of the study design, and length of follow-up), basic characteristics of participants (such as age, percentage male, duration of diabetes, baseline of BMI, and FBG), and intervention characteristics (such as type of intervention, dose, and type of control) and reported the outcomes of interest.
Assessing the quality of the methodology used
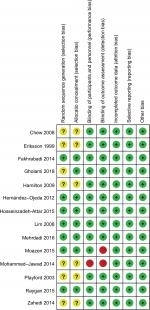
Two of the investigators independently used the Cochrane Collaboration bias risk analysis tool to assess the quality of the trials included.26 Randomization sequence generation, allocation concealment, blinding of participants and study personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other biases (defined as baseline imbalance in our present study) were classified as high, low, or unclear for each domain of the studies included.
Grading quality of evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to rate the quality of evidence and generate absolute estimates of the effect of CoQ10 for the primary and secondary outcomes.27
Statistical analyses
We used STATA software (version 12; StataCorp LP, College Station, TX, USA) to combine the individual study results for all outcomes studied. For each marker, we calculated the effect sizes as a weighted mean difference (WMD) and a 95% CI between the CoQ10 intervention and control groups using a random-effects model. We used the I2 statistic to assess the degree of statistical heterogeneity among studies, with a value of <25%, 26%–50%, and >50% being considered as a low, moderate, and high levels of heterogeneity, respectively.28 To evaluate the robustness of our findings, sensitivity analyses were performed by removing one study each time and repeating the analysis (the “leave-one-out” approach). To explore the influence of various factors on the cardiovascular risk factors of CoQ10 intervention, a priori subgroup analyses were then carried out according to the mean age of the patients, the duration of T2DM, the baseline level of BMI, CoQ10 dose, and intervention duration. We investigated the possibility of publication bias using funnel plot asymmetry and Egger’s weighted regression test.29 A P-value of <0.05 was considered to be statistically significant.
Meta-regression
In the present study, we applied a restricted maximum likelihood (REML)-based random-effects meta-regression analysis to explore whether the potential confounders of treatment response, such as the dose (mg/d) and duration (number of weeks) of supplementation with CoQ10, were associated with the source of heterogeneity.30
Results
Search results and trial flow
A PRISMA study flow chart of the selected trials is shown in Figure S1. We initially identified 375 potential records from the original literature search, of which 44 were duplicate articles. Three hundred thirty-one potentially relevant articles were screened based on their titles and abstracts, and then 45 articles were selected for detailed evaluation. After full-text assessment, 31 of these studies were excluded, most of them due to CoQ10 being mixed with other components (n=3); reported from the same population (n=2); the outcomes of interest not being reported (n=3); duplicate data (n=2); not being an RCT study (n=4); letters, reviews, or meta-analysis (n=6); mixed patients (n=5); subjects being younger than 18 years (n=3); and participants with a BMI< 25 kg/m2 (n=3). Overall, a total of 14 studies with 693 participants were finally selected for the meta-analysis.20–24,31–39
Study and participant characteristics
Detailed characteristics of the resulting 14 trials are listed in Table 1. Most studies included were single-center studies published between 1999 and 2018. The mean baseline BMI of the T2DM patients varied from 25.0 to 30.4 kg/m2. The population sizes included in these studies ranged from 23 to 80 subjects. Overall, 730 subjects were randomly assigned in these trials, while 693 (94.9%) subjects completed the studies. Of the 14 trials used in the meta-analysis, 10 trials included both men and women,20–22,24,31,33–36,39 one included only women,38 and three did not indicate the sex composition of the sample.23,32,37 The dose of CoQ10 varied from 100 to 400 mg/d (median, 185.7 mg/d). The follow-up duration of CoQ10 intervention was 8, 12, or 24 weeks (median, 12.86 weeks). The mean age of the participants ranged from 47 to 68 years (median, 55.78 years). The mean baseline FBG ranged from 7.15 to 11.5 mmol/L (median, 8.88 mmol/L). All trials were randomized, with 92.8% (13 trials) utilizing a parallel design and 7.2% (one trials) utilizing a crossover design. Among the studies included, 11 of the 14 studies included subjects with only T2DM. Of the remaining three trials, one was performed on subjects with T2DM and dyslipidemia, one was conducted on T2DM subjects with left ventricular diastolic dysfunction, and one was conducted on T2DM subjects with coronary heart disease.
Assessment of quality and potential bias
A detailed risk-of-bias summary is shown in Figure 1. Among the 14 studies included, only seven studies reported random sequence generation.21,22,33–35,37,39 Two trials did not describe whether the blind method was adopted and were categorized as at high risk of bias.20,21 Overall, six trials were considered as at low risk of bias,22,33–35,37,39 two as at a high risk of bias,20,21 and six as unclear.23,24,31,32,36,37
Changes in glycemic control, insulin levels, and sensitivity
Overall, the pooled result showed that the consumption of CoQ10 significantly decreased the FBG (12 studies; WMD=–0.59 mmol/L; 95% CI=–1.05 to –0.12; P=0.01) and HbA1c levels (13 studies; WMD=–0.28%; 95% CI=–0.53 to –0.03; P=0.03) in overweight and obese patients with T2DM. Heterogeneity tests results of these outcomes were 37% and 33%, respectively. However, FBI (five studies; WMD=–1.87 μIU/mL; 95% CI=–4.51 to 0.77; P=0.17; I2=71%) and HOMA-IR (five studies; WMD=–1.03; 95% CI=–2.06 to –0.00; P=0.05; I2=71%) levels did not change significantly with the consumption of CoQ10. Forest plots for each measured parameter are shown in Figure 2.
Changes in lipid concentrations, blood pressure, and BMI
As shown in Figure 3, the consumption of CoQ10 by overweight and obese patients with T2DM caused a significant reduction in TG concentrations compared with the control (eight studies; WMD=–0.17 mmol/L; 95% CI=–0.32 to –0.03; P=0.02; I2=0%). However, no significant changes were found in the concentrations of TC (10 studies; WMD=–0.18 mmol/L; 95% CI=–0.33 to 0.16; P=0.50; I2=40%), LDL-C (10 studies; WMD=0.05 mmol/L; 95% CI=–0.30 to 0.20; P=0.69; I2=77%), and HDL-C (10 studies; WMD=0.03 mmol/L; 95% CI=–0.01 to 0.08; P=0.17; I2=29%) values of the diabetic participants.
Six studies investigated the impact of CoQ10 on BP in overweight and obese patients with T2DM. CoQ10 supplementation did not result in a significant reduction in SBP (six studies; WMD=1.84 mmHg; 95% CI=–5.60 to 1.92; P=0.34; I2=21%) and DBP levels (five studies; WMD=–1.38 mmHg; 95% CI=–5.48 to 2.72; P=0.51; I2=80%). Similarly, no significant difference was seen in BMI with CoQ10 intake in the three studies that evaluated this outcome (WMD=–0.35 kg/m2; 95% CI=–1.58 to 0.88; P=0.58; I2=0%).
Meta-regression analyses
The results from the univariate weighted random-effects meta-regression analysis demonstrate that the duration of CoQ10 supplementation was not a source of heterogeneity of FBG (coefficient=0.068; 95% CI=–0.069 to 0.206; P=0.294) and HbA1c levels (coefficient=–0.034; 95% CI=–0.038 to 0.106; P=0.294; Figure S2). Similarly, as shown in Figure S3, the amount of CoQ10 consumed per day was also not a source of heterogeneity of FBG (coefficient=0.002; 95% CI=–0.006 to 0.010; P=0.626) and HbA1c levels (coefficient=–0.0003; 95% CI=–0.005 to 0.004; P=0.877).
Subgroup analysis and sensitivity analysis
The subgroup analysis results based on the CoQ10 dose demonstrated that CoQ10 consumption significantly decreased the levels of FBG (P=0.006), HbA1c (P=0.005), FBI (P=0.01), HOMA-IR (P=0.0001), and TG (P=0.02) in the low-dose CoQ10 supplementation group (<200 mg/d). In the subgroup analysis based on the baseline patient BMI, the pooled result showed that CoQ10 supplementation significantly decreased FBG, HbA1c, and TG levels of overweight and obese patients with diabetes mellitus by 0.67 mmol/L, 0.36%, and 0.16 mmol/L, respectively. We layered the trials according to mean age (<55.7 or ≥55.7 years), and a significant change in FBG (P=0.02) and HbA1c levels (P=0.01) was observed in subjects with a mean age of <55.7 years, whereas a significant change in FBI (P=0.001) and HOMA-IR levels (P=0.03) was observed in subjects with mean age of ≥55.7 years. A post hoc subgroup analysis was also performed to examine the effect of intervention duration on the overall effects of CoQ10 on CVD risk factors in overweight and obese patients with T2DM. The post hoc subgroup analysis suggested that CoQ10 consumption significantly decreased the levels of FBG (P=0.01), HbA1c (P=0.02), and TG (P=0.04) at supplemental duration of ≤12 weeks. There were no statistically significant differences in the pooled effects of CoQ10 on CVD risk markers in the subgroups stratified by the duration of diabetes. Complete results of subgroup analysis are shown in Table S1.
The sensitivity analysis results show that the exclusion of any single study each time did not influence the significance of our pooled effect size for either outcome (data not shown).
Publication bias
No obvious publication bias was found in the visual inspection of funnel plots. Figure 4 shows the funnel plots of these study outcomes. Similarly, Egger’s tests showed that no statistical evidence of publication bias was observed in relation to levels of FBG (P=0.275), HbA1c (P=2.53), FBI (P=0.836), HOMA-IR (P=0.618), TC (P=0.518), LDL-C (P=0.560), HDL-C (P=0.627), TG (P=0.069), SBP (P=0.977), DBP (P=0.896), or BMI (P=0.093).
GRADE profile evidence evaluation
GRADE evidence profiles for the primary and secondary outcomes are shown in Table S2. There were 11 outcomes regarding efficacy in this meta-analysis. The GRADE Working Group grade levels of evidence are of high quality for levels of TG, SBP and BMI; of moderate quality for levels of FBG, HbA1c, and HDL-C; and low quality for levels of HOMA-IR, FBI, TC, LDL-C, and DBP.
Discussion
Summary of main results
The results from our pooled analysis demonstrate that CoQ10 interventions significantly reduced the levels of FBG, HbA1c, and TG by 0.59 mmol/L, 0.28%, and 0.17 mmol/L, respectively. These changes varied substantially depending on the treatment dose of CoQ10, the intervention duration, the mean age of the subjects, and the initial level of BMI. Subgroup analysis also demonstrated that consumption of lower dose of CoQ10 (<200 mg/d) caused a significant reduction in levels of FBG, HbA1c, FBI, HOMA-IR, and TG in overweight and obese individuals with T2DM.
Agreements and disagreements with other studies
The impact of CoQ10 supplementation for improving glucose and insulin responses, various lipid parameters, and blood pressure in patients with diabetes have been inconsistent. Therefore, there is not yet sufficient synthesis regarding the efficacy and risks for clinicians to make evidence-based decisions regarding the supplementation of CoQ10 in the management of diabetes, especially in overweight and obese patients with diabetes mellitus. Two meta-analyses regarding this topic have been published.40,41 A previous meta-analysis that included seven trials involving 356 patients pointed out that CoQ10 supplementation has no beneficial effects on glycemic control, lipid profile, or blood pressure in patients with diabetes. However, TG levels may be reduced with CoQ10 ingestion.41 A further meta-analysis based on 14 eligible studies regarding the effects of CoQ10 supplementation on diabetes biomarkers in healthy (healthy subjects without diabetes mellitus) and unhealthy subjects by Moradi et al40 concluded that CoQ10 supplementation slightly but significantly reduced FBG levels, but not fasting insulin and HbA1c levels in the overall analysis. A subgroup analysis based on the type of disease was also performed in this study, with no beneficial effects of CoQ10 on FBG and HbA1c levels being found in patients with diabetes. However, the primary outcomes included were the change in HbA1c, FBI, and FPG levels, while CVD risk factors, such as levels of HOMA-IR, lipid profile and BP, were not determined in this study. In our present study, nine studies included were overlapped with the two previous meta-analyses. By using the previous meta-analysis as a base, we included five other recent RCTs, giving a greater power to identify and quantify the effects of CoQ10 on cardiovascular and metabolic biomarkers in overweight and obese patients with established T2DM. Furthermore, several strengths of our study should be noted: first, our meta-analysis paid attention to only overweight and obese patients with established T2DM and included 14 clinical RCTs totaling 693 patients, which was highly homogeneous and selective. Second, largely due to lenient inclusion criteria, there is indirectness of evidence in terms of the differences in population and the differences in intervention and inconsistency in the effect and effect size across studies. To evaluate the strength of the available evidence and make more objective decisions regarding the results, subgroup analyses according to putative moderators, such as baseline BMI, CoQ10 dose, mean age, and intervention duration, were performed to distinguish the influence of different factors on treatment efficacy. Third, our current meta-analysis was the latest and the most comprehensive one, which generally redefined and reinforces earlier results of previous meta-analyses. We examined the dose response and time response of CoQ10 consumption by using linear meta-regression. Publication bias and sensitivity analysis were also performed to test the robustness of our findings. Furthermore, the GRADE methodology was used to rate the level of evidence. Finally, we further summarized the reported side effects of CoQ10 consumption in the eligible trials, which had not been investigated in previous reviews.
Potential explanation of findings
The exact mechanisms responsible for the glycemic control and lipid-lowering effects of CoQ10 in diabetes are not yet completely understood. Increasing evidence suggests that oxidative stress contributes to and also results from hyperglycemia, insulin resistance, and malfunction of β-cell function.42,43 The β-cell function as well as glucose and fatty acid metabolism in the liver may be deteriorated as a result of CoQ10 deficiency resulting in impaired insulin action and hyperinsulinemia. Supplemental use of CoQ10 improves insulin sensitivity and hyperglycemia- or hyperinsulinemia-associated metabolic disorders by modulating insulin and adiponectin receptors, as well as tyrosine kinase (TK), phosphatidylinositol 3 kinase (PI3K), and glucose transporters 2 (GLUT2), while improving the lipid profile, redox system, soluble receptor of advanced glycated end products (sRAGEs), and adipocytokines (eg, adiponectin and visfatin).44
Our results also show that participants with established diabetes receiving CoQ10 had statistically significantly lower levels of TG level after treatment compared with control individuals. This positive effect was supported by in vivo mechanistic study finding, in which CoQ10 has been shown to increase fatty acid oxidation through AMP-activated protein kinase (AMPK)-mediated peroxisome proliferator-activated receptor alpha (PPARα) induction.45 Moreover, CoQ10 increased the lipolysis of TGs by decreasing the oxidative stresses and endothelial metabolism of the participants.46
Supplementation with CoQ10 could also improve hypertension and coronary artery disease. Some investigators found that CoQ10 consumption can decrease BP in subjects with uncontrolled or poorly controlled hypertension.47,48 The primary mode of action of CoQ10 in clinical hypertension is vasodilatation, via a direct effect on the endothelium and vascular smooth muscle.47 CoQ10 may also reduce BP by reducing peripheral resistance through the preservation of nitric oxide.49 In addition, CoQ10 is beneficial in improving prostaglandin prostacyclin production, promoting vasodilation and inhibiting platelet aggregation.50 The effect of CoQ10 on the change of BP in diabetes has not yet been systematically examined. Therefore, we further assessed the effect of CoQ10 consumption on SBP and DBP outcomes, but failed to find any significant effects.
Safety concerns
Some adverse events reported to be associated with CoQ10 were gastrointestinal effects (ie, abdominal discomfort, nausea, vomiting, diarrhea, and anorexia), allergic rash, headache, and the risk of bleeding.51 In our pooled analysis, CoQ10 treatment was well tolerated, and no drug-related adverse reactions were reported among the eligible trials during the follow-up period.
Implications for clinical practice
The persistence of excess adiposity in children and adults can lead to metabolic abnormality. Insulin resistance and progressive β-cell dysfunction are regarded as the key pathophysiologic mechanisms of T2DM development, and most T2DM patients who are overweight or obese also have CVD comorbidities/risk factors such as hypertension, dyslipidemia, and hypercoagulability.52,53 Our findings may have major clinical implications, given that the metabolic biomarkers evaluated in our present study are clinically relevant for monitoring the treatment and progression of diabetes across multiple organ systems. Glycemic control is fundamental to the management of diabetes. The pooled estimates suggest that CoQ10 significantly affected the FBG level. FBG level is considered a key variable in the diagnosis of diabetes and is also adopted by the Food and Drug Administration (FDA) to evaluate the efficacy of dietary supplements and glycemic-lowering drugs. Moreover, our results show a significant benefit for HbA1c levels in overweight and obese individuals with T2DM. Large clinical trials have firmly established that a reduction in HbA1c level, a long-term indicator of glycemic control, is associated with a decreased risk for multiple diabetic complications and death.3 Patients with T2DM also have an increased prevalence of lipid abnormalities in visceral organs and ectopic fat depots that contribute to higher rates of CVD outcomes. Lipid management has been shown to reduce macrovascular disease and mortality in patients with T2DM, particularly in those who have had prior cardiovascular events.54 Our pooled result also shows that CoQ10 intervention significantly lowered TG levels. Therefore, our study provided evidence for clinicians and researchers to incorporate CoQ10 treatment into the management of T2DM in overweight and obese patients.
Limitations
Our present meta-analysis has some limitations in interpreting the current results. First, our search was limited to studies published in English, and non-English or unpublished reports may exist. Second, despite the numerous subgroup and sensitivity analyses that were carried out, there was unavoidable heterogeneity in many of the reported outcomes, indicating variation between the studies in the estimates of the effect of CoQ10 on the measured outcomes. Moreover, due to the lack of information in most of the existing studies, influences of other covariates such as the smoking habits could not be fully determined. Therefore, we hardly considered this point in the subgroup analysis and ruled out heterogeneity thoroughly. Third, our pooled result was obtained with unadjusted estimates, and the precise effect of CoQ10 on CVD biomarkers in overweight and obese patients with T2DM could have been impacted by other confounders (ie, other lifestyle interventions, alcohol consumption). The synergistic effects of other coexisting substances on the clinical outcomes should be excluded during the study period. Finally, since we performed multiple comparisons in this meta-analysis, the Bonferroni method, which controls false-positive error rate, was used to adjust for multiple comparisons. The threshold for significance was set at a P-value of <0.015. After Bonferroni correction, no significant effect was observed for the HbA1c and TG outcomes.
Conclusion
The present pooled analysis provides evidence for the beneficial effects of daily CoQ10 supplementation on concentrations of FBG, HbA1c, and TG levels in overweight and obese patients with established T2DM. In a subgroup analysis, CoQ10 effectively reduced the values of FBG, HbA1c, FBI, HOMA-IR, and TG in the low-dose of CoQ10 (<200 mg/d) consumption group. CoQ10 was well tolerated, and drug-related adverse reactions were not reported among the eligible trials during the follow-up period. The present results should be interpreted cautiously due to the unadjusted estimates (Bonferroni correction). Further adequately powered studies investigating the effects of CoQ10 consumption on clinical endpoints, such as diabetes-related complications morbidity (ie, coronary artery disease, stroke, blindness, and end-stage kidney disease) and all-cause mortality, are needed.
Acknowledgment
This work was supported by the Social Development Project of Dongguan City under grants 2016108101021 and 2014108101054.
Disclosure
Haohai Huang and Honggang Chi contributed equally to this work. The authors report no conflicts of interest in this work.
References
Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–321. | ||
Copeland KC, Silverstein J, Moore KR, et al. Management of newly diagnosed type 2 Diabetes Mellitus (T2DM) in children and adolescents. Pediatrics. 2013;131(2):364–382. | ||
Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003;26(9):2653–2664. | ||
Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. | ||
Davidson MH, Hauptman J, DiGirolamo M, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA. 1999;281(3):235–242. | ||
Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925–1932. | ||
Eckel RH, Kahn SE, Ferrannini E, et al. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? J Clin Endocrinol Metab. 2011;96(6):1654–1663. | ||
Kim SH, Reaven G. Obesity and insulin resistance: an ongoing saga. Diabetes. 2010;59(9):2105–2106. | ||
Xi P, Liu RH. Whole food approach for type 2 diabetes prevention. Mol Nutr Food Res. 2016;60(8):1819–1836. | ||
Hausenblas HA, Schoulda JA, Smoliga JM. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus--systematic review and meta-analysis. Mol Nutr Food Res. 2015;59(1):147–159. | ||
Huang H, Chen G, Dong Y, Zhu Y, Chen H. Chromium supplementation for adjuvant treatment of type 2 diabetes mellitus: results from a pooled analysis. Mol Nutr Food Res. 2018;62(1):1700438. | ||
Lamson DW, Plaza SM. Mitochondrial factors in the pathogenesis of diabetes: a hypothesis for treatment. Altern Med Rev. 2002;7(2):94–111. | ||
Joseph AM, Joanisse DR, Baillot RG, Hood DA. Mitochondrial dysregulation in the pathogenesis of diabetes: potential for mitochondrial biogenesis-mediated interventions. Exp Diabetes Res. 2012;2012:642038. | ||
Garrido-Maraver J, Cordero MD, Oropesa-Avila M, et al. Clinical applications of coenzyme Q10. Front Biosci (Landmark Ed). 2014;19(4):619–633. | ||
Hernández-Camacho JD, Bernier M, López-Lluch G, Navas P. Coenzyme Q10 supplementation in aging and disease. Front Physiol. 2018;9:44. | ||
Hargreaves IP. Coenzyme Q10 as a therapy for mitochondrial disease. Int J Biochem Cell Biol. 2014;49:105–111. | ||
Ates O, Bilen H, Keles S, et al. Plasma coenzyme Q10 levels in type 2 diabetic patients with retinopathy. Int J Ophthalmol. 2013;6(5):675–679. | ||
Hasegawa G, Yamamoto Y, Zhi JG, et al. Daily profile of plasma %CoQ10 level, a biomarker of oxidative stress, in patients with diabetes manifesting postprandial hyperglycaemia. Acta Diabetol. 2005;42(4):179–181. | ||
Mezawa M, Takemoto M, Onishi S, et al. The reduced form of coenzyme Q10 improves glycemic control in patients with type 2 diabetes: an open label pilot study. Biofactors. 2012;38(6):416–421. | ||
Kadhim Mohammed-Jawad N, Al- Sabbagh M, Al-Jezaeri KA. Role of L-carnitine and coenzyme Q10 as adjuvant therapy in patients with type 2 diabetes mellitus. Am J Pharmacol Sci. 2014;2(5):82–86. | ||
Moazen M, Mazloom Z, Ahmadi A, Dabbaghmanesh MH, Roosta S. Effect of coenzyme Q10 on glycaemic control, oxidative stress and adiponectin in type 2 diabetes. J Pak Med Assoc. 2015;65(4):404–408. | ||
Mehrdadi P, Kolahdouz Mohammadi R, Alipoor E, Eshraghian MR, Esteghamati A, Hosseinzadeh-Attar MJ. The effect of coenzyme Q10 supplementation on circulating levels of novel adipokine Adipolin/CTRP12 in overweight and obese patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2017;125(3):156–162. | ||
Eriksson JG, Forsén TJ, Mortensen SA, Rohde M. The effect of coenzyme Q10 administration on metabolic control in patients with type 2 diabetes mellitus. Biofactors. 1999;9(2-4):315–318. | ||
Zahedi H, Eghtesadi S, Seifirad S, et al. Effects of CoQ10 supplementation on lipid profiles and glycemic control in patients with type 2 diabetes: a randomized, double blind, placebo-controlled trial. J Diabetes Metab Disord. 2014;13:81. | ||
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. | ||
Higgins JP, Green S [homepage on the Internet]. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. Available from: http://handbook.cochrane.org/. Accessed October 26, 2018. | ||
Guyatt GH, Oxman AD, Vist GE, et al; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | ||
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. | ||
Morton SC, Adams JL, Suttorp MJ, Shekelle PG. Meta-regression Approaches: What, Why, When, and How? Rockville, MD USA: Agency for Healthcare Research and Quality; 2004. | ||
Chew GT, Watts GF, Davis TM, et al. Hemodynamic effects of fenofibrate and coenzyme Q10 in type 2 diabetic subjects with left ventricular diastolic dysfunction. Diabetes Care. 2008;31(8):1502–1509. | ||
Hamilton SJ, Chew GT, Watts GF. Coenzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes Care. 2009;32(5):810–812. | ||
Hernández-Ojeda J, Cardona-Muñoz EG, Román-Pintos LM, et al. The effect of ubiquinone in diabetic polyneuropathy: a randomized double-blind placebo-controlled study. J Diabetes Complications. 2012;26(4):352–358. | ||
Hosseinzadeh-Attar M, Kolahdouz Mohammadi R, Eshraghian M, et al. Reduction in asymmetric dimethylarginine plasma levels by coenzyme Q10 supplementation in patients with type 2 diabetes mellitus. Minerva Endocrinol. 2015;40(4):259–266. | ||
Lim SC, Lekshminarayanan R, Goh SK, et al. The effect of coenzyme Q10 on microcirculatory endothelial function of subjects with type 2 diabetes mellitus. Atherosclerosis. 2008;196(2):966–969. | ||
Playford DA, Watts GF, Croft KD, Burke V. Combined effect of coenzyme Q10 and fenofibrate on forearm microcirculatory function in type 2 diabetes. Atherosclerosis. 2003;168(1):169–179. | ||
Raygan F, Rezavandi Z, Dadkhah Tehrani S, Farrokhian A, Asemi Z. The effects of coenzyme Q10 administration on glucose homeostasis parameters, lipid profiles, biomarkers of inflammation and oxidative stress in patients with metabolic syndrome. Eur J Nutr. 2016;55(8):2357–2364. | ||
Gholami M, Rezvanfar MR, Delavar M, Abdollahi M, Khosrowbeygi A. Effects of coenzyme Q10 supplementation on serum values of gamma-glutamyl transferase, pseudocholinesterase, bilirubin, ferritin, and high-sensitivity c-reactive protein in women with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2018. | ||
Akbari Fakhrabadi M, Zeinali Ghotrom A, Mozaffari-Khosravi H, Hadi Nodoushan H, Nadjarzadeh A. Effect of coenzyme Q10 on oxidative stress, glycemic control and inflammation in diabetic neuropathy: a double blind randomized clinical trial. Int J Vitam Nutr Res. 2014;84(5–6):252–260. | ||
Moradi M, Haghighatdoost F, Feizi A, Larijani B, Azadbakht L. Effect of coenzyme Q10 supplementation on diabetes biomarkers: a systematic review and meta-analysis of randomized controlled clinical trials. Arch Iran Med. 2016;19(8):588–596. | ||
Suksomboon N, Poolsup N, Juanak N. Effects of coenzyme Q10 supplementation on metabolic profile in diabetes: a systematic review and meta-analysis. J Clin Pharm Ther. 2015;40(4):413–418. | ||
Ceriello A. Oxidative stress and glycemic regulation. Metabolism. 2000;49(2 Suppl 1):27–29. | ||
Keane KN, Cruzat VF, Carlessi R, de Bittencourt PI, Newsholme P. Molecular events linking oxidative stress and inflammation to insulin resistance and β-cell dysfunction. Oxid Med Cell Longev. 2015;2015:181643. | ||
Amin MM, Asaad GF, Abdel Salam RM, El-Abhar HS, Arbid MS. Novel CoQ10 antidiabetic mechanisms underlie its positive effect: modulation of insulin and adiponectine receptors, tyrosine kinase, PI3K, glucose transporters, sRAGE and visfatin in insulin resistant/diabetic rats. PLoS One. 2014;9(2):e89169. | ||
Lee SK, Lee JO, Kim JH, et al. Coenzyme Q10 increases the fatty acid oxidation through AMPK-mediated PPARα induction in 3T3-L1 preadipocytes. Cell Signal. 2012;24(12):2329–2336. | ||
Skoglund-Andersson C, Karpe F, Hellénius ML, Regnström J, Hamsten A, Tornvall P. In vitro and in vivo lipolysis of plasma triglycerides increases the resistance to oxidative modification of low-density lipoproteins. Eur J Clin Invest. 2003;33(1):51–57. | ||
Singh RB, Niaz MA, Rastogi SS, Shukla PK, Thakur AS. Effect of hydrosoluble coenzyme Q10 on blood pressures and insulin resistance in hypertensive patients with coronary artery disease. J Hum Hypertens. 1999;13(3):203–208. | ||
Digiesi V, Cantini F, Oradei A, et al. Coenzyme Q10 in essential hypertension. Mol Aspects Med. 1994;15(Suppl):s257–s263. | ||
Pepe S, Marasco SF, Haas SJ, Sheeran FL, Krum H, Rosenfeldt FL. Coenzyme Q10 in cardiovascular disease. Mitochondrion. 2007;7(Suppl):S154–S167. | ||
Lönnrot K, Pörsti I, Alho H, Wu X, Hervonen A, Tolvanen JP. Control of arterial tone after long-term coenzyme Q10 supplementation in senescent rats. Br J Pharmacol. 1998;124(7):1500–1506. | ||
Hidaka T, Fujii K, Funahashi I, Fukutomi N, Hosoe K. Safety assessment of coenzyme Q10 (CoQ10). Biofactors. 2008;32(1-4):199–208. | ||
Haas AV, McDonnell ME. Pathogenesis of cardiovascular disease in diabetes. Endocrinol Metab Clin North Am. 2018;47(1):51–63. | ||
Carr ME. Diabetes mellitus: a hypercoagulable state. J Diabetes Complications. 2001;15(1):44–54. | ||
Watson KE, Peters Harmel AL, Matson G. Atherosclerosis in type 2 diabetes mellitus: the role of insulin resistance. J Cardiovasc Pharmacol Ther. 2003;8(4):253–260. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.